Abstract
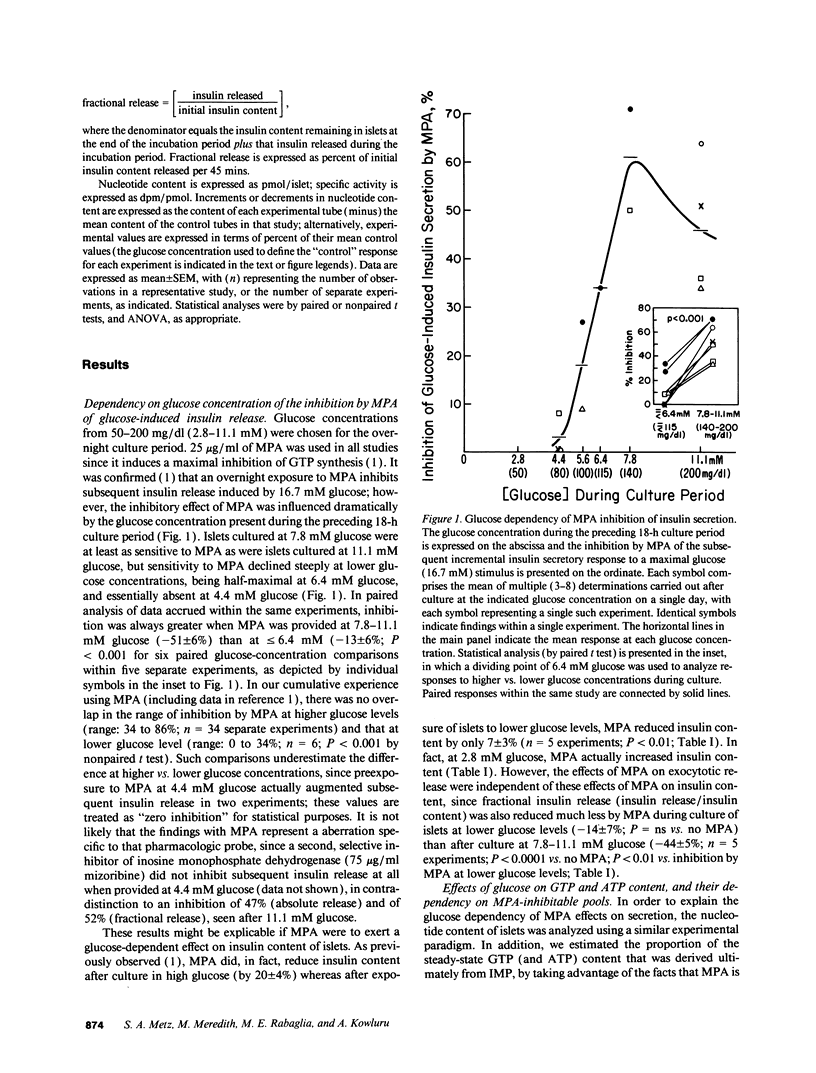
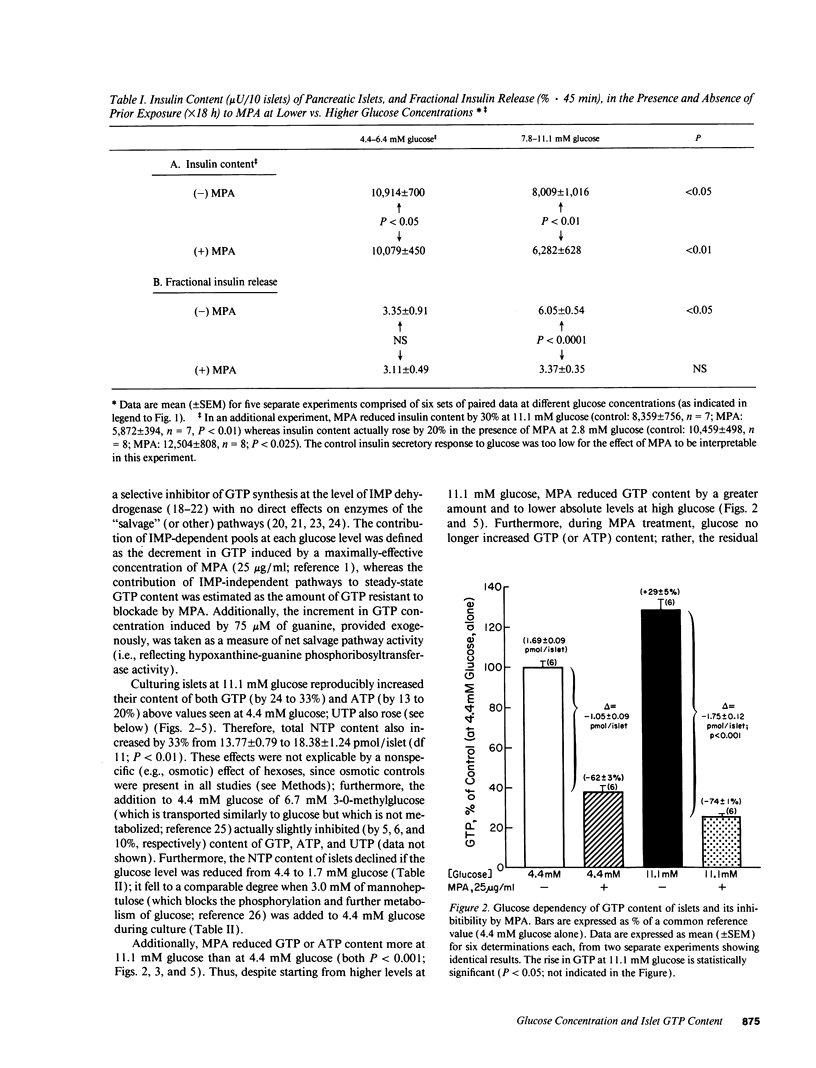
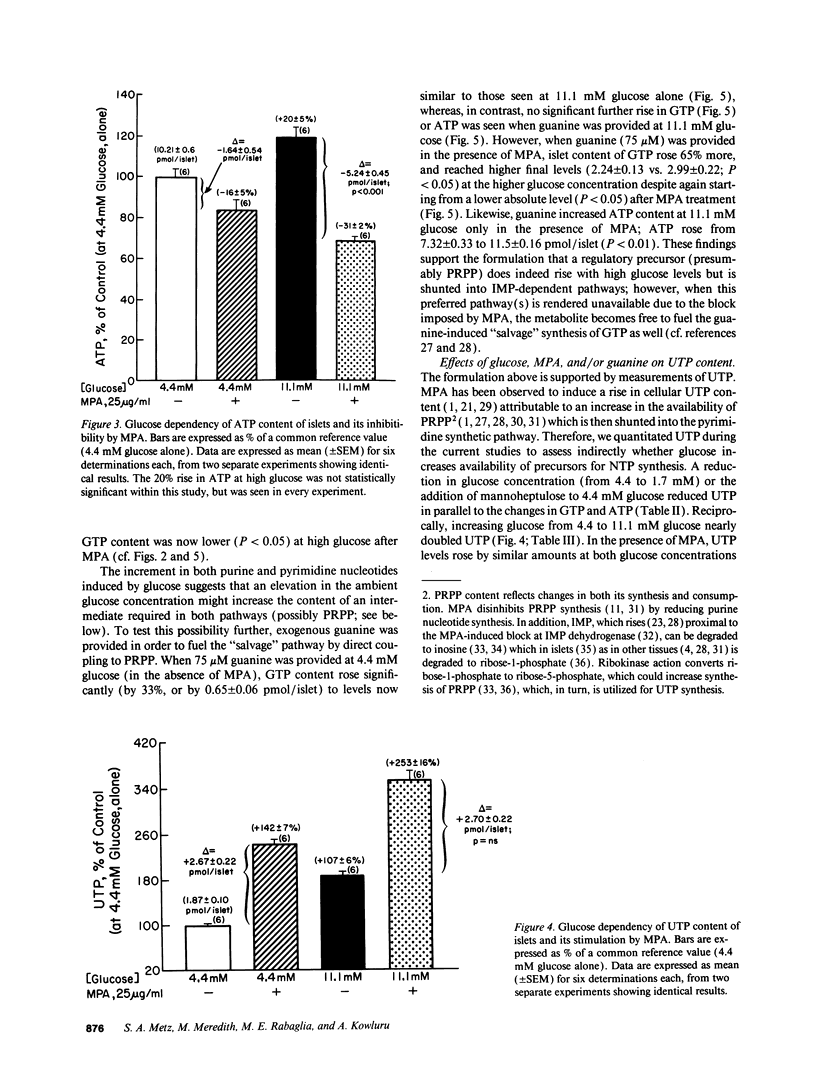
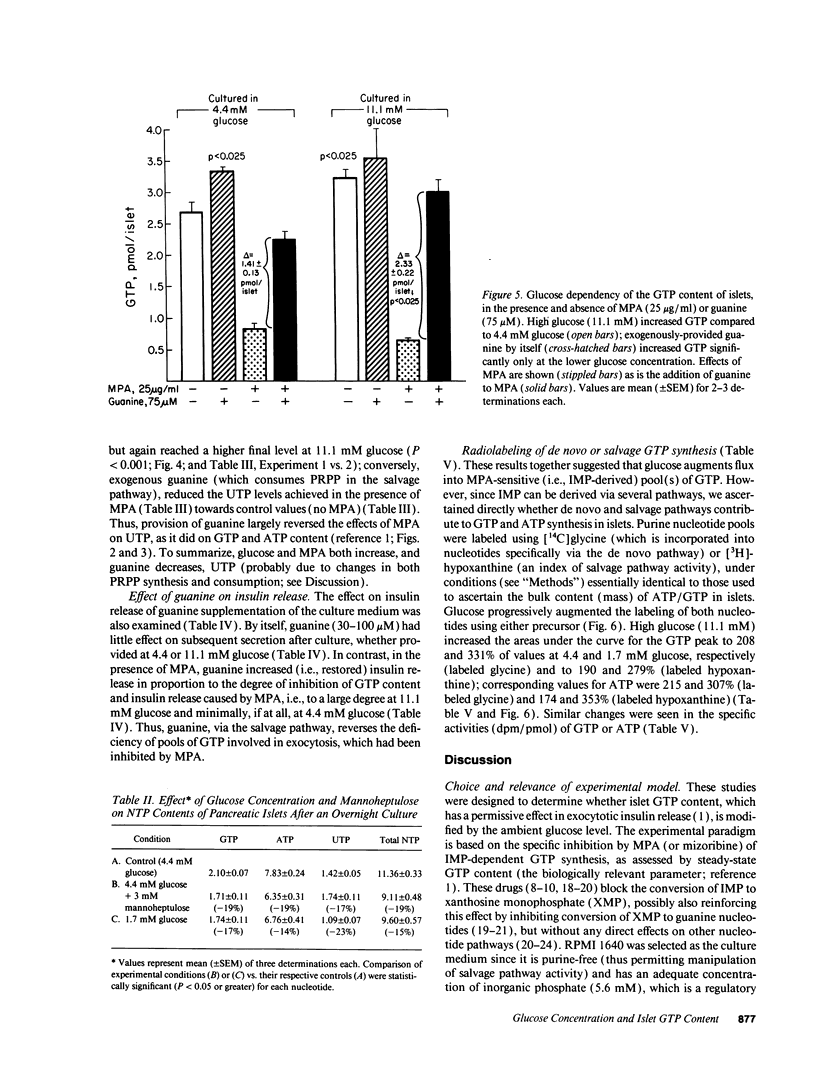
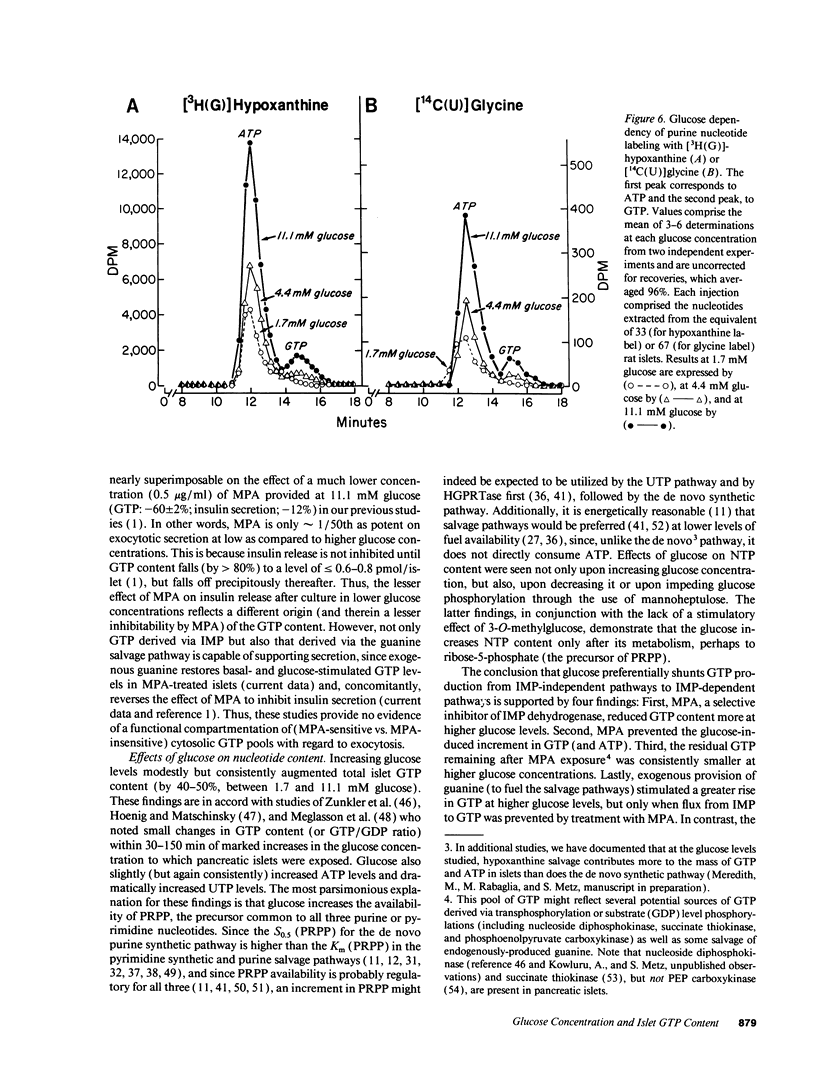
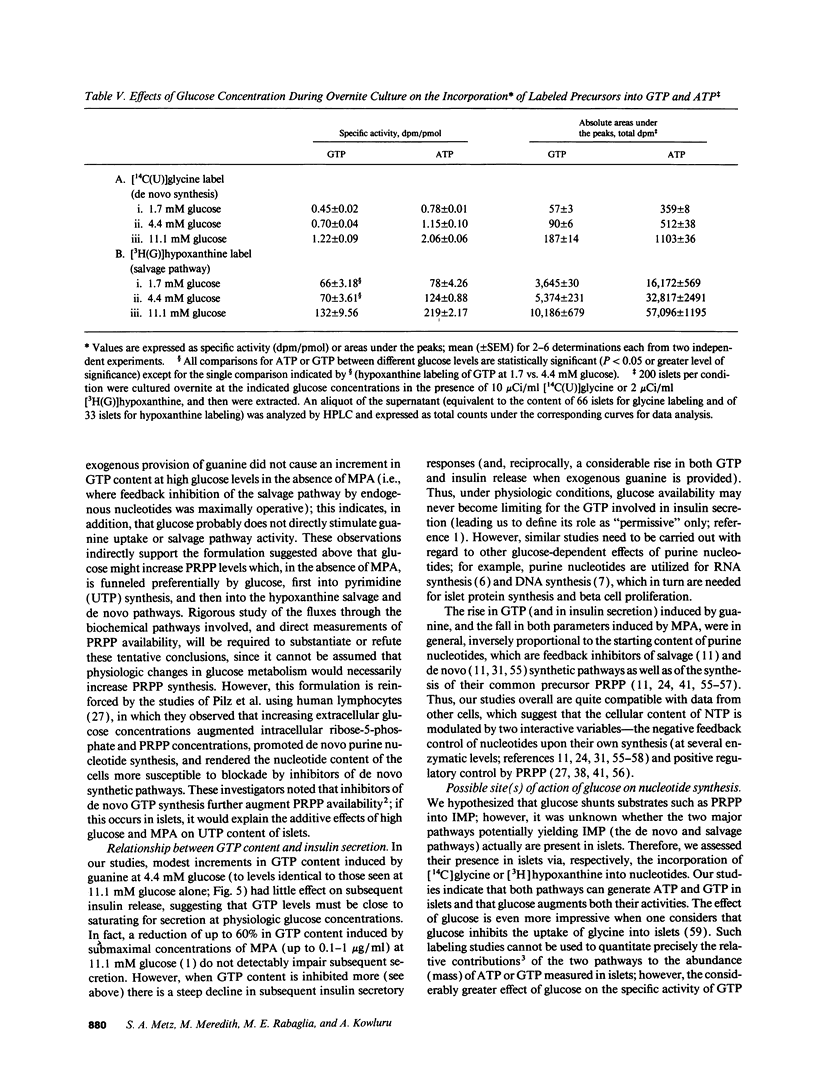
Recent studies suggest a permissive requirement for guanosine 5'-triphosphate (GTP) in insulin release, based on the use of GTP synthesis inhibitors (such as myocophenolic acid) acting at inosine monophosphate (IMP) dehydrogenase; herein, we examine the glucose dependency of GTP synthesis. Mycophenolic acid inhibited insulin secretion equally well after islet culture at 7.8 or 11.1 mM glucose (51% inhibition) but its effect was dramatically attenuated when provided at < or = 6.4 mM glucose (13% inhibition; P < 0.001). These observations were explicable by a stimulation of islet GTP synthesis derived from IMP since, at high glucose: (a) total GTP content was augmented; (b) a greater decrement in GTP (1.75 vs. 1.05 pmol/islet) was induced by mycophenolic acid; and (c) a smaller "pool" of residual GTP persisted after drug treatment. Glucose also accelerated GTP synthesis from exogenous guanine ("salvage" pathway) and increased content of a pyrimidine, uridine 5'-triphosphate (UTP), suggesting that glucose augments production of a common regulatory intermediate (probably 5-phosphoribosyl-1-pyrophosphate). Pathway-specific radiolabeling studies confirmed that glucose tripled both salvage and de novo synthesis of nucleotides. We conclude that steep changes in the biosynthesis of cytosolic pools of GTP occur at modest changes in glucose concentrations, a finding which may have relevance to the adaptive (patho) physiologic responses of islets to changes in ambient glucose levels.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978 Jun;14(6):397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- Bagnara A. S., Letter A. A., Henderson J. F. Multiple mechanisms of regulation of purine biosynthesis de novo in intact tumor cells. Biochim Biophys Acta. 1974 Dec 20;374(3):259–270. doi: 10.1016/0005-2787(74)90247-0. [DOI] [PubMed] [Google Scholar]
- Barankiewicz J., Henderson J. F. Effect of lowered intracellular ATP and GTP concentrations on purine ribonucleotide synthesis and interconversion. Can J Biochem. 1977 Mar;55(3):257–262. doi: 10.1139/o77-036. [DOI] [PubMed] [Google Scholar]
- Barankiewicz J., Henderson J. F. Ribose 1-phosphate metabolism in Ehrlich ascites tumor cells in vitro. Biochim Biophys Acta. 1977 Dec 14;479(4):371–377. doi: 10.1016/0005-2787(77)90030-2. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Kim M. Regulation of purine synthesis de novo in human fibroblasts by purine nucleotides and phosphoribosylpyrophosphate. J Biol Chem. 1987 Oct 25;262(30):14531–14537. [PubMed] [Google Scholar]
- Becker M. A., Losman M. J., Kim M. Mechanisms of accelerated purine nucleotide synthesis in human fibroblasts with superactive phosphoribosylpyrophosphate synthetases. J Biol Chem. 1987 Apr 25;262(12):5596–5602. [PubMed] [Google Scholar]
- Becker M. A., Raivio K. O., Seegmiller J. E. Synthesis of phosphoribosylpyrophosphate in mammalian cells. Adv Enzymol Relat Areas Mol Biol. 1979;49:281–306. doi: 10.1002/9780470122945.ch7. [DOI] [PubMed] [Google Scholar]
- Bergsten P. Significance of the calcium content of mouse beta cells in the preservation of glucose-induced insulin release during culture. J Endocrinol. 1987 Oct;115(1):27–34. doi: 10.1677/joe.0.1150027. [DOI] [PubMed] [Google Scholar]
- Boer P., Lipstein B., De Vries A., Sperling O. The effect of ribose 5-phosphate and 5-phosphoribosyl-1-pyrophosphate availability on de novo synthesis of purine nucleotides in rat liver slices. Biochim Biophys Acta. 1976 Apr 15;432(1):10–17. doi: 10.1016/0005-2787(76)90036-8. [DOI] [PubMed] [Google Scholar]
- Boss G. R. Decreased phosphoribosylpyrophosphate as the basis for decreased purine synthesis during amino acid starvation of human lymphoblasts. J Biol Chem. 1984 Mar 10;259(5):2936–2941. [PubMed] [Google Scholar]
- Brosh S., Sperling O., Dantziger E., Sidi Y. Metabolism of guanine and guanine nucleotides in primary rat neuronal cultures. J Neurochem. 1992 Apr;58(4):1485–1490. doi: 10.1111/j.1471-4159.1992.tb11368.x. [DOI] [PubMed] [Google Scholar]
- Bökkerink J. P., Bakker M. A., Hulscher T. W., De Abreu R. R., Schretlen E. D., van Laarhoven J. P., De Bruyn C. H. Sequence-, time- and dose-dependent synergism of methotrexate and 6-mercaptopurine in malignant human T-lymphoblasts. Biochem Pharmacol. 1986 Oct 15;35(20):3549–3555. doi: 10.1016/0006-2952(86)90625-8. [DOI] [PubMed] [Google Scholar]
- Capito K., Hedeskov C. J. Inosine-stimulated insulin release and metabolism of inosine in isolated mouse pancreatic islets. Biochem J. 1976 Aug 15;158(2):335–340. doi: 10.1042/bj1580335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. B., Sadee W. Contributions of the depletions of guanine and adenine nucleotides to the toxicity of purine starvation in the mouse T lymphoma cell line. Cancer Res. 1983 Apr;43(4):1587–1591. [PubMed] [Google Scholar]
- Crabtree G. W., Henderson J. F. Rate-limiting steps in the interconversion of purine ribonucleotides in Ehrlich ascites tumor cells in vitro. Cancer Res. 1971 Jul;31(7):985–991. [PubMed] [Google Scholar]
- Fahien L. A., MacDonald M. J., Kmiotek E. H., Mertz R. J., Fahien C. M. Regulation of insulin release by factors that also modify glutamate dehydrogenase. J Biol Chem. 1988 Sep 25;263(27):13610–13614. [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. The inhibition of nucleic acid synthesis by mycophenolic acid. Biochem J. 1969 Jul;113(3):515–524. doi: 10.1042/bj1130515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDFINGER S., KLINENBERG J. R., SEEGMILLER J. E. THE RENAL EXCRETION OF OXYPURINES. J Clin Invest. 1965 Apr;44:623–628. doi: 10.1172/JCI105175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. B., Thompson L., Johnson L. A., Emmerson B. T. Regulation of purine de novo synthesis in cultured human fibroblasts: the role of P-ribose-PP. Biochim Biophys Acta. 1979 Mar 28;562(1):162–176. doi: 10.1016/0005-2787(79)90135-7. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. F. Feedback inhibition of purine biosynthesis in ascites tumor cells. J Biol Chem. 1962 Aug;237:2631–2635. [PubMed] [Google Scholar]
- HENDERSON J. F., KHOO M. K. AVAILABILITY OF 5-PHOSPHORIBOSYL 1-PYROPHOSPHATE FOR RIBONUCLEOTIDE SYNTHESIS IN EHRLICH ASCITES TUMOR CELLS IN VITRO. J Biol Chem. 1965 Jun;240:2358–2362. [PubMed] [Google Scholar]
- Hershfield M. S., Seegmiller J. E. Regulation of de novo purine synthesis in human lymphoblasts. Similar rates of de novo synthesis during growth by normal cells and mutants deficient in hypoxanthine-guanine phosphoribosyltransferase activity. J Biol Chem. 1977 Sep 10;252(17):6002–6010. [PubMed] [Google Scholar]
- Hershko A., Razin A., Mager J. Regulation of the synthesis of 5-phosphoribosyl-I-pyrophosphate in intact red blood cells and in cell-free preparations. Biochim Biophys Acta. 1969 Jun 17;184(1):64–76. doi: 10.1016/0304-4165(69)90099-3. [DOI] [PubMed] [Google Scholar]
- Hoenig M., Matschinsky F. M. HPLC analysis of nucleotide profiles in glucose-stimulated perifused rat islets. Metabolism. 1987 Mar;36(3):295–301. doi: 10.1016/0026-0495(87)90192-2. [DOI] [PubMed] [Google Scholar]
- Holmes E. W., McDonald J. A., McCord J. M., Wyngaarden J. B., Kelley W. N. Human glutamine phosphoribosylpyrophosphate amidotransferase. Kinetic and regulatory properties. J Biol Chem. 1973 Jan 10;248(1):144–150. [PubMed] [Google Scholar]
- Howell S. B., Herbst K., Boss G. R., Frei E., 3rd Thymidine requirements for the rescue of patients treated with high-dose methotrexate. Cancer Res. 1980 Jun;40(6):1824–1829. [PubMed] [Google Scholar]
- Kagawa D., Nakamura T., Ueda T., Ando S., Tsutani H., Uchida M., Domae N., Sasada M., Uchino H. Reverse effect of guanine on the inhibitory action of mycophenolic acid during nucleic acid synthesis. Anticancer Res. 1986 Jul-Aug;6(4):643–648. [PubMed] [Google Scholar]
- Kim Y. A., King M. T., Teague W. E., Jr, Rufo G. A., Jr, Veech R. L., Passonneau J. V. Regulation of the purine salvage pathway in rat liver. Am J Physiol. 1992 Mar;262(3 Pt 1):E344–E352. doi: 10.1152/ajpendo.1992.262.3.E344. [DOI] [PubMed] [Google Scholar]
- Koyama H., Tsuji M. Genetic and biochemical studies on the activation and cytotoxic mechanism of bredinin, a potent inhibitor of purine biosynthesis in mammalian cells. Biochem Pharmacol. 1983 Dec 1;32(23):3547–3553. doi: 10.1016/0006-2952(83)90301-5. [DOI] [PubMed] [Google Scholar]
- Lee H. J., Pawlak K., Nguyen B. T., Robins R. K., Sadée W. Biochemical differences among four inosinate dehydrogenase inhibitors, mycophenolic acid, ribavirin, tiazofurin, and selenazofurin, studied in mouse lymphoma cell culture. Cancer Res. 1985 Nov;45(11 Pt 1):5512–5520. [PubMed] [Google Scholar]
- Liang Y., Najafi H., Smith R. M., Zimmerman E. C., Magnuson M. A., Tal M., Matschinsky F. M. Concordant glucose induction of glucokinase, glucose usage, and glucose-stimulated insulin release in pancreatic islets maintained in organ culture. Diabetes. 1992 Jul;41(7):792–806. doi: 10.2337/diab.41.7.792. [DOI] [PubMed] [Google Scholar]
- Lui M. S., Faderan M. A., Liepnieks J. J., Natsumeda Y., Olah E., Jayaram H. N., Weber G. Modulation of IMP dehydrogenase activity and guanylate metabolism by tiazofurin (2-beta-D-ribofuranosylthiazole-4-carboxamide). J Biol Chem. 1984 Apr 25;259(8):5078–5082. [PubMed] [Google Scholar]
- MacDonald M. J., McKenzie D. I., Walker T. M., Kaysen J. H. Lack of glyconeogenesis in pancreatic islets: expression of gluconeogenic enzyme genes in islets. Horm Metab Res. 1992 Apr;24(4):158–160. doi: 10.1055/s-2007-1003284. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Nelson J., Nelson D., Erecinska M. Bioenergetic response of pancreatic islets to stimulation by fuel molecules. Metabolism. 1989 Dec;38(12):1188–1195. doi: 10.1016/0026-0495(89)90158-3. [DOI] [PubMed] [Google Scholar]
- Metz S. A., Dunlop M. Stimulation of insulin release by phospholipase D. A potential role for endogenous phosphatidic acid in pancreatic islet function. Biochem J. 1990 Sep 1;270(2):427–435. doi: 10.1042/bj2700427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz S. A. Exogenous arachidonic acid promotes insulin release from intact or permeabilized rat islets by dual mechanisms. Putative activation of Ca2+ mobilization and protein kinase C. Diabetes. 1988 Nov;37(11):1453–1469. doi: 10.2337/diab.37.11.1453. [DOI] [PubMed] [Google Scholar]
- Metz S. A. Glucose increases the synthesis of lipoxygenase-mediated metabolites of arachidonic acid in intact rat islets. Proc Natl Acad Sci U S A. 1985 Jan;82(1):198–202. doi: 10.1073/pnas.82.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz S. A., Rabaglia M. E., Pintar T. J. Selective inhibitors of GTP synthesis impede exocytotic insulin release from intact rat islets. J Biol Chem. 1992 Jun 25;267(18):12517–12527. [PubMed] [Google Scholar]
- Morris R. E., Wang J., Blum J. R., Flavin T., Murphy M. P., Almquist S. J., Chu N., Tam Y. L., Kaloostian M., Allison A. C. Immunosuppressive effects of the morpholinoethyl ester of mycophenolic acid (RS-61443) in rat and nonhuman primate recipients of heart allografts. Transplant Proc. 1991 Apr;23(2 Suppl 2):19–25. [PubMed] [Google Scholar]
- Natsumeda Y., Prajda N., Donohue J. P., Glover J. L., Weber G. Enzymic capacities of purine de Novo and salvage pathways for nucleotide synthesis in normal and neoplastic tissues. Cancer Res. 1984 Jun;44(6):2475–2479. [PubMed] [Google Scholar]
- Pilz R. B., Willis R. C., Boss G. R. The influence of ribose 5-phosphate availability on purine synthesis of cultured human lymphoblasts and mitogen-stimulated lymphocytes. J Biol Chem. 1984 Mar 10;259(5):2927–2935. [PubMed] [Google Scholar]
- Puig J. G., Jiménez M. L., Mateos F. A., Fox I. H. Adenine nucleotide turnover in hypoxanthine-guanine phosphoribosyl-transferase deficiency: evidence for an increased contribution of purine biosynthesis de novo. Metabolism. 1989 May;38(5):410–418. doi: 10.1016/0026-0495(89)90189-3. [DOI] [PubMed] [Google Scholar]
- Raivio K. O., Seegmiller J. E. Role of glutamine in purine synthesis and in guanine nucleotide formation in normal fibroblasts and in fibroblasts deficient in hypoxanthine phosphoribosyltransferase activity. Biochim Biophys Acta. 1973 Mar 19;299(2):283–292. doi: 10.1016/0005-2787(73)90351-1. [DOI] [PubMed] [Google Scholar]
- Rizzo M. T., Tricot G., Hoffman R., Jayaram H. N., Weber G., Garcia J. G., English D. Inosine monophosphate dehydrogenase inhibitors. Probes for investigations of the functions of guanine nucleotide binding proteins in intact cells. Cell Signal. 1990;2(6):509–519. doi: 10.1016/0898-6568(90)90073-j. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K., Tsujino M., Yoshizawa M., Mizuno K., Hayano K. Action of bredinin on mammalian cells. Cancer Res. 1975 Jul;35(7):1643–1648. [PubMed] [Google Scholar]
- Sehlin J. Transport and oxidation of glycine in mammalian pancreatic islets with reference to the mechanism of amino acid-induced insulin release. Hormones. 1972;3(3):144–155. doi: 10.1159/000178264. [DOI] [PubMed] [Google Scholar]
- Smith C. M., Henderson J. F. Relative importance of alternative pathways of purine nucleotide biosynthesis in Ehrlich ascites tumor cells in vivo. Can J Biochem. 1976 Apr;54(4):341–349. doi: 10.1139/o76-050. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Henderson J. F., Cook D. A. Inhibition of purine metabolism--computer-assisted analysis of drug effects. Biochem Pharmacol. 1972 Sep 1;21(17):2351–2357. doi: 10.1016/0006-2952(72)90386-3. [DOI] [PubMed] [Google Scholar]
- Sollinger H. W., Deierhoi M. H., Belzer F. O., Diethelm A. G., Kauffman R. S. RS-61443--a phase I clinical trial and pilot rescue study. Transplantation. 1992 Feb;53(2):428–432. doi: 10.1097/00007890-199202010-00031. [DOI] [PubMed] [Google Scholar]
- Sweeney M. J., Hoffman D. H., Esterman M. A. Metabolism and biochemistry of mycophenolic acid. Cancer Res. 1972 Sep;32(9):1803–1809. [PubMed] [Google Scholar]
- Thompson L. F., Willis R. C., Stoop J. W., Seegmiller J. E. Purine metabolism in cultured human fibroblasts derived from patients deficient in hypoxanthine phosphoribosyltransferase, purine nucleoside phosphorylase, or adenosine deaminase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3722–3726. doi: 10.1073/pnas.75.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turka L. A., Dayton J., Sinclair G., Thompson C. B., Mitchell B. S. Guanine ribonucleotide depletion inhibits T cell activation. Mechanism of action of the immunosuppressive drug mizoribine. J Clin Invest. 1991 Mar;87(3):940–948. doi: 10.1172/JCI115101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh N., Sandler S., Welsh M., Hellerström C. Regulation of RNA metabolism in relation to insulin production and oxidative metabolism in mouse pancreatic islets in vitro. Biochim Biophys Acta. 1986 Jun 16;887(1):58–68. doi: 10.1016/0167-4889(86)90122-9. [DOI] [PubMed] [Google Scholar]
- Wood A. W., Becker M. A., Seegmiller J. E. Purine nucleotide synthesis in lymphoblasts cultured from normal subjects and a patient with Lesch-Nyhan syndrome. Biochem Genet. 1973 Jul;9(3):261–274. doi: 10.1007/BF00485739. [DOI] [PubMed] [Google Scholar]
- Yen R. C., Raivio K. O., Becker M. A. Inhibition of phosphoribosylpyrophosphate synthesis in human fibroblasts by 6-methylthioinosinate. J Biol Chem. 1981 Feb 25;256(4):1839–1845. [PubMed] [Google Scholar]
- Zawalich W. S. Intermediary metabolism and insulin secretion from isolated rat islets of Langerhans. Diabetes. 1979 Mar;28(3):252–262. doi: 10.2337/diab.28.3.252. [DOI] [PubMed] [Google Scholar]


