Abstract
Spontaneous preterm birth is a frequent complication of pregnancy and a common cause of morbidity in childhood. Obstetricians suspect abnormalities of the cervix are implicated in a significant number of preterm births. The cervix is composed of fibrous connective tissue and undergoes significant remodeling in preparation for birth. We hypothesized that a tissue engineering strategy could be used to develop three-dimensional cervical-like tissue constructs that would be suitable for investigating cervical remodeling. Cervical cells were isolated from two premenopausal women undergoing hysterectomy for a benign gynecological condition, and the cells were seeded on porous silk scaffolds in the presence or absence of dynamic culture and with 10% or 20% serum. Morphological, biochemical, and mechanical properties were measured during the 8-week culture period. Cervical cells proliferated in three-dimensions and synthesized an extracellular matrix with biochemical constituents and morphology similar to native tissue. Compared to static culture, dynamic culture was associated with significantly increased collagen deposition (p < 0.05), sulfated glycosaminoglycan synthesis (p < 0.05), and mechanical stiffness (p < 0.05). Serum concentration did not affect measured variables. Relevant human tissue-engineered cervical-like constructs constitute a novel model system for a range of fundamental and applied studies related to cervical remodeling.
Introduction
In the United States, preterm birth occurs in greater than 500,000 pregnancies per year or 12.7% of all pregnancies.1 Preterm birth is a common contributor to morbidity and mortality in newborn infants2 and an important cause of childhood learning disability and cerebral palsy.3 Preterm birth is also associated with significant cost. The Institute of Medicine estimates the average hospital cost per newborn admission is $51,000, resulting in an annual healthcare cost in the United States of $26 billion.4 Despite a substantial research effort aimed at identifying the causes of preterm birth, the incidence of preterm birth is increasing5 and an effective therapy to prevent preterm birth remains elusive.6
Obstetricians suspect that abnormalities of the cervix are implicated in a significant number of preterm births.7 The cervix is composed of fibrous connective tissue and is located in anatomic continuity with the bottom of the uterus and top of the vagina.8 In normal pregnancy, the cervix has an important load-bearing function because it maintains its anatomic shape during fetal and uterine growth. In pregnancies at risk for preterm birth, the cervix shortens and dilates prematurely.7,9 Hence, a short cervix is used to identify women at high risk for preterm birth10 and identify women who benefit from therapy to prevent preterm birth.11–13
The disease that relates most directly to an impaired cervix is cervical insufficiency (also known as cervical incompetence). In patients with cervical insufficiency, the cervix is impaired because the cervix opens prematurely even though uterine contractions are absent.14 The treatment for cervical insufficiency is cervical cerclage, which is a suture placed around the cervix. The purpose of a cerclage is to prevent premature dilation by providing load-bearing support. Although the efficacy of cerclage is controversial in the clinical literature,15 cerclage appears to benefit carefully selected patients,13,15,16 especially in the absence of intrauterine infection or hemorrhage.17,18
In engineering terms, an impaired cervix relates most specifically to impaired mechanical properties of cervical stroma. The mechanical properties of the cervical stroma are derived from its extracellular matrix (ECM),19,20 which undergoes complex remodeling during pregnancy.21 Whereas the uterus is primarily composed of smooth muscle, the cervix is a fibrous connective tissue with over 50% of the dry weight as collagen.20 Proteoglycans22 and hyaluronan23 are present in significant quantities and are suspected to regulate collagen organization and tissue hydration. Cervical elastin is also present, which provides tissue elasticity.24 Changes in collagen organization,25 proteoglycan composition,26 and cervical inflammation27 have been linked to cervical remodeling during pregnancy.
The connection between cervical remodeling, cervical mechanical properties, and preterm birth is not well defined because current model systems have important limitations. In previous work, we used human cervical tissue from hysterectomy specimens (pregnant and nonpregnant) to investigate cervical remodeling associated with pregnancy.19,25 However, our prior work was limited because it was difficult to obtain cervical tissue from pregnancy of sufficient size to permit both biochemical and mechanical characterization. Many animal models (mouse,28 rat,29 primate,30 rabbit,31 and guinea pig32) have been investigated to study the cervix during pregnancy, but cervical remodeling in animal models may not reflect changes in the human cervix. Therefore, a tissue engineering model using human cells may permit studies previously unavailable using conventional model systems. To our knowledge, investigation of cervical remodeling using a tissue engineering system has not been attempted.
The purpose of this study was to explore the feasibility of using a tissue engineering strategy to develop three-dimensional cervical-like constructs. For this purpose, primary human cervical cells were cultured for 8 weeks on porous silk scaffolds in a spinner flask bioreactor system followed by biochemical and mechanical characterization.
Materials and Methods
Cell culture
Informed consent was obtained from two Caucasian women scheduled for hysterectomies for benign gynecological conditions. Patient #1 was 41 years old, and her obstetric history was notable for a prior cesarean delivery at term. Patient #2 was 34 years old, and her obstetric history was notable for a prior term vaginal delivery. The study protocol was approved by the Institutional Review Board at Tufts Medical Center. Cervical cells were isolated using previously published procedures.33 In brief, immediately after the surgical specimen was removed, a wedge biopsy was obtained from the cervical stroma (mid canal region) under sterile conditions. The cervical epithelium was removed from the biopsy and discarded. The biopsy was divided into two portions and used for (1) isolation of cervical cells and (2) assessment of native tissue morphology and biochemical content. The portion used for cell isolation was transferred to a tissue culture hood in growth medium consisting of Dulbecco's modified Eagle's medium (#11995-065; Invitrogen, Carlsbad CA), supplemented with 10% fetal bovine serum (FBS) (#16000-044; Invitrogen), 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin B (#15240; Invitrogen). The biopsy was minced and maintained in a humidified tissue culture incubator at 37°C, 5% CO2/95% air, and 95% relative humidity. At day 14, cells were confluent around the explants at which point cells were passaged and culture-expanded in 185 cm2 tissue culture flasks (#144903; Nunc, Rochester, NY). The population doubling time was 3 days. Cells from passage 5 were used to seed scaffolds.
Silk protein sponge scaffold
Silk was chosen for the scaffold biomaterial for two reasons: (1) techniques for controlling the morphological and functional properties of the silk protein scaffolds, including support for cell and tissue growth, are well known to the laboratory,34 and (2) by controlling the pore size, the mechanical properties of scaffolds can be adjusted to mimic native tissue.19,35 Briefly, cocoons of the Bombyx mori silkworm (Tajima Shoji, Yokohami, Japan) were cut into 2 mm pieces and boiled for 30 min in an aqueous solution of 0.02 M Na2CO3, which yielded purified silk fibroin protein. Silk protein was solubilized in 9.3 M LiBr solution at 60°C for 4 h, dialyzed against distilled water for 2 days, and diluted to obtain a 6% (w/v) silk fibroin solution. Silk sponges were prepared by adding 44 g of granular NaCl (particle size 610–725 μm) to 22 mL silk fibroin solution in a rectangular Delrin mold (115 × 72 mm). The container was covered, left at room temperature for 24 h, and then moved to an oven at 60°C until solidified, usually overnight. The lid was removed, and the sponge scaffold was immersed in water for 2 days to extract the NaCl. After removal from the mold, the sponge scaffold was washed for 24 h in water. The sponge scaffold was cut into 18 smaller, rectangular scaffolds (12 × 35 × 3–4 mm) with a cutting tool (Chopper II, NWSL, Seattle, WA). To facilitate nutrition delivery and create scaffolds of uniform thickness, scaffolds were further processed such that the thickness was 1.0–1.2 mm. To create thin scaffolds, they were embedded in paraffin wax (Histowax™ LT; Leica, Bannockburn, IL), mounted in a rotary microtome (RMT-30; Radical Instruments, Haryana, India), and sliced to the desired thickness. The wax was removed by three washes in hexane (#15671; Sigma-Aldrich, St. Louis, MO) followed by washes of 2 min each of 100%, 95%, 70% ethanol, and water to rehydrate the scaffold. Scaffolds were autoclaved for sterilization (Fig. 1A). Before seeding, sterile scaffolds were rehydrated overnight in phosphate-buffered saline (PBS) in a six-well plate.
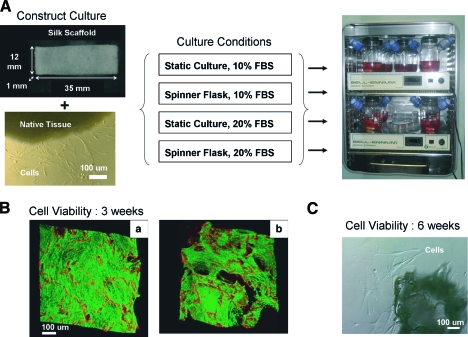
FIG. 1.
(A) Cervical cells isolated from native tissue were seeded on a rectangular porous silk scaffold (dimensions 35 × 12 × 1 mm) in the presence or absence of media mixing and with 10% or 20% serum. (B) After 3 weeks, viable cells proliferated to fill the pores of the scaffold. Improved proliferation was seen with a more concentrated [20 × 106 cells/mL, (a)] versus a less concentrated [10 × 106 cells/mL, (b)] cell suspension. (C) After 6 weeks, viable cells grew out from cultured scaffold explants. FBS, fetal bovine serum. Color images available online at www.liebertonline.com/ten.
Construct seeding
Preliminary experiments revealed that cells proliferated poorly on the silk surface. However, when silk was coated with a collagen solution, cell proliferation was equivalent to tissue culture plastic. Hence, all silk scaffolds were collagen coated by immersion in a 0.5 mg/mL collagen solution for 5 min (lyophilized rat tail collagen [Roche Diagnostics, Indianapolis, IN] in 0.02 N glacial acetic acid), drying in a culture hood for 2 h, washing with PBS, and rehydrating in culture media for 30 min. To seed the scaffold, 750 μL of concentrated cell suspension (20 × 106 cells/mL, 15 million cells per scaffold) was applied in a drop-wise fashion over the scaffold surface in a six-well plate. The plate was transferred to the incubator for 2 h to promote cell attachment, after which 5 mL of media were added to each well and the scaffolds were cultured overnight. After 24 h, the scaffolds were moved to new six-well plates. After 2 days, the scaffolds were transferred to the bioreactor for long-term culture.
Bioreactor
Scaffolds were cultured in a previously described spinner flask system.35,36 Briefly, scaffolds were suspended on steel wire and sandwiched between two small pieces of C-flex tubing. The wire was inserted into a solid rubber stopper (Fisher Scientific, Pittsburgh, PA) and placed in a 250 mL spinner flask bioreactor (#1967-10250; Bellco Glass, Vineland, NJ). The side arms of the spinner flask were covered with vented screw caps (#165-00032; Bellco Glass) attached to disposable filter units (Millex-GS 0.22 μm pore size, #SLGS033SS; Millipore, Billerica, MA) to allow gas exchange. To promote dynamic culture, a magnetic stir bar turned at 50 rpm (Bell-Ennium D2005; Bellco Glass). Static culture conditions were identical to spinner flask conditions except that no stir bar was used. Scaffolds were cultured in 150 mL of media supplemented with freshly prepared 50 μg/mL ascorbic acid 2-phosphate (A8960; Sigma-Aldrich) in a humidified incubator at 37°C, 5% CO2/95% air. Three times per week, 75 mL (50% volume) of the media was replaced. Every 2 weeks, the scaffolds were transferred to a newly autoclaved spinner flask. No contamination issues were observed over the course of the experiment.
Scaffolds were divided into four groups (Fig. 1A): static versus spinner flask and 10% versus 20% FBS. To ensure consistency, serum from only one lot number (#448265) was used. Scaffolds were harvested at 2, 4, 6, and 8 weeks and tested immediately (mechanical testing), processed for histology, or stored at −80°C until biochemical analysis.
Cell viability
The viability of the cervical cells on the scaffold was assessed with two methods: (1) a two-color fluorescence assay (LIVE/DEAD viability/cytotoxicity kit; Invitrogen) and (2) an explant method. For the LIVE/DEAD assay, a scaffold (3-week culture time) was incubated with two probes: 2 mM calcein AM and 1 mM ethidium homodimer-1 for 30 min at room temperature. In the presence of viable cells, calcein AM is converted to calcein, a green fluorescent product. Ethidium homodimer-1 is a red fluorescent stain that preferentially binds to both silk scaffold as well as DNA of membrane compromise dead cells (Fig. 1B). The fluorescence signal was observed using a confocal microscope (DM IRE2 microscope; Leica, Wetzlar, Germany). For the explant method, a 6-week scaffold was minced and further cultured for 1 week in a six-well plate (Fig. 1C).
Histology and immunohistochemistry
Histology and immunohistochemistry were performed to compare construct morphology and cell phenotype to native tissue. Staining protocols were performed on 5-μm-thick slices taken from formalin-fixed, paraffin-embedded blocks. Hematoxylin and eosin (H&E) staining was performed using standard procedures. For immunohistochemistry, the following antibodies were used: anti-Vimentin (#790-2917; Ventana Medical Systems, Tucson, AZ), anti pan-Keratin (#760-2595; Ventana Medical Systems), and anti-actin smooth muscle (#AM128-10M; Biogenex, San Ramon, CA) using the manufacturer's protocol on the Benchmark XT (Ventana Medical Systems). As a positive control, the same staining protocols were performed on native cervical tissue from which the cells were cultured (Fig. 2).
FIG. 2.
Immunohistochemistry for cell markers in native cervical tissue versus 8-week constructs. In the constructs, cells stained positive for vimentin and α-smooth muscle actin, which suggests a mix of fibroblasts and myofibroblast phenotypes. No epithelial cells were observed in the constructs. Scale bars are 100 μm. Color images available online at www.liebertonline.com/ten.
Biochemical characterization
Cultured scaffolds and native tissue were assayed for (1) hydration, (2) collagen concentration, (3) sulfated glycosaminoglycan (S-GAG) concentration, and (4) DNA concentration. To measure hydration, pieces of scaffold or tissue (20–30 mg) were homogenized in a Bessman tissue pulverizer that was precooled in liquid nitrogen. The frozen, crushed powder was placed in a preweighed 1.5 mL microcentrifuge tube, and weighed. After overnight lyophilization, the powder was re-weighed and hydration was determined: hydration = (wet weight − dry weight)/wet weight. To measure collagen, the dry powder was hydrolyzed in sealed Kimax tubes in 6 M HCl at 115°C overnight (1 mL acid per 2.5 mg dry weight). Acid was evaporated at 95°C and the hydrolysate was resuspended in 1.5 mL water. Collagen concentration was determined using a standard assay for hydroxyproline.37 To measure S-GAG and DNA concentration, minced pieces of scaffold or native tissue (20–30 mg wet weight) were digested in 400 μL of 1.0 mg/mL Proteinase K (#03115887001; Roche Diagnostics) in digestion buffer (50 mM Tris HCl, 1 mM EDTA, pH 8.0) at 50°C overnight. The digested solutions were clarified by centrifugation at 10,000 g for 10 min, and the supernatants were assayed. S-GAG concentrations was determined using a 1,9-dimethylmethylene blue dye label (Blyscan Assay kit; Biocolor, Carrick Fergus, United Kingdom) and chondroitin-6-sulfate for the standard curve. DNA concentration was determined using a fluorescent nucleic acid stain (Quant-iT PicoGreen dsDNA kit; Invitrogen) and Lambda DNA as the standard curve.
Second harmonic imaging
Second harmonic imaging (SHG) was used to observe collagen deposition and quantify the relative collagen content of the constructs based on our recent method.38 SHG micrographs were obtained from 5-μm-thick histologic sections after H&E staining. A Leica DM IRE2 microscope with a TCS SP2 scanner (Wetzlar, Germany) was used. The excitation light source was a Mai Tai tunable (710–920 nm) titanium sapphire laser (Spectra Physics, Mountain View, CA). SHG images were acquired in the forward direction with 800 nm incident light through a bandpass filter centered at 400 nm (Chroma hq400/20m-2p) using a 10 × 0.4 NA objective. Analysis was performed with Leica Confocal Software (Wetzlar, Germany) and ImageJ (Rasband, W.S., ImageJ; U.S. National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997–2008). Three SHG micrographs from each construct were obtained. The image size was 1.5 × 1.5 mm. SHG images were thresholded to remove random noise and converted to a binary format (pixel values either 0 or 254), and the collagen content was determined by measuring the average image intensity. To observe the full thickness of the scaffold, individual images were combined into a single mosaic (Fig. 3).
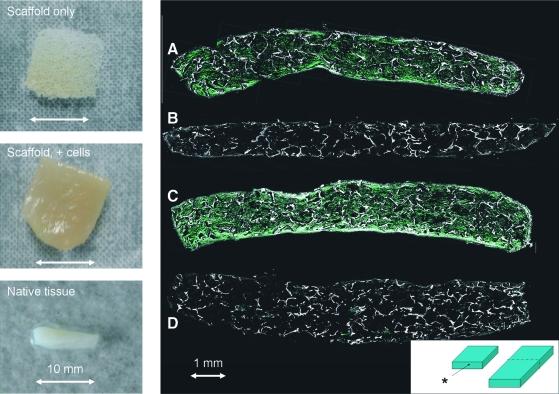
FIG. 3.
The left panels show the gross appearance of an unseeded scaffold, a seeded scaffold, and native tissue. The scale bar is 10 mm. The right panel shows second harmonic images from (A) spinner flask, 10% serum, (B) static culture, 10% serum, (C) spinner flask, 20% serum, and (D) static culture, 20% serum. The graphic at the bottom right shows the section from which the images were taken. The asterisk indicates the section of the image. Scale bar is 1 mm. Color images available online at www.liebertonline.com/ten.
Real-time quantitative reverse transcription–polymerase chain reaction
Gene expression was compared (1) between culture conditions (static vs. spinner flask) and (2) over time (relative to 1 day of culture). Gene expression was measured using 10% FBS concentration in the media. Constructs used for RNA extraction were flash frozen in liquid nitrogen and stored at −80°C until time of assay. Total RNA was extracted from constructs using the RNeasy Fibrous Tissue Kit (Qiagen, Valencia, CA). Small pieces of frozen scaffold (20–30 mg) were homogenized using a Bessman tissue pulverizer that was precooled with liquid nitrogen. The frozen, crushed powder was placed in a guanidine-based lysis solution (Buffer RLT containing β-Mercaptoethanol) and further homogenized using an 18-gauge needle. The lysate was incubated in Proteinase K for 10 min at 55°C following the manufacturer's protocol. RNA was purified on the RNeasy spin column, and an on-column DNase digestion was performed following the manufacturer's protocol. Purified RNA was eluted with 50 μL of water and stored at −80°C. The A260/A280 ratio was above 2.0 for all samples tested, and the RNA concentration was 0.05–0.1 μg/μL. Reverse transcription reactions (50 μL RNA: 50 μL reverse transcription Master Mix) were performed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) following the manufacturer's instructions. cDNA was stored at −20°C.
Quantitative gene expression was measured with the Stratagene Mx 3000P QPCR System (Stratagene, La Jolla, CA), and primer and probe sets using TaqMan® chemistry (Applied Biosystems). Each 50 μL reaction consisted of 25 μL TaqMan Gene Expression Master Mix + 5 μL cDNA template + 17.5 μL RNase-free water + 2.5 μL TaqMan Gene Expression Assay. Reactions took place at 50°C for 2 min, 95°C for 10 min, followed by 50 amplification cycles consisting of a denaturation step at 95°C for 15 s, and an extension step at 60°C for 1 min. The following six genes associated with cervical ECM remodeling were measured: collagen type 1 (COL1A1; assay ID Hs00164004_m1), collagen type 3 (COL3A1; assay ID Hs00164103_m1), decorin (DCN; assay ID Hs00370384_m1), hyaluronan synthase 2 (HAS2; assay ID Hs00193435_m1), matrix metallopeptidase 1 (MMP1; assay ID Hs00233958_m1), and matrix metallopeptidase 3 (MMP3; assay ID Hs00233962_m1). In addition, two genes served as negative controls: collagen type 2 (COL2A1; assay ID Hs00156568_m1) and aggrecan (ACAN; assay ID Hs00153936_m1). All genes were normalized by the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH; assay ID Hs99999905_m1). The cycle threshold was calculated from baseline-corrected, normalized fluorescence data using instrument software. Each data point represented the mean ± standard error of the mean of four biological replicates. Quantification was expressed as fold change relative to gene expression at day 1 of static culture using the 2−(ΔΔCt) method.
Mechanical testing
To determine whether synthesis of ECM resulted in a measurable mechanical signal, a uniaxial tension test was performed on seeded and unseeded scaffolds. Tension tests were performed using a Zwick/Roell Z2.5/TS1S materials testing machine and TestXpert V10.1 master software (Ulm, Germany) with a 20 N load cell. All scaffolds were hydrated in PBS, and all tests were conducted at room temperature. Tensile tests consisted of three uniaxial load–unload cycles at a constant nominal strain rate of 0.5%/s. The gauge length was 10 mm, and scaffolds were loaded to 10% tensile strain (1 mm) for each cycle. The cross-sectional area was measured with calipers. Each scaffold was fixed into stainless steel tension grips with silicon carbide sandpaper to prevent slipping. Extensive preliminary testing of unseeded scaffolds was conducted to optimize specimen preparation and testing protocols. Average peak stress at 10% strain for the first cycle was recorded. Control scaffolds consisted of unseeded scaffolds cultured for the same time period and in the same culture conditions as seeded scaffolds.
Model repeatability
As noted above, cervical cells from two patients were cultured. The model system described above was developed with cells from patient #1. To verify that the model system was functional with cells from more than one patient, cells from patient #2 were cultured in static and spinner flask conditions for 7 weeks. The serum concentration was 10%. Constructs were harvested at 4 and 7 weeks and were evaluated for gross morphology and histology (H&E).
Statistical analysis
Data are expressed as mean values ± standard error of the mean. Mean values were calculated from three to five replicates as indicated. Mean values were compared using Student's t-test or one-way analysis of variance with Tukey's multiple comparison procedure (Minitab 15, State College, PA) as appropriate. Results were considered significant when p < 0.05.
Results
Evaluation of cell viability and phenotype
After 3 weeks in static culture, the cervical cells proliferated to fill the pores of the scaffold (Fig. 1B). Seeding the scaffolds at a lower cell density (10 million cells/mL) resulted in nonuniform cell distribution (compare Fig. 1B, left vs. right). At 6 weeks of culture, cervical cells grew out of the explants (Fig. 1C), confirming that the cells remained viable. Regarding cell phenotype, the native cervical tissue cell population is known to consist of fibroblasts (positive vimentin, negative α-smooth muscle actin, and negative cytokeratin), epithelial cells (negative vimentin, negative α-smooth muscle actin, and positive cytokeratin), and smooth muscle cells (positive vimentin, positive α-smooth muscle actin, and negative cytokeratin), as shown in Figure 2. Constructs stained positive for both vimentin and α-smooth muscle actin, which is most consistent with a mixed fibroblast and myofibroblast phenotype. Epithelial cells were not observed because (1) the cervical epithelium was discarded before explant culture and (2) the culture media favored fibroblast growth over epithelial cell growth.
Cervical-like tissue morphology
After 8 weeks in spinner flask conditions, the gross appearance of the scaffold resembled native tissue (Fig. 3). In spinner flask conditions, collagen synthesis occurred throughout the full thickness of the scaffolds (Fig. 3A, C). In contrast, static culture conditions resulted in limited collagen synthesis (Fig. 3B, D). Histological examination confirmed that spinner flask conditions were associated with improved tissue synthesis compared to static culture conditions (Fig. 4). Tissue morphology was most similar to native tissue in the first 100 μm of the scaffold surface. In contrast, static conditions were associated with thin layer of tissue at the scaffold surface and little tissue formation in the scaffold interior. The concentration of serum in the media did not appear to affect tissue synthesis, as assessed by histology or second harmonic generation.
FIG. 4.
Hematoxylin and eosin staining of constructs as a function of time. The graphic at the bottom right shows the section from which the slide was made. Compared to static culture, spinner flask conditions were associated with tissue morphology most similar to native tissue. Serum concentration did not affect tissue morphology. Black scale bars are 100 μm. The experiment shown above was performed twice with cells from two different subjects and similar results were seen. Color images available online at www.liebertonline.com/ten.
When scaffolds were cultured with cells from a second patient, similar results were observed. Namely, the gross appearance of the scaffold resembled native tissue and, compared to static culture, spinner flask conditions were associated with improved tissue synthesis throughout the full thickness of the scaffold.
Measurement of biochemical constituents
Collagen synthesis was significantly increased at all time points in spinner flask conditions compared to static culture (Fig. 5). In spinner flask conditions, collagen synthesis appeared to increase in a linear fashion up to 6 weeks. In contrast, collagen synthesis did not increase in static culture conditions.
FIG. 5.
Collagen synthesis as a function of time as assessed using a hydroxyproline assay (left panel) or second harmonic generation (right panel). At all time points, spinner flask conditions were associated with significantly increased collagen synthesis (a, p < 0.05). In contrast, serum concentration did not affect collagen synthesis as assessed by the hydroxyproline assay. Using second harmonic generation, 10% serum was associated with increased collagen synthesis at 6 weeks (b, p < 0.05) and decreased synthesis at 8 weeks (c, p < 0.05). Data are expressed at mean ± standard error of the mean (SEM) of four replicates.
Table 1 shows hydration and concentrations of DNA, S-GAG, and collagen in both 6-week constructs and native tissue. Spinner flask culture resulted in significantly higher S-GAG and collagen synthesis compared to static culture (p < 0.05). The concentration of serum in the media did not affect any of the measured constituents. There were no significant differences in hydration or DNA concentration between the four culture conditions. Comparing native tissue to spinner flask conditions, spinner flask culture demonstrated significantly increased hydration, decreased DNA, decreased S-GAG, and decreased collagen (p < 0.05).
Table 1.
Biochemical Constituents After 6 Weeks of Culture
| Culture condition | Hydration (% water), n = 5 | DNA concentration (μg/mg wet weight), n = 4 | Sulfated GAG (μg/mg wet weight), n = 4 | % Collagen (mg/mg dry weight), n = 5 |
|---|---|---|---|---|
| Static culture, 10% FBS | 90.1 ± 0.25 | 0.13 ± 0.012 | 0.11 ± 0.054 | 1.1 ± 0.11 |
| Static culture, 20% FBS | 89.3 ± 0.61 | 0.14 ± 0.017 | 0.19 ± 0.050 | 1.1 ± 0.10 |
| Spinner flask, 10% FBS | 87.8 ± 0.51 | 0.22 ± 0.077 | 0.50 ± 0.050a | 4.7 ± 0.51a |
| Spinner flask, 20% FBS | 88.7 ± 0.63 | 0.20 ± 0.030 | 0.68 ± 0.096a | 5.3 ± 0.16a |
| Native tissue | 77.7 ± 2.6b | 2.1 ± 0.059b | 1.1 ± 0.151b | 38.6 ± 5.6b |
| Silk scaffold, no cells | 64.0 ± 2.3 | Not detected | Not detected | Not detected |
Data presented as mean ± SEM of four or five replicates as indicated.
p < 0.05 versus static culture.
p < 0.05 versus constructs.
GAG, glycosaminoglycan; FBS, fetal bovine serum; SEM, standard error of the mean.
Gene expression
COL1A and COL3A were significantly upregulated at least fourfold in spinner flask conditions at weeks 2, 4, 6, and 8 (p < 0.05, Fig. 6). Decorin was significantly upregulated at weeks 4, 6, and 8 in spinner flask conditions (p < 0.05). MMP1 and MMP3 were not affected by culture conditions but were downregulated as the experiment proceeded. HAS2 was upregulated only at the 2 week time point in spinner flask conditions. No expression of collagen type 2 or aggrecan was observed.
FIG. 6.
Real-time quantitative reverse transcription–polymerase chain reaction of matrix-associated genes. COL1A and COL3A were significantly upregulated in spinner flask conditions at all time points. Expression of MMP1 and MMP3 was not affected by spinner flask conditions but were downregulated as the experiment proceeded. HAS2 was significantly upregulated acutely but downregulated at later time points. Expression of decorin showed a pattern similar to collagen expression. Gene expression was normalized by glyceraldehyde-3-phosphate dehydrogenase and reported as a fold change relative to gene expression at day 1 of static culture. Data are expressed as mean ± SEM of four biological replicates. *Spinner flask significantly different than static culture, p < 0.05. #Spinner flask significantly different than day 1 culture, p < 0.05. COL1A, collagen type I, alpha 1; COL3A, collagen type III, alpha 1; HAS2, hyaluronan synthase 2; MMP1, matrix metallopeptidase 1; MMP3, matrix metallopeptidase 3.
Tension test of seeded and unseeded scaffolds
No difference was found between seeded and unseeded scaffolds at week 4. After 6 weeks in culture, the peak tensile stress at 10% strain for scaffolds cultured in spinner flask conditions was significantly increased with respect to control (unseeded) scaffolds (Fig. 7). Owing to the low statistical power of this preliminary investigation, the difference between seeded and unseeded scaffolds at week 8 was not statistically significant. Scaffolds from static cultures did not exhibit significant increases in peak tensile stress as compared to control scaffolds at any time point (data not shown).
FIG. 7.
Tension test of seeded versus unseeded scaffolds. In spinner flask conditions, seeded scaffolds demonstrated significantly increased stiffness after 6 weeks of culture (*p < 0.05). Data expressed as mean ± SEM of tests from three independent scaffolds.
Discussion
In this study, we found that human cervical cells proliferated on a three-dimensional scaffold and synthesized an ECM with biochemical constituents and morphology similar to native tissue. Compared to static culture, dynamic culture was associated with increased collagen and S-GAG synthesis, which translated to the development of an ECM with a measurable mechanical response. In contrast, increasing the serum content from 10% to 20% did not affect ECM synthesis.
In the current experiment, tissue synthesis was markedly dependent on (1) dynamic culture and (2) cell location within the construct. Compared to static culture conditions, spinner flask conditions demonstrated increased matrix synthesis, which correlated with increased expression of matrix-associated genes. By analogy to tissue-engineered cartilage systems,39,40 it is likely that dynamic culture improves mass transport of dissolved gases (O2 and CO2) and bioactive molecules (nutrients and growth factors), resulting in an improved nutritional environment. Of note, tissue morphology in spinner flask conditions was most similar to native tissue at the first 100 μm of scaffold surface. This finding is consistent with well-known diffusional mass transport limitations in three-dimensional tissue culture.41–43
The serum content was varied in this experiment because preliminary studies suggested that high serum concentration had a positive effect on cervical cell proliferation. In addition, others have noted robust matrix synthesis with high serum concentration.44 However, high serum concentration was not an important variable in our model system under the conditions of the study.
Limitations of the current model system are acknowledged. First, the amount of collagen measured in the construct was approximately 10% of the value of native tissue, probably because of the poor nutritional environment that was present in the scaffold interior. This issue can be addressed via a number of options, including the use of larger pores in the matrix, more robust perfusion of media, or cocultures with vascular conduits.45 Second, cervical cells cultured on constructs appeared to adopt a mixed fibroblast/myofibroblast phenotype; no epithelial cells were seen. In native tissue, multiple cell types are present (fibroblasts, smooth muscle cells, and epithelial cells), which may affect synthesis and remodeling of matrix proteins.21 Coculturing would be a logical step to address this issue. Third, the current experiment was performed with cells from nonpregnant patients with no history of preterm delivery, an issue that can be addressed by culturing cells of different lineages and/or varying obstetric histories of the selected donors.
The long-term goal of this research is to use cervical-like constructs as a novel model system for investigating cell–matrix interactions under conditions that mimic pregnancy. Most prior studies of cervical remodeling during pregnancy have utilized the following three model systems: (1) biopsies of the human cervix, (2) animal models, and (3) two-dimensional culture of isolated cells. These model systems have been used to suggest that both hormonal mediators (prostaglandins,46,47 progesterone,23 nitric oxide,32,48 androgens,29 and relaxin49) and mechanical strain50–52 have important roles in cervical remodeling. However, three-dimensional culture models have important benefits. It is known that ECM-related gene expression and matrix adhesions are modified in three-dimensional versus two-dimensional culture of fibroblasts.53–55 Further, three-dimensional models may be used to investigate the effects of biologically relevant stress states on cell activity. The three-dimensional constructs can be deformed in tension, compression, and shear to recreate a mechanical microenvironment for the cells that closely mimic in vivo conditions. Elucidation of mechanotransduction pathways in cervical cells is a critical step to identify appropriate intervention strategies. Finally, three-dimensional constructs are ideally suited to investigate structure–property relationships in cervical tissue. The preliminary data presented in this study demonstrate that it is possible to synthesize ECM with measurable mechanical properties. Direct quantitative correlations between biochemical and mechanical properties in the same model system can provide valuable insight in the role of ECM constituents and structure in determining the macroscopic mechanical properties of the tissue. Studies of this nature are difficult to perform using biopsy specimens or two-dimensional cell culture.
Investigators classify pregnancy-associated cervical changes into two phases: cervical “softening” and cervical “ripening.”21,27,56 Cervical softening occurs slowly over the course of gestation and refers to changes in the biochemical constituents and mechanical properties of the cervical stroma. Biochemical changes associated with softening include decreased collagen concentration, increased collagen solubility, decreased decorin concentration, and increased hydration.20 These biochemical changes are associated with decreased tissue stiffness.19,57 Cervical ripening is a more general term. Ripening refers not only to softening but also to anatomic changes associated with pregnancy (i.e., effacement, funneling, and dilation).58 Studies of biopsies of human cervical stroma at different stages of cervical ripening support the hypothesis that ripening is associated with an inflammatory response27,59 However, the explicit connection between softening, ripening, and cervical mechanical properties remain poorly defined, in part because of the complexity of the in vivo environment. Tissue-engineered cervical constructs, by virtue of complete control of the in vitro environment, may reduce complexity and help elucidate structure–function relationships in cervical tissue.
The long-term goal of this research is to utilize cervical-like constructs to investigate cervical remodeling under conditions that mimic pregnancy. We expect to pursue this goal in the following future investigations. First, the importance of stromal–epithelial interactions has been demonstrated in the mouse cervix.56,60 Hence, future constructs will incorporate both fibroblasts and epithelial cells in a coculture model. Second, mechanical cues are suspected to play an important role in the way cervical cells synthesize and remodel the local matrix environment. Mechanical strain will be investigated as a regulator of matrix protein expression and organization. Last, pregnancy is associated with marked increases in local blood flow,61 which likely modulates cell nutrition and phenotype. Cervical-like constructs are ideally suited for investigating how cell nutrition affects cell–matrix interactions.
In summary, we report the feasibility of using a tissue engineering strategy to develop a cervical-like construct that is promising as a model system for investigating cervical remodeling during pregnancy. We expect that a robust three-dimensional model of cervical ECM will help elucidate the role of an impaired cervix in spontaneous preterm birth.
Footnotes
Work performed in the Department of Biomedical Engineering, Tufts University, Medford, Massachusetts. Abstract of this work was presented at the Society for Gynecologic Investigation, Glasgow, Scotland, 2009. Abstract no. 753.
Acknowledgments
This work was supported by the Reproductive Scientist Development Program (NIH grant #2K12HD000849-21) and the March of Dimes Birth Defects Foundation. Additional funding from the Tissue Engineering Resource Center (TERC) from the National Institute of Biomedical Imaging and Bioengineering (EB002520) is gratefully acknowledged.
Disclosure Statement
No competing financial interests exist.
References
- 1.Martin J.A. Hamilton B.E. Sutton P.D. Ventura S.J. Kirmeyer S. Munson M.L. National vital statistics reports. 6. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2007. Births: final data for 2005. [PubMed] [Google Scholar]
- 2.Saigal S. Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 3.Marlow N. Wolke D. Bracewell M.A. Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 4.Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 5.Martin J.A. Kochanek K.D. Strobino D.M. Guyer B. Macdorman M.F. Annual summary of vital statistics—2003. Pediatrics. 2005;115:619. doi: 10.1542/peds.2004-2695. [DOI] [PubMed] [Google Scholar]
- 6.Simhan H.N. Caritis S.N. Prevention of preterm delivery. N Engl J Med. 2007;357:477. doi: 10.1056/NEJMra050435. [DOI] [PubMed] [Google Scholar]
- 7.Iams J.D. Goldenberg R.L. Meis P.J. Mercer B.M. Moawad A. Das A. Thom E. Mcnellis D. Copper R.L. Johnson F. Roberts J.M. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 8.Leppert P.C. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Owen J. Yost N. Berghella V. Thom E. Swain M. Dildy G.A., 3rd Miodovnik M. Langer O. Sibai B. Mcnellis D. National Institute of Child Health and Human Development, M.-F.M.U.N. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 10.American College of Obstetricians and Gynecologists. Ultrasonography in Pregnancy. ACOG Practice Bulletin No. 101. Obstet Gynecol. 2009;113:451. doi: 10.1097/AOG.0b013e31819930b0. [DOI] [PubMed] [Google Scholar]
- 11.Fonseca E.B. Celik E. Parra M. Singh M. Nicolaides K.H. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 12.Defranco E.A. O'brien J.M. Adair C.D. Lewis D.F. Hall D.R. Fusey S. Soma-Pillay P. Porter K. How H. Schakis R. Eller D. Trivedi Y. Vanburen G. Khandelwal M. Trofatter K. Vidyadhari D. Vijayaraghavan J. Weeks J. Dattel B. Newton E. Chazotte C. Valenzuela G. Calda P. Bsharat M. Creasy G.W. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 13.Owen J. Hankins G. Iams J.D. Berghella V. Sheffield J.S. Perez-Delboy A. Egerman R.S. Wing D.A. Tomlinson M. Silver R. Ramin S.M. Guzman E.R. Gordon M. How H.Y. Knudtson E.J. Szychowski J.M. Cliver S. Hauth J.C. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened mid-trimester cervical length. Am J Obstet Gynecol. 2009;201:375 e1. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.House M. Socrate S. The cervix as a biomechanical structure. Ultrasound Obstet Gynecol. 2006;28:745. doi: 10.1002/uog.3850. [DOI] [PubMed] [Google Scholar]
- 15.Berghella V. Odibo A.O. To M.S. Rust O.A. Althuisius S.M. Cerclage for short cervix on ultrasonography: meta-analysis of trials using individual patient-level data. Obstet Gynecol. 2005;106:181. doi: 10.1097/01.AOG.0000168435.17200.53. [DOI] [PubMed] [Google Scholar]
- 16.Daskalakis G. Papantoniou N. Mesogitis S. Antsaklis A. Management of cervical insufficiency and bulging fetal membranes. Obstet Gynecol. 2006;107:221. doi: 10.1097/01.AOG.0000187896.04535.e6. [DOI] [PubMed] [Google Scholar]
- 17.Lee K.Y. Jun H.A. Kim H.B. Kang S.W. Interleukin-6, but not relaxin, predicts outcome of rescue cerclage in women with cervical incompetence. Am J Obstet Gynecol. 2004;191:784. doi: 10.1016/j.ajog.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Weiner C.P. Lee K.Y. Buhimschi C.S. Christner R. Buhimschi I.A. Proteomic biomarkers that predict the clinical success of rescue cerclage. Am J Obstet Gynecol. 2005;192:710. doi: 10.1016/j.ajog.2004.10.588. [DOI] [PubMed] [Google Scholar]
- 19.Myers K.M. Paskaleva A.P. House M. Socrate S. Mechanical and biochemical properties of human cervical tissue. Acta Biomater. 2008;4:104. doi: 10.1016/j.actbio.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 20.House M. Kaplan D.L. Socrate S. Relationships between mechanical properties and extracellular matrix constituents of the cervical stroma during pregnancy. Semin Perinatol. 2009;33:300. doi: 10.1053/j.semperi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Word R.A. Li X.H. Hnat M. Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 22.Hjelm A.M. Barchan K. Malmstrom A. Ekman-Ordeberg G.E. Changes of the uterine proteoglycan distribution at term pregnancy and during labour. Eur J Obstet Gynecol Reprod Biol. 2002;100:146. doi: 10.1016/s0301-2115(01)00476-6. [DOI] [PubMed] [Google Scholar]
- 23.Straach K.J. Shelton J.M. Richardson J.A. Hascall V.C. Mahendroo M.S. Regulation of hyaluronan expression during cervical ripening. Glycobiology. 2005;15:55. doi: 10.1093/glycob/cwh137. [DOI] [PubMed] [Google Scholar]
- 24.Leppert P.C. Yu S.Y. Keller S. Cerreta J. Mandl I. Decreased elastic fibers and desmosine content in incompetent cervix. Am J Obstet Gynecol. 1987;157:1134. doi: 10.1016/s0002-9378(87)80277-6. [DOI] [PubMed] [Google Scholar]
- 25.Myers K. Socrate S. Tzeranis D. House M. Changes in the biochemical constituents and morphologic appearance of the human cervical stroma during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2009;144(Suppl 1):S82. doi: 10.1016/j.ejogrb.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Uldbjerg N. Malmstrom A. The role of proteoglycans in cervical dilatation. Semin Perinatol. 1991;15:127. [PubMed] [Google Scholar]
- 27.Sennstrom M.B. Ekman G. Westergren-Thorsson G. Malmstrom A. Bystrom B. Endresen U. Mlambo N. Norman M. Stabi B. Brauner A. Human cervical ripening, an inflammatory process mediated by cytokines. Mol Hum Reprod. 2000;6:375. doi: 10.1093/molehr/6.4.375. [DOI] [PubMed] [Google Scholar]
- 28.Mahendroo M.S. Porter A. Russell D.W. Word R.A. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 29.Ji H. Dailey T.L. Long V. Chien E.K. Androgen-regulated cervical ripening: a structural, biomechanical, and molecular analysis. Am J Obstet Gynecol. 2008;198:543 e1. doi: 10.1016/j.ajog.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 30.Simon C. Einspanier A. The hormonal induction of cervical remodeling in the common marmoset monkey (Callithrix jacchus) Reproduction. 2009;137:517. doi: 10.1530/REP-08-0417. [DOI] [PubMed] [Google Scholar]
- 31.El Maradny E. Kanayama N. Kobayashi H. Hossain B. Khatun S. Liping S. Kobayashi T. Terao T. The role of hyaluronic acid as a mediator and regulator of cervical ripening. Hum Reprod. 1997;12:1080. doi: 10.1093/humrep/12.5.1080. [DOI] [PubMed] [Google Scholar]
- 32.Chwalisz K. Shao-Qing S. Garfield R.E. Beier H.M. Cervical ripening in guinea-pigs after a local application of nitric oxide. Hum Reprod. 1997;12:2093. doi: 10.1093/humrep/12.10.2093. [DOI] [PubMed] [Google Scholar]
- 33.Cavaille F. Cabrol D. Ferre F. Jones G.E. Methods in Molecular Medicine: Human Cell Culture Protocols. Totowa, NJ: Humana Press Inc.; 1996. Human myometrial smooth muscle cells and cervical fibroblasts in culture; p. 335. [DOI] [PubMed] [Google Scholar]
- 34.Kim U.J. Park J. Kim H.J. Wada M. Kaplan D.L. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 35.Kim H.J. Kim U.J. Leisk G.G. Bayan C. Georgakoudi I. Kaplan D.L. Bone regeneration on macroporous aqueous-derived silk 3-D scaffolds. Macromol Biosci. 2007;7:643. doi: 10.1002/mabi.200700030. [DOI] [PubMed] [Google Scholar]
- 36.Freed L.E. Vunjak-Novakovic G. Culture of organized cell communities. Adv Drug Deliv Rev. 1998;33:15. doi: 10.1016/s0169-409x(98)00017-9. [DOI] [PubMed] [Google Scholar]
- 37.Stegeman H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 38.Bayan C. Levitt J.M. Miller E. Kaplan D. Georgakoudi I. Fully automated, quantitative, noninvasive assessment of collagen fiber content and organization in thick collagen gels. J Appl Phys. 2009;105:102042. doi: 10.1063/1.3116626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vunjak-Novakovic G. Freed L.E. Biron R. Langer R. Effects of mixing on the composition and morphology of tissue-engineered cartilage. AIChE J. 1996;42:850. [Google Scholar]
- 40.Gooch K.J. Kwon J.H. Blunk T. Langer R. Freed L.E. Vunjak-Novakovic G. Effects of mixing intensity on tissue-engineered cartilage. Biotechnol Bioeng. 2001;72:402. doi: 10.1002/1097-0290(20000220)72:4<402::aid-bit1002>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 41.Jain R.K. Au P. Tam J. Duda D.G. Fukumura D. Engineering vascularized tissue. Nat Biotechnol. 2005;23:821. doi: 10.1038/nbt0705-821. [DOI] [PubMed] [Google Scholar]
- 42.Zhao F. Pathi P. Grayson W. Xing Q. Locke B.R. Ma T. Effects of oxygen transport on 3-d human mesenchymal stem cell metabolic activity in perfusion and static cultures: experiments and mathematical model. Biotechnol Prog. 2005;21:1269. doi: 10.1021/bp0500664. [DOI] [PubMed] [Google Scholar]
- 43.Radisic M. Yang L. Boublik J. Cohen R.J. Langer R. Freed L.E. Vunjak-Novakovic G. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004;286:H507. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 44.L'heureux N. Dusserre N. Konig G. Victor B. Keire P. Wight T.N. Chronos N.A. Kyles A.E. Gregory C.R. Hoyt G. Robbins R.C. Mcallister T.N. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang J.H. Gimble J.M. Kaplan D.L. In vitro 3D model for human vascularized adipose tissue. Tissue Eng Part A. 2009;15:2227. doi: 10.1089/ten.tea.2008.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitz T. Leroy M.J. Dallot E. Breuiller-Fouche M. Ferre F. Cabrol D. Interleukin-1beta induces glycosaminoglycan synthesis via the prostaglandin E2 pathway in cultured human cervical fibroblasts. Mol Hum Reprod. 2003;9:1. doi: 10.1093/molehr/gag007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tornblom S.A. Patel F.A. Bystrom B. Giannoulias D. Malmstrom A. Sennstrom M. Lye S.J. Challis J.R. Ekman G. 15-Hydroxyprostaglandin dehydrogenase and cyclooxygenase 2 messenger ribonucleic acid expression and immunohistochemical localization in human cervical tissue during term and preterm labor. J Clin Endocrinol Metab. 2004;89:2909. doi: 10.1210/jc.2003-031149. [DOI] [PubMed] [Google Scholar]
- 48.Tornblom S.A. Maul H. Klimaviciute A. Garfield R.E. Bystrom B. Malmstrom A. Ekman-Ordeberg G. mRNA expression and localization of bNOS, eNOS and iNOS in human cervix at preterm and term labour. Reprod Biol Endocrinol. 2005;3:33. doi: 10.1186/1477-7827-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palejwala S. Stein D.E. Weiss G. Monia B.P. Tortoriello D. Goldsmith L.T. Relaxin positively regulates matrix metalloproteinase expression in human lower uterine segment fibroblasts using a tyrosine kinase signaling pathway. Endocrinology. 2001;142:3405. doi: 10.1210/endo.142.8.8295. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida M. Sagawa N. Itoh H. Yura S. Takemura M. Wada Y. Sato T. Ito A. Fujii S. Prostaglandin F(2alpha), cytokines and cyclic mechanical stretch augment matrix metalloproteinase-1 secretion from cultured human uterine cervical fibroblast cells. Mol Hum Reprod. 2002;8:681. doi: 10.1093/molehr/8.7.681. [DOI] [PubMed] [Google Scholar]
- 51.Takemura M. Itoh H. Sagawa N. Yura S. Korita D. Kakui K. Hirota N. Fujii S. Cyclic mechanical stretch augments both interleukin-8 and monocyte chemotactic protein-3 production in the cultured human uterine cervical fibroblast cells. Mol Hum Reprod. 2004;10:573. doi: 10.1093/molehr/gah077. [DOI] [PubMed] [Google Scholar]
- 52.Takemura M. Itoh H. Sagawa N. Yura S. Korita D. Kakui K. Kawamura M. Hirota N. Maeda H. Fujii S. Cyclic mechanical stretch augments hyaluronan production in cultured human uterine cervical fibroblast cells. Mol Hum Reprod. 2005;11:659. doi: 10.1093/molehr/gah229. [DOI] [PubMed] [Google Scholar]
- 53.Webb K. Li W. Hitchcock R.W. Smeal R.M. Gray S.D. Tresco P.A. Comparison of human fibroblast ECM-related gene expression on elastic three-dimensional substrates relative to two-dimensional films of the same material. Biomaterials. 2003;24:4681. doi: 10.1016/s0142-9612(03)00368-5. [DOI] [PubMed] [Google Scholar]
- 54.Cukierman E. Pankov R. Stevens D.R. Yamada K.M. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 55.Serban M.A. Liu Y. Prestwich G.D. Effects of extracellular matrix analogues on primary human fibroblast behavior. Acta Biomater. 2008;4:67. doi: 10.1016/j.actbio.2007.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Read C.P. Word R.A. Ruscheinsky M.A. Timmons B.C. Mahendroo M.S. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction. 2007;134:327. doi: 10.1530/REP-07-0032. [DOI] [PubMed] [Google Scholar]
- 57.Myers K.M. Socrate S. Paskaleva A.P. House M. A study of the anisotropy and tension/compression behavior of human cervical tissue. J Biomech Eng. 2010;132:021003. doi: 10.1115/1.3197847. [DOI] [PubMed] [Google Scholar]
- 58.Bishop E.H. Pelvic scoring for elective induction. Obstet Gynecol. 1964;244:266. [PubMed] [Google Scholar]
- 59.Junqueira L.C. Zugaib M. Montes G.S. Toledo O.M. Krisztan R.M. Shigihara K.M. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol. 1980;138:273. doi: 10.1016/0002-9378(80)90248-3. [DOI] [PubMed] [Google Scholar]
- 60.Timmons B.C. Mitchell S.M. Gilpin C. Mahendroo M.S. Dynamic changes in the cervical epithelial tight junction complex and differentiation occur during cervical ripening and parturition. Endocrinology. 2007;148:1278. doi: 10.1210/en.2006-0851. [DOI] [PubMed] [Google Scholar]
- 61.Palmer S.K. Zamudio S. Coffin C. Parker S. Stamm E. Moore L.G. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet Gynecol. 1992;80:1000. [PubMed] [Google Scholar]