Abstract
We have developed an in vitro endothelial cell (EC)–smooth muscle cell (SMC) coculture platform that can mimic either the healthy or diseased state of blood vessels. Transforming growth factor-β1 (TGF-β1) and heparin were introduced to the SMC cultures to upregulate the SMC differentiation markers, α-smooth muscle actin (α-SMA) and calponin (homotypic model). Interestingly, seeding of near-confluent concentrations of ECs on the SMCs (heterotypic model) induced higher levels of α-SMA and calponin expression in the SMC cultures than did the addition of heparin and TGF-β1 alone. The expression levels increased further on pretreating the SMCs with TGF-β1 and heparin before adding a near-confluent monolayer of ECs. In contrast, seeding of sparse concentrations of ECs forced the SMCs into a more hyperplastic state as determined by α-SMA and calponin expression. This study highlights the importance of both soluble factors and EC seeding densities when considering culture conditions; in vivo SMCs are in close proximity with and interact with a monolayer of ECs. Our study suggests that this architecture is important for healthy vascular tissue function. In addition, it shows that disruption of this architecture can be used to mimic diseased states. As the EC-SMC coculture model can mimic either a diseased or a healthy blood vessel it may be useful as a test bed for evaluating cardiovascular therapeutics.
Introduction
Cardiovascular disease-related deaths constitute a major cause of mortality in the United States. Main causes of cardiovascular disease-related diseases are restenosis and late in-stent thrombosis after the deployment of cardiovascular stents. Uncontrolled proliferation of smooth muscle cells (SMCs) and the lack of endothelial cell (EC) growth contribute to in-stent restenosis and late-stent thrombosis. We are interested in developing a high-throughput model that can be used to screen potential drugs and combinations of drugs for preventing this SMC proliferation after implantation of cardiovascular stents and for enabling the regeneration of the endothelium.
In vitro models that allow improved screening of new devices and candidate drugs to advance the quality of care standards have the potential to significantly reduce the cost of therapeutic development and increase the speed by which companies can get effective therapies to patients. With this in mind, the goal of this work was to develop an in vitro EC-SMC coculture model that displays many of the characteristics of either healthy intimal and medial layers, or damaged and disease-like arterial layers.
The medial layer of the vessel wall is composed of SMCs and the surrounding extracellular matrix (ECM). The functions of these mature SMCs are manifold and include maintenance of proper vascular tone, contractile ability in response to environmental stimuli, and provision of mechanical stability to the vessel wall. Notably, even mature SMCs are not terminally differentiated and retain the ability to dedifferentiate from their normal quiescent contractile phenotype to a synthetic phenotype characterized by high proliferation, migration, and altered ECM composition.1 In fact, SMCs normally remain in the G0/G1 phase of the cell cycle.2,3 The phenotypic modulation resulting in the cells passing the restriction point in the late G1 phase can occur in response to local environmental cues stemming from factors, such as altered levels of growth factors in the event of a disease or injury as in the aftermath of balloon angioplasty4 or prosthetic arterial grafting.5 Although the synthetic functionality of SMCs is essential for vessel wall development (vasculogenesis) and repair, it is implicated in the pathophysiology of major forms of vascular diseases such as atherosclerosis and hypertension.6 Importantly, SMCs cultured in vitro dedifferentiate rapidly in culture with a corresponding decrease in the expression of differentiation-specific markers7 such as α-smooth muscle actin (α-SMA)8 and calponin.9,10 This makes it difficult to study the effect of vasoactive therapeutics in an in vitro replicate of the healthy in vivo SMC microenvironment.
The SMC phenotype is an important criterion in replicating the functional status of the vessel wall as demonstrated here and by others.11,12 Researchers aim to control the phenotype of SMCs with the goal to generate the more differentiated phenotype to mimic the healthy arterial wall or the less differentiated phenotype to mimic the diseased arterial wall. Some of the strategies can be enumerated as follows: first, by altering the properties of the substrate on which the SMCs are cultured13,14; second, by altering the mechanical environment in which SMCs are cultured via the use of different types of mechanical forces, including pulsatile or cyclic strain15 and shear stress16,17; third, by introducing exogenous compounds in the culture medium18,19 or even affording a repertoire of growth factors stemming from the use of media conditioned by other cell types,20,21 including ECs22; and fourth, by coculturing SMCs with ECs for triggering a more native phenotype,11,12,23,24 with other cell types or with media conditioned with other cell types including monocytes, activated monocytes,25 macrophages,26 or even with platelet-derived factors27 to artificially simulate a procoagulant, prothrombotic injury environment to study various pathophysiologic conditions.
The aim of this study was to control the phenotype of SMCs primarily by combining the third and fourth strategies described earlier. Specifically, this involved pretreating SMCs with transforming growth factor-β1 (TGF-β1) (2.5 and 5 ng/mL) and heparin (30 μg/mL) in a low-serum environment (1% [v/v] fetal bovine serum [FBS]),27 and then coculturing SMCs with ECs. One of the primary reasons for selecting TGF-β1 as the growth factor for pretreating SMCs was that TGF-β1 is activated in EC-SMC cocultures.28,29 This indicates that TGF-β1, in the right concentrations,30 may be one of the cytokines responsible for the upregulation of SMC differentiation factors in coculture.31–34 The upregulation of differentiation factors in SMCs as a result of coculture has been observed in the past12,35 and was further reinforced in our findings. In addition, heparin is also known to suppress SMC proliferation, possibly through a combination of direct effects on SMCs36,37 and indirectly through its action on clotting factors, which could be mitogenic.27,36
Here, we first describe a hyperplastic cell culture model, with proliferative SMCs, which results in lower EC coverage, and second, two healthy cell culture models with contractile SMCs, which result in stable EC-SMC cocultures. Of the two healthy cell culture models, the homotypic model relies on low serum levels, heparin, and TGF-β1 to maintain the SMC contractile phenotype. The heterotypic, healthy EC-SMC culture model presents both cells in a more native configuration. Through the addition of soluble factors and control of the EC seeding density, the SMC phenotype can be modulated, thus creating a platform that may be useful for in vitro therapeutic screening.
Materials and Methods
EC-SMC culture conditions
Primary human aortic SMCs (ASMCs), primary human coronary SMCs (CSMCs), and primary human aortic ECs were obtained from Cascade Biologics, Portland, OR (now a part of Invitrogen). All cultures were maintained in 25 cm2 tissue culture (TC)-treated flasks in a 5% CO2/95% air environment at 37°C with appropriate media supplemented with gentamicin/amphotericin B solution (Cascade Biologics). All media components (Table 1) were purchased from Cascade Biologics unless specified otherwise. Cells were used within four passages of receipt for consistency. All SMC studies were carried out with ASMCs unless specified otherwise. All experiments were performed in TC-treated eight-well μ-slides or 35 mm μ-dishes from Ibidi, Martinsried, Germany (ibiTreat) for high-resolution microscopy (www.ibidi.de/). Long-term experiments were performed on TC-treated polystyrene surfaces from BD Biosciences, San Jose, CA. For SMCs the initial seeding density was 3–5 × 104 cells/cm2, and for ECs the initial seeding density was 8–10 ×104 cells/cm2.
Table 1.
Media Components for Culturing Endothelial Cells and Smooth Muscle Cells in Homotypic Formats and in Heterotypic Endothelial Cell–Smooth Muscle Cell Coculture Formats
| Desired cell phenotype | Medium | Comments |
|---|---|---|
| Proliferative SMCs | SMC growth medium: M231 supplemented with SMGS, containing FBS (4.9%, v/v), human bFGF (2 ng/mL), human EGF (0.5 ng/mL), heparin (5 ng/mL), insulin (5 μg/mL), and bovine serum albumin (0.2 μg/mL) | To form 1–3 layers of SMCs over time. |
| Contractile SMCs | SMC differentiation medium: M231 with SMDS, containing FBS (1%, v/v) and heparin (30 μg/mL) | To inhibit SMC proliferation and induce redifferentiation; for greater differentiation/contractility, 2.5 ng/mL (2.5TGF-β1-treated SMCs) and 5 ng/mL TGF-β1 (5TGF-β1-treated SMCs) (TGF-β1 obtained from Invitrogen) were added to the SMC differentiation medium to form the “enhanced SMC differentiation medium.” This formed the homotypic SMC culture model. |
| Proliferating ECs | EC growth medium: M200 supplemented with low-serum growth supplement, containing FBS (2%, v/v), HC (1 μg/mL), human EGF (10 ng/mL), bFGF (3 ng/mL), and heparin (10 μg/mL) | To result in EC proliferation to form an EC monolayer over the underlying SMC layers. |
| Native-like EC-SMC direct contact cocultures | EC-SMC coculture medium: M200 supplemented with FBS (2%, v/v), HC (1 μg/mL), and heparin (30 μg/mL) | This formed the heterotypic EC-SMC direct contact coculture model. |
bFGF, basic fibroblast growth factor; ECs, endothelial cells; EGF, epidermal growth factor; FBS, fetal bovine serum; HC, hydrocortisone; M231, Medium 231; M200, Medium 200; SMCs, smooth muscle cells; SMDS, smooth muscle differentiation supplement; SMGS, smooth muscle growth supplement; TGF-β1, transforming growth factor-β1.
Hyperplastic cell culture model
ASMCs were cultured in smooth muscle growth supplement-supplemented media, consisting of 4.9% (v/v) FBS. In addition, to create a diseased state, a low density of ECs (3 × 104 cells/cm2) were seeded directly on the ASMCs. Further, a seeding density of ECs sufficient to create a confluent endothelium was also added to the proliferative ASMCs to see the effect of the SMC phenotype and the in situ generated ECM on initial EC coverage.
Healthy cell culture models
For creating healthy cell culture models, we used TGF-β1 and heparin in the homotypic SMC culture model, and TGF-β1, heparin pretreatment, and a confluent layer of ECs in the heterotypic EC-SMC culture model, to better mimic the in vivo phenotypes. In addition, we used CSMCs to see if the effect of TGF-β1 and heparin was also observed in the coronary phenotype of SMCs.
Homotypic cell culture model
Differentiated ASMC cultures were generated by the addition of TGF-β1 to smooth muscle differentiation supplement-supplemented SMC basal medium as described in Table 2. In addition, ECs were treated with 5 ng/mL TGF-β1, in addition to other EC media additives, to see the effect of TGF-β1 inclusion on the proliferation of ECs. Proliferation of ECs with and without the inclusion of TGF-β1 was determined using a modified MTT assay (CellTiter 96®AQueous One Solution Reagent; Promega, Madison, WI); the MTS reagent was used as per the manufacturer's specifications.
Table 2.
Differentially Pretreated Smooth Muscle Cell Cultures That Were Used as Controls to Compare with the Endothelial Cell–Smooth Muscle Cell Cocultures
| Desired cell phenotype | Medium | Experimental setup |
|---|---|---|
| Hyperplastic SMCs | SMGS-supplemented M231 (SMC growth medium) | Negative control |
| Contractile SMCs | SMDS-supplemented M231 (SMC differentiation medium) | Test for differentiation indices (e.g., SMA, calponin) |
| More contractile SMCs | 2.5TGF-β1-treated SMCs or 5TGF-β1-treated SMCs (enhanced SMC differentiation medium) | Test for differentiation indices (e.g., SMA, calponin) |
SMA, smooth muscle actin.
Heterotypic EC-SMC coculture model
The heterotypic EC-SMC cultures were set up as described in Table 3. ASMCs were first cultured in SMC growth medium beyond confluence. Then, the medium was switched to SMC differentiation medium for inducing SMC differentiation. Alternately, for inducing greater differentiation, the TGF-β1-enhanced differentiation medium was used. As a negative control, the medium was refreshed with the SMC growth medium. After 36–48 h of altering the SMC medium, ECs were directly seeded, on top of the confluent ASMCs, in a suspension formed in EC growth medium. The time window before seeding ECs was to allow the SMCs to generate an in situ matrix to serve as a basement lamina-like substrate for the ECs. After 24 to 36 h of seeding ECs on ASMCs, the medium was switched to the EC-SMC coculture medium; medium was thereafter refreshed every 36–48 h. Cocultures were maintained for over 30 days (n = 3). For immunocytochemistry (ICC), the cocultures and monoculture controls were fixed, permeabilized, and immunostained for phenotype assessment.
Table 3.
Setup of the Heterotypic Endothelial Cell–Smooth Muscle Cell Coculture Model
| Pretreated substrate for ECs | Experiment initialization | Experimental setup |
|---|---|---|
| Hyperplasic SMCs | 8–10 × 104 ECs/cm2 added directly on top of the SMCs | Negative control (coculture) |
| Contractile SMCs | 8–10 × 104 ECs/cm2 added directly on top of the SMCs | Test coculture |
| 2.5TGF-β1-treated SMCs | 8–10 × 104 ECs/cm2 added directly on top of the SMCs | Test coculture |
| 5TGF-β1-treated SMCs | 8–10 × 104 ECs/cm2 added directly on top of the SMCs | Test coculture |
| No substrate; ECs added directly to ibiTreat | 8–10 × 104 ECs/cm2 added to ibiTreat | EC homotypic culture (control) |
Live imaging SMCs and ECs in cocultures for determining long-term stability of cocultures and for assessing EC coverage
For live tracking and imaging, ASMCs were labeled with 5-chloromethylfluorescein diacetate (CTG) (CellTracker™ Green CMFDA; Invitrogen, Carlsbad, CA) by incubating the cells with 10 μM of CTG for 45 min. ECs were incubated with 10 μg/mL acetylated low-density lipoprotein (Ac-LDL), tagged with 1,1′-dioctadecyl-3,3,3',3'-tetramethyl-indocarbocyanine perchlorate (DiI) for 4 h (DiI-Ac-LDL; Biomedical Technologies, Stoughton, MA), and then seeded directly on top of CTG-labeled SMCs.
To detect SMCs and ECs in mono- or coculture, z-sections of cultured cells were obtained using a Radiance 2100MP Rainbow (BioRad, Hemel Hempstead, United Kingdom) on a TE2000 (Nikon, Tokyo, Japan) inverted microscope using a 10 × fluor (0.5 NA) objective when imaging cocultures in BD Falcon's 12.5 cm2 TC flasks or 48-well TC plates and with a 60 × oil-immersion (1.4 NA) objective when imaging cocultures in Ibidi's μ-slide surfaces or gridded dishes. Sequential imaging was done in multitrack mode with filter sets to prevent cross-talk between the fluorescent signals from CTG and DiI-Ac-LDL. The cocultures were monitored for at least 30 days.
To estimate extent of EC coverage after ∼40 days of coculture, a 60-μm-thick z-series was acquired at four random locations of three samples using the same instrument settings for all images. A top–down, maximum value projection of each z-series was created. For the DiI-Ac-LDL channel, a threshold for positive staining was empirically established and all images were converted to binary using the same threshold. The number of pixels above the threshold in each acquired image was normalized as a percentage of the average number of pixels above the threshold from images acquired under identical conditions of a confluent layer of ECs (EC monocultures) on a TC surface.
ICC studies to assess the SMC and EC phenotypes
For ICC studies, cultures were fixed using neutral-buffered formalin for ∼30 min and permeabilized using 0.5% Triton-X for ∼20 min. Blocking steps before adding the primary antibodies were performed using 3% bovine serum albumin. Table 4 lists the antibodies used for immunostaining. The final concentration of the secondary antibodies tagged to Alexa Fluor dyes (Invitrogen) was 5 μg/mL in all experiments. The final concentrations of the primary antibodies used in the assays are listed in Table 4. Primary antibodies were incubated for 1.5 h at room temperature, whereas secondary antibodies were incubated for 45 min at room temperature in the dark. SlowFade® Gold antifade reagent (Invitrogen) was used for enhanced resistance to photobleaching. 4′,6-Diamidino-2-phenylindole (DAPI) or Hoechst 33342, trihydrochloride, trihydrate nucleic acid stain (Invitrogen) was used for nuclear visualization.
Table 4.
Target Cellular or Extracellular Matrix Proteins and Primary and Secondary Antibodies for Detecting Them via Immunocytochemistry
| Target protein | Primary antibodies | Secondary antibodies |
|---|---|---|
| SMA and calponin in SMCs | SMA (mouse IgG2a [Invitrogen], ready to use) and calponin (mouse IgG1 [Dako], 1:50 dilution) | Isotype-specific secondary antibodies tagged to AF 488 and AF 647, respectively |
| vWF and eNOS or NOS3 in ECs | vWF (mouse IgG1 [Dako], 1:25 dilution) and eNOS (NOS3–C20; rabbit polyclonal IgG [Santa Cruz Biotechnology, Santa Cruz, CA], 1:50 dilution) | Isotype-specific (anti-IgG1) secondary antibodies tagged to AF 647 and species-specific (anti-rabbit) secondary antibodies tagged to AF 555 |
| FN and LN generated by SMCs | FN (chicken IgY [Invitrogen], 1:100 dilution) and LN (mouse IgG1 [Invitrogen], 1:200 dilution) | Species-specific secondary antibodies tagged to AF 555 and isotype-specific secondary antibodies tagged to AF 647 |
AF, Alexa Fluor; eNOS, endothelial nitric oxide synthase; FN, fibronectin; LN, laminin; vWF, von Willebrand factor.
To visualize the target proteins in SMCs or ECs, for cells cultured in Ibidi's μ-slide surfaces or gridded dishes, z-sections of cultured cells were obtained using the inverted microscope described earlier with a 60 × oil-immersion (1.4 NA) objective. For assessing the SMC phenotype, SMCs were probed for α-SMA and calponin. For testing the EC phenotype, ECs were double-immunostained for von Willebrand factor (vWF) and endothelial nitric oxide synthase (eNOS). For comparison of the ECM generated by proliferative and heparin-differentiated SMCs, fixed and permeabilized, proliferative and contractile SMCs were immunostained for fibronectin (FN) and laminin (LN) after culturing them in SMC growth medium or SMC differentiation medium for at least 48 h after attaining confluence.
For establishing the SMC phenotype, five 25-μm-thick z-series of images were captured per sample using the same instrument settings. α-SMA, calponin, and DAPI images were simultaneously acquired. A top–down, maximum value projection of each z-series was created. Single-channel images were converted to binary using the thresholds established on controls. The number of pixels above the threshold in each acquired image was determined using Adobe Photoshop CS2 (San Jose, CA).
In-Cell Westerns™ to assess the SMC phenotype in SMC monocultures and EC-SMC cocultures
To corroborate the data obtained using ICC followed by confocal microscopy, we carried out In-Cell Westerns for the in situ quantification of α-SMA and calponin in the SMC monocultures and cocultures. Before performing In-Cell Westerns, specificity of the antibodies for α-SMA and calponin was verified using traditional western-blotting techniques (data not shown). For In-Cell Westerns, both ASMC monocultures and EC-ASMC cocultures cultured in Optilux plates (BD Biosciences) were fixed using neutral-buffered formalin for ∼30 min and permeabilized using 0.5% Triton-X for ∼20 min. Blocking steps before adding the primary antibodies were performed using Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 1 h. Next, primary antibodies for α-SMA (diluted 1:200; Abcam) and calponin (diluted 1:50; Dako, Glostrup, Denmark), in Odyssey blocking buffer, were incubated with the samples for 2.5 h. Finally, goat anti-rabbit IRDye 800 (diluted 1:400 in blocking buffer; LI-COR Biosciences) was added, along with cell count normalization agents, DRAQ5 (diluted 1:2000) and Sapphire700 (diluted 1:1000), and incubated for 1 h. Before every antibody incubation step, the plates were washed with Tween-20 washing buffer. After the final wash, postsecondary antibody incubation, the wash solution was aspirated and the dry wells were immediately imaged at 700 and 800 nm using an Odyssey infrared scanner. The integrated intensity of the IRDye bound to the primary antibody, α-SMA or calponin (800 nm channel), was normalized to the total cell count (700 nm channel) for each well to acquire the quantitative protein expression levels for each treatment.
Statistical analysis
Results are expressed by mean ± standard deviation. Analysis of variance was used when performing multiple comparisons and a Student's t-test was used as a post hoc test when appropriate. In all analyses, a p-value of <0.05 was considered statistically significant.
Results
Qualitative morphology of SMCs and proliferation of ECs in different culture media
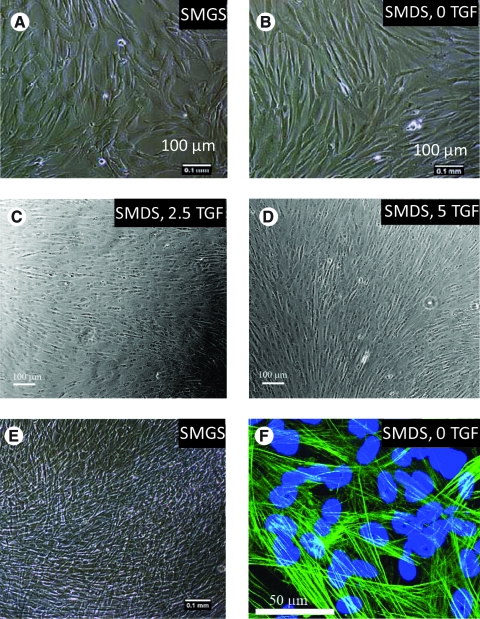
ASMCs, seeded at a density of 5 × 104 cells/cm2, proliferated in the SMC growth medium to attain confluence within 48 h. In the presence of higher serum (4.9%, v/v) and growth factors, such as basic fibroblast growth factor in the growth medium, they appeared polygonal, as observed qualitatively. The SMC differentiation medium resulted in a population of cells predominantly of spindle-shaped morphology. Further, the enhanced differentiation medium consisting of different doses of TGF-β1 (2.5 and 5 ng/mL) also resulted in a population of predominantly spindle-shaped cells (Fig. 1A–E). For ECs, 5 ng/mL TGF-β1 resulted in 52% higher proliferation with respect to regular EC growth medium (p-value <0.05).
FIG. 1.
Qualitative assessment of smooth muscle cell (SMC) morphology after treatment with different media supplements (added to Medium 231) for the same time period is depicted in the top two rows (A–D). The media supplements resulting in various morphologies are smooth muscle growth supplement (SMGS) (A), smooth muscle differentiation supplement (SMDS) and 0 ng/mL transforming growth factor-β1 (TGF-β1) (SMDS, 0 TGF) (B), SMDS and 2.5 ng/mL TGF-β1 (SMDS, 2.5 TGF) (C), and smooth muscle differentiation supplement and 5 ng/mL TGF-β1 (SMDS, 5 TGF) (D). (E) A phase-contrast image depicting the formation of one to three layers of SMCs when allowed to proliferate in the SMC growth medium (SMGS-supplemented Medium 231) beyond confluence. (F) A confocal image demonstrating α-smooth muscle actin (α-SMA) expression by SMCs cocultured with endothelial cells (ECs), imaged 10 days after the initiation of the coculture. Nuclei are counterstained with 4′,6-diamidino-2-phenylindole. The scale bars on the phase-contrast images are all 100 μm. The scale bar on the confocal image is 50 μm. Color images available online at www.liebertonline.com/ten.
SMC phenotype analysis under different culture conditions
The phenotype of SMCs was ascertained when using one or a combination of the following differentiation medium treatments: TGF-β1, heparin, and coculture with ECs, or when culturing SMCs in growth medium alone. SMC phenotype was assessed by studying the expression of SMC differentiation markers, calponin and α-SMA, and the patterning of SMC-secreted ECM proteins, LN and FN.
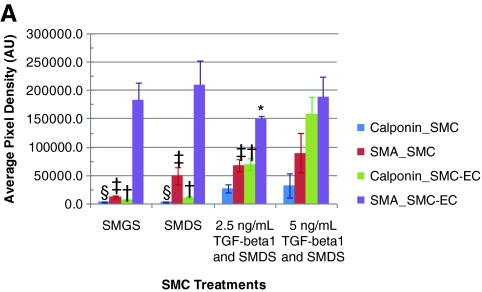
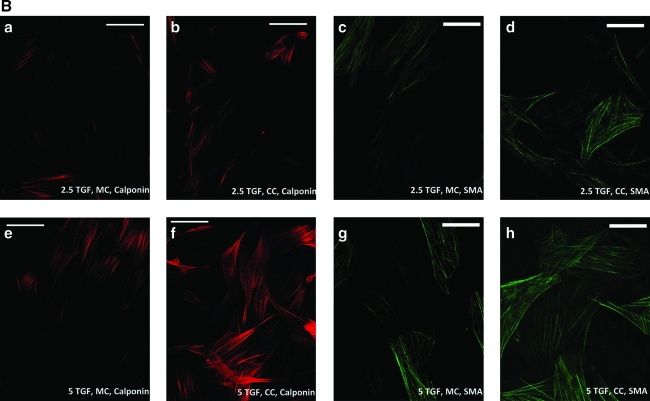
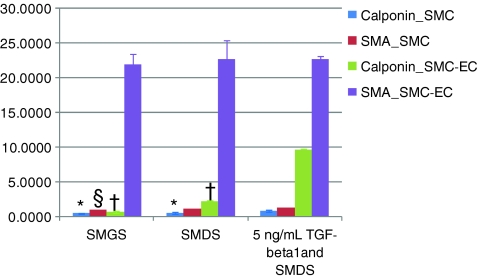
The expression of both α-SMA and calponin was modulated by the alteration of the media components for SMCs grown in monoculture (Figures 2 and 3). The presence of heparin in the differentiation medium resulted in 19% higher expression of α-SMA by western analysis when compared with α-SMA expression levels seen in the proliferating SMCs, and further addition of 5 ng/mL TGF-β1, but not 2.5 ng/mL TGF-β1, resulted in an approximately 35% increase in α-SMA expression. ICC analysis confirmed these relative α-SMA expression levels. An appreciable degree of calponin expression was observed only with the introduction of both heparin and TGF-β1 into the culture medium. In fact, there was an approximately sixfold increase in calponin expression with the addition of both heparin and 5 ng/mL TGF-β1 to the SMC medium. This indicated that the presence of TGF-β1, in addition to heparin, enabled the SMCs to differentiate to a significantly greater extent compared with the presence of heparin alone (Figs. 2 and 3). This statistically significant increase in calponin expression was also seen in CSMCs (data not shown).
FIG. 2.
(A) Increased expression of α-SMA and calponin mediated by TGF-β1 treatment and further increase in expression levels by coculturing differentially pretreated SMCs with ECs. (a–d) Cultures kept with 2.5 ng/mL TGF-β1; (e–h) cultures kept with 5 ng/mL TGF-β1; (a, e, c, g) monocultures; (b, f, d, h) cocultures. *Statistically significant difference (p-value <0.05) of coculture (CC) α-SMA expression from α-SMA expression in 5TGF, CC (i.e., 5 ng/mL TGF-β1-treated cocultures); †statistical difference of CC calponin expression from calponin expression in 5TGF, CC; ‡statistical difference of SMC monoculture (MC) α-SMA expression from α-SMA expression in 5TGF, MC (i.e., 5 ng/mL TGF-β1-treated monocultures); §statistical difference of MC calponin expression from calponin expression in 5TGF, MC. (B) Representative images of calponin (red) and α-SMA (green) expression by differentially treated SMCs in monoculture (MC) and in coculture (CC). 5 ng/mL TGF-β1 (5 TGF-β1, MC) resulted in greater expression of calponin and α-SMA than 2.5 ng/mL TGF-β1 (2.5 TGF-β1, MC); in both cases the expression of calponin and α-SMA further increased in coculture, for both 2.5 ng/mL TGF-β1 and 5 ng/mL TGF-β1 (i.e., 2.5 TGF-β1, CC and 5 TGF-β1, CC). Scale bars = 50 μm on all images. Calponin and α-SMA expression was also upregulated in the heterotypic culture of SMCs and ECs compared with the SMC monocultures in the cultures not treated with TGF-β1. Color images available online at www.liebertonline.com/ten.
FIG. 3.
Increased expression of α-SMA and calponin mediated by 5 ng/mL TGF-β1 treatment and further increase in expression levels by coculturing differentially pretreated SMCs with ECs, as quantified by In-Cell Westerns™. All symbols represent statistically significant difference (p-value <0.05) from the corresponding 5 ng/mL TGF-β1-treated cells. Color images available online at www.liebertonline.com/ten.
The degree of α-SMA and calponin expression further increased in the EC-SMC cocultures. Coculture of SMCs with ECs resulted in an approximately threefold increase in calponin expression for heparin-treated cocultures and ninefold increase in calponin expression for the 5 ng/mL TGF-β1-treated cocultures as determined by western analysis (Fig. 3), indicating a more mature phenotype in coculture. Increased calponin expression under these conditions was confirmed with ICC (Fig. 2). Interestingly, coculture conditions resulted in an order of magnitude increased α-SMA expression when compared with SMCs in monoculture (Fig. 1F). Taken together, combinations of heparin, TGF-β1, and coculture conditions result in more highly differentiated SMCs.
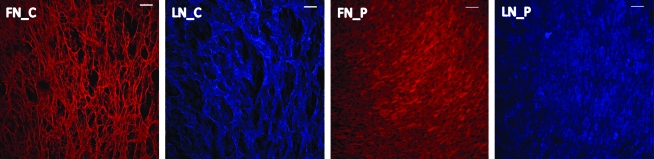
We hypothesized that significant differences in the ECM protein expression and patterning generated by the different SMC phenotypes may partially contribute to the differences in the initial EC adhesion, complete monolayer formation, and subsequent functional integrity of the monolayer. Our studies validate this hypothesis; immunocytochemical techniques revealed the fibrillar architecture of FN and LN when secreted by differentiated SMCs in contrast to diffuse FN and LN architecture when secreted by proliferative SMCs (Fig. 4).
FIG. 4.
Distribution of FN and LN secreted by contractile SMCs (panels FN_C and LN_C) and proliferative SMCs (panels FN_P and LN_P). Scale bars = 100 μm. FN, fibronectin; LN, laminin. Color images available online at www.liebertonline.com/ten.
Long-term coculture stability
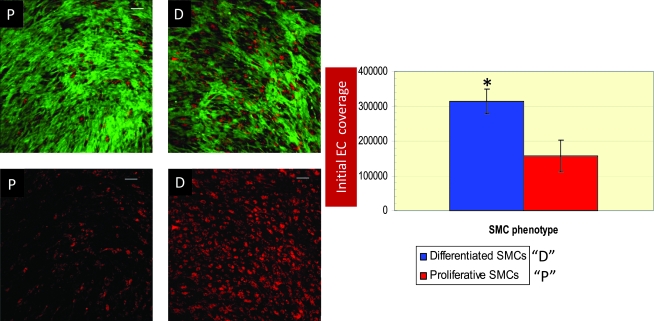
The initial EC coverage approximately doubled when seeded on heparin-differentiated SMCs when compared with proliferating SMCs (Fig. 5). Further, slowly proliferating ECs, when seeded at a low density, resulted in dedifferentiation of the differentiated SMCs and consequently destabilized the cocultures within 48 h of initiation of coculture. In fact, seeding low densities of ECs even on highly differentiated SMCs resulted in loss of SMC differentiation markers after 48 h, with a greater tendency of delamination of the EC-SMC cocultures from the surface of the Ibidi μ-slides. In contrast to the low EC densities, confluent or near-confluent EC densities (seeding densities in the range of 8–10 × 104 cells/cm2) resulted in the enhancement of the differentiated state of SMCs (Figs. 1F and 2B). Thus, a reciprocally beneficial response was observed when a differentiated bed of SMCs (with the in situ generated ECM) was used as a substrate for a confluent, or near-confluent, monolayer-forming density of ECs. Figure 6A and B, depicting the SMC and EC sections, respectively, imaged at different levels of the same photographic field of the EC-SMC coculture, indicate that the SMCs and ECs retain their phenotypes in coculture. Specifically, Figure 6 represents the coculture in which the SMCs were pretreated with 30 μg/mL heparin, 2.5 ng/mL TGF-β1, and 1% (v/v) FBS, before adding a near-confluent monolayer of ECs.
FIG. 5.
Representative confocal images depicting the difference in initial EC coverage on proliferative (P) and differentiated (D) SMCs. The images on the top panels depict z-sections of both ECs (red) and SMCs (green); the ones on the bottom panels depict z-sections of the ECs alone cultured on SMCs. Images were acquired 48 h after initiation of coculture to assess initial EC coverage. SMCs were tracked via CellTracker™ Green CMFDA and ECs were tracked by via DiI-Ac-LDL. Scale bars on all images are 100 μm. The image on the right demonstrates a graphical representation of initial EC coverage on proliferative and differentiated SMCs. *Statistical (significant) difference (p < 0.05) from initial EC coverage to proliferative SMCs. DiI-Ac-LDL, 1,1′-dioctadecyl-3,3,3',3'-tetramethyl-indocarbocyanine perchlorate acetylated low-density lipoprotein. Color images available online at www.liebertonline.com/ten.
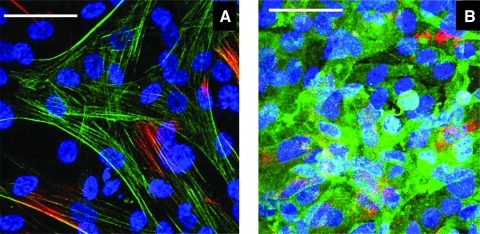
FIG. 6.
Representative confocal images of 48 h established cocultures where SMCs were pretreated with 2.5 ng/mL TGF-β1 before seeding a near-confluent density of ECs. (A) The SMCs alone in the EC-SMC cocultures where the SMCs were immunostained for α-SMA (green) and calponin (red). (B) The ECs alone in the EC-SMC cocultures where the ECs were immunostained for endothelial nitric oxide synthase (green) and von Willebrand factor (red). The SMCs and ECs were imaged at different levels of the same photographic field to test their phenotypes in coculture. Scale bars = 50 μm. Color images available online at www.liebertonline.com/ten.
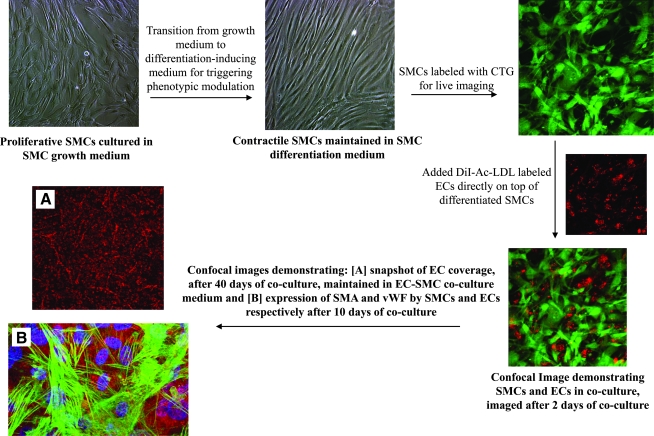
The EC-SMC cocultures were maintained for over 40 days (n = 3) with a distinct endothelium over the long term, as evidenced by specific uptake of DiI-Ac-LDL via scavenger receptors (Fig. 7)38 and underlying layer(s) of CTG-labeled SMCs. Further, the long-term coverage of ECs in coculture was found to be significantly higher relative to EC coverage on TC-treated plastic. Specifically, EC coverage, normalized as a function of monocultured ECs, at day 40 of coculture was found to be 235.78% ± 42.80% and at day 50 of coculture was found to be 148.34% ± 26.42%. Further, ECs exhibited normal cobblestone morphology in monoculture, whereas the EC-SMC cocultures resulted in the ECs being more elongated, which has also been observed by Wallace et al. in their coculture model.12
FIG. 7.
Setup of the EC-SMC coculture system for live imaging of the coculture over at least 30 days of coculture (n = 3). SMCs were tracked via CellTracker™ Green CMFDA and ECs were tracked by specifically labeling them with DiI-Ac-LDL. CTG, chloromethylfluorescein diacetate. Color images available online at www.liebertonline.com/ten.
Discussion
The need for testing new candidate drugs for use in drug eluting stent (DES) platforms cannot be overemphasized given the life-threatening risk of late in-stent thrombosis with an incidence of about 1% after implantation of DESs.39 The results in our study reflect the first step in the development of an in vitro test bed for evaluating vasoactive therapeutics. In this article, arterial vessel models with either a diseased medial layer or an intact, functional medial layer were explored. Endothelial denudation precipitates at least a temporary loss of the differentiated SMC phenotype.40–42 Thus, simply a loss of ECs can trigger a vicious chain of events contributing to greater neointimal proliferation.43,44 This is consistent with the observation in the described coculture model where a low density of EC seeding resulted in unstable cocultures with an apparent shift to a proliferative SMC phenotype as determined by at least a short-term loss of SMC differentiation markers.
As described herein and by Lavender et al.,11 ECs display a more elongated shape and exhibit enhanced coverage when cultured on differentiated SMCs than on proliferative SMCs. This may be due to altered cell–cell and cell–ECM connections and potentially due to soluble signals. It has recently been shown that ECs form focal adhesions when seeded on FN-coated plastic and form fibrillar adhesions on SMCs.24 Certainly, the morphology of the FN and LN produced by contractile and proliferative SMCs is distinct and the fibrillar morphology seen with contractile SMCs may contribute to improved EC coverage in the described direct coculture model because ECs are effectively seeded on the in situ SMC-generated ECM.
In our studies, the lower initial EC coverage on proliferative SMCs (Fig. 5) coupled with the distinct ECM generation by proliferative and contractile SMCs (Fig. 4) may suggest that hyperplastic or scar-like SMCs, such as those formed after balloon angioplasty, are not conducive to the generation of an endothelialization-favoring ECM and soluble signals in the aftermath of vascular injury.45–47 Therefore, it may be important to therapeutically retain the more differentiated SMC phenotype in vivo to favor the adhesion of migrating ECs or, potentially, the capture and maturation of circulating EC progenitor cells.48–50 Reciprocally, the quick resurfacing of the luminal surface of the blood vessel with ECs will naturally promote SMC mitogenic quiescence44 and reestablish the functionally active EC–EC51 and EC–SMC connections.52
Paradoxically, after vascular injury, proliferating ECs at the wound edge trigger the migration of SMCs via the release of platelet-derived growth factor-B53,54 as a part of the healing response. In this regard, two of the major functions of DES drugs after balloon angioplasty-related trauma should involve, first, maintenance of the contractile SMC phenotype to favor initial adhesion of migrating ECs or circulating EC progenitor cells, and second, induction of quick migration and proliferation of ECs to cover the EC denuded area on top of the contractile SMC layer to restore native function. This is similar to the described approach where the differentiated SMC phenotype was first restored using a cocktail of heparin, TGF-β1, and low serum, and then the resultant contractile SMCs were naturally maintained in their contractile state via the complex communications between the SMC layer(s) and the overlying EC monolayer (in the absence of the cocktail). In fact, despite the absence of the exogenous growth factors for the ECs and exogenously added heparin and TGF-β1 for SMC differentiation, the SMCs displayed enhanced differentiation and the ECs retained their functional phenotype. In our model, preliminary testing of EC functionality was achieved by immunostaining for eNOS protein expression because eNOS is a marker for a healthy endothelium55–58 (Fig. 6).
Several different models have been used to coculture SMCs and ECs, including culturing ECs and SMCs on opposite sides of transwell filters or semipermeable membranes,22,28,59–62 microcarrier63 or spheroidal64 coculture modules, culturing ECs on collagen gels containing SMCs,65,66 direct coculture models where ECs are directly cultured on SMCs,23,67–69 and more recently, direct coculture models where ECs are cultured on differentiated or more contractile SMCs.11,12 The direct coculture model, described herein, in which ECs are seeded directly on a differentiated SMC substrate, is useful to recapitulate some of the signal transduction events in the healthy artery transitioning to abnormal events with the advent of vascular injury-triggered EC denudation. We believe that this in vitro model will be useful for evaluating therapeutics and future work will focus on this testing.
Currently, a limitation of our model is the absence of hemodynamic forces operating in it. However, in this study we aimed to recreate a portion of the vascular microenvironment for understanding some of the biochemical effects and the effects of the in situ generated ECM on the stability and phenotype of the EC-SMC cocultures. Future improvements of the model will involve introducing hemodynamic variants in the model.
Conclusion
This article describes the validation of an in vitro EC-SMC coculture model, which provides the opportunity to study both SMCs and ECs in a range of phenotypes. This model will be useful for testing new cardiovascular therapies in a more native-like in vitro environment and is the focus of our future work. In this model, TGF-β1, in addition to low serum and heparin, induced SMCs to transition from their proliferative state to their more differentiated, contractile state. Interestingly, TGF-β1 also resulted in statistically higher proliferation of ECs in EC monocultures. Further, the described coculture model manifested higher initial EC coverage when ECs were seeded on differentiated SMCs in contrast to proliferative SMCs. In addition, coculture in the presence of a confluent EC layer induced a further increase in the expression of α-SMA and calponin, manifesting a reciprocal relationship between differentiated SMCs and a complete monolayer of ECs. Thus, the described coculture model elucidates the importance of differentiated SMCs and the ability of a confluent layer of ECs to retain and further enhance the differentiated state of the underlying SMCs. This model may be useful to test new therapies for cardiovascular ailments including complications arising from DES implantations after balloon angioplasty.
Acknowledgments
The authors thank Jennifer Sturgis at the Bindley Bioscience Center for her excellent technical assistance with confocal microscopy. This study was supported in part by the National Institutes of Health through grant HL078715 and by the Showalter Trust Fund.
Disclosure Statement
No competing financial interests exist.
References
- 1.Owens G.K. Regulation of differentiation of vascular smooth muscle cells. Physiol Rev. 1995;75:487. doi: 10.1152/physrev.1995.75.3.487. [DOI] [PubMed] [Google Scholar]
- 2.Sakai T. Inoue S. Otsuka T. Matsuyama T.-A. Saito T. Murakami M. Ota H. Katagiri T. Cell cycle regulator expression after coronary stenting in humans: immunohistochemical examination. Jpn Heart J. 2004;45:133. doi: 10.1536/jhj.45.133. [DOI] [PubMed] [Google Scholar]
- 3.Gordon D. Reidy M.A. Benditt E.P. Schwartz S.M. Cell proliferation in human coronary arteries. Proc Natl Acad Sci U S A. 1990;87:4600. doi: 10.1073/pnas.87.12.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karas S.P. Gravanis M.B. Santoian E.C. Robinson K.A. Anderberg K.A. King S.B. Coronary intimal proliferation after balloon injury and stenting in swine—an animal-model of restenosis. J Am Coll Cardiol. 1992;20:467. doi: 10.1016/0735-1097(92)90119-8. [DOI] [PubMed] [Google Scholar]
- 5.Willis D.J. Kalish J.A. Li C. Deutsch E.R. Contreras M.A. LoGerfo F.W. Quist W.C. Temporal gene expression following prosthetic arterial grafting. J Surg Res. 2004;120:27. doi: 10.1016/j.jss.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Ross R. The pathogenesis of atherosclerosis—a perspective for the 1990s. Nature. 1993;362:801. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 7.Worth N.F. Rolfe B.E. Song J. Campbell G.R. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell Motil Cytoskeleton. 2001;49:130. doi: 10.1002/cm.1027. [DOI] [PubMed] [Google Scholar]
- 8.Skalli O. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi K. Hiwada K. Kokubu T. Isolation and characterization of a 34000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem Biophys Res Commun. 1986;141:20. doi: 10.1016/s0006-291x(86)80328-x. [DOI] [PubMed] [Google Scholar]
- 10.Frid M.G. Shekhonin B.V. Koteliansky V.E. Glukhova M.A. Phenotypic changes of human smooth muscle cells during development: late expression of heavy caldesmon and calponin. Dev Biol. 1992;153:185. doi: 10.1016/0012-1606(92)90104-o. [DOI] [PubMed] [Google Scholar]
- 11.Lavender M.D. Pang Z. Wallace C.S. Niklason L.E. Truskey G.A. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials. 2005;26:4642. doi: 10.1016/j.biomaterials.2004.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace C. Champion J. Truskey G. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann Biomed Eng. 2007;35:375. doi: 10.1007/s10439-006-9230-5. [DOI] [PubMed] [Google Scholar]
- 13.Nikolovski J. Mooney D.J. Smooth muscle cell adhesion to tissue engineering scaffolds. Biomaterials. 2000;21:2025. doi: 10.1016/s0142-9612(00)00079-x. [DOI] [PubMed] [Google Scholar]
- 14.Mann B.K. West J.L. Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. J Biomed Mater Res. 2002;60:86. doi: 10.1002/jbm.10042. [DOI] [PubMed] [Google Scholar]
- 15.Isenberg B.C. Tranquillo R.T. Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng. 2003;31:937. doi: 10.1114/1.1590662. [DOI] [PubMed] [Google Scholar]
- 16.Stegemann J.P. Nerem R.M. Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Ann Biomed Eng. 2003;31:391. doi: 10.1114/1.1558031. [DOI] [PubMed] [Google Scholar]
- 17.Tada S. Tarbell J.M. Interstitial flow through the internal elastic lamina affects shear stress on arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2000;278:H1589. doi: 10.1152/ajpheart.2000.278.5.H1589. [DOI] [PubMed] [Google Scholar]
- 18.Ignarro L.J. Lippton H. Edwards J.C. Baricos W.H. Hyman A.L. Kadowitz P.J. Gruetter C.A. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218:739. [PubMed] [Google Scholar]
- 19.Ross J.J. Hong Z. Willenbring B. Zeng L. Isenberg B. Lee E.H. Reyes M. Keirstead S.A. Weir E.K. Tranquillo R.T. Cytokine-induced differentiation of multipotent adult progenitor cells into functional smooth muscle cells. J Clin Invest. 2006;116:3139. doi: 10.1172/JCI28184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee E. Grodzinsky A.J. Libby P. Clinton S.K. Lark M.W. Lee R.T. Human vascular smooth muscle cell–monocyte interactions and metalloproteinase secretion in culture. Arterioscler Thromb Vasc Biol. 1995;15:2284. doi: 10.1161/01.atv.15.12.2284. [DOI] [PubMed] [Google Scholar]
- 21.Toshio I. David K.M.H. Leonard H. Takuzo H. Ichiro N. Liles W.C. Alan M.G. Stephen M.S. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from monocytes/macrophage. Atherosclerosis. 2002;161:143. doi: 10.1016/s0021-9150(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 22.Fillinger M.F. Sampson L.N. Cronenwett J.L. Powell R.J. Wagner R.J. Coculture of endothelial cells and smooth muscle cells in bilayer and conditioned media models. J Surg Res. 1997;67:169. doi: 10.1006/jsre.1996.4978. [DOI] [PubMed] [Google Scholar]
- 23.Heydarkhan-Hagvall S. Helenius G. Johansson B.R. Li J.Y. Mattsson E. Risberg B. Co-culture of endothelial cells and smooth muscle cells affects gene expression of angiogenic factors. J Cell Biochem. 2003;89:1250. doi: 10.1002/jcb.10583. [DOI] [PubMed] [Google Scholar]
- 24.Wallace C.S. Strike S.A. Truskey G.A. Smooth muscle cell rigidity and extracellular matrix organization influence endothelial cell spreading and adhesion formation in coculture. Am J Physiol Heart Circ Physiol. 2007;293:H1978. doi: 10.1152/ajpheart.00618.2007. [DOI] [PubMed] [Google Scholar]
- 25.Seshiah P.N. Kereiakes D.J. Vasudevan S.S. Lopes N. Su B.Y. Flavahan N.A. Goldschmidt-Clermont P.J. Activated monocytes induce smooth muscle cell death: role of macrophage colony-stimulating factor and cell contact. Circulation. 2002;105:174. doi: 10.1161/hc0202.102248. [DOI] [PubMed] [Google Scholar]
- 26.Boyle J.J. Bowyer D.E. Weissberg P.L. Bennett M.R. Human blood-derived macrophages induce apoptosis in human plaque-derived vascular smooth muscle cells by Fas-ligand/Fas interactions. Arterioscler Thromb Vasc Biol. 2001;21:1402. doi: 10.1161/hq0901.094279. [DOI] [PubMed] [Google Scholar]
- 27.Ross R. Glomset J. Kariya B. Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974;71:1207. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell R.J. Bhargava J. Basson M.D. Sumpio B.E. Coculture conditions alter endothelial modulation of TGF-beta 1 activation and smooth muscle growth morphology. Am J Physiol Heart Circ Physiol. 1998;274:H642. doi: 10.1152/ajpheart.1998.274.2.H642. [DOI] [PubMed] [Google Scholar]
- 29.Nunes I. Munger J.S. Harpel J.G. Nagano Y. Shapiro R.L. Gleizes P.E. Rifkin D.B. Structure and activation of the large latent transforming growth factor-beta complex. Int J Obes Relat Metab Disord 20 Suppl. 1996;3:S4. [PubMed] [Google Scholar]
- 30.Battegay E.J. Raines E.W. Seifert R.A. Bowen-Pope D.F. Ross R. TGF-beta induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell. 1990;63:515. doi: 10.1016/0092-8674(90)90448-n. [DOI] [PubMed] [Google Scholar]
- 31.Rama A. Matsushita T. Charolidi N. Rothery S. Dupont E. Severs N.J. Up-regulation of connexin43 correlates with increased synthetic activity and enhanced contractile differentiation in TGF-β-treated human aortic smooth muscle cells. Eur J Cell Biol. 2006;85:375. doi: 10.1016/j.ejcb.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Deaton R.A. Su C. Valencia T.G. Grant S.R. Transforming growth factor-{beta}1-induced expression of smooth muscle marker genes involves activation of PKN and p38 MAPK. J Biol Chem. 2005;280:31172. doi: 10.1074/jbc.M504774200. [DOI] [PubMed] [Google Scholar]
- 33.Lien S.C. Usami S. Chien S. Chiu J.J. Phosphatidylinositol 3-kinase/Akt pathway is involved in transforming growth factor-ß1-induced phenotypic modulation of 10T1/2 cells to smooth muscle cells. Cell Signal. 2006;18:1270. doi: 10.1016/j.cellsig.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 34.Su C. Deaton R.A. Iglewsky M.A. Valencia T.G. Grant S.R. PKN activation via transforming growth factor-beta 1 (TGF-beta 1) receptor signaling delays G2/M phase transition in vascular smooth muscle cells. Cell Cycle. 2007;6:739. doi: 10.4161/cc.6.6.3985. [DOI] [PubMed] [Google Scholar]
- 35.Brown D.J. Rzucidlo E.M. Merenick B.L. Wagner R.J. Martin K.A. Powell R.J. Endothelial cell activation of the smooth muscle cell phosphoinositide 3-kinase/Akt pathway promotes differentiation. J Vasc Surg. 2005;41:509. doi: 10.1016/j.jvs.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 36.Clowes A.W. Karnowsky M.J. Suppression by heparin of smooth muscle cell proliferation in injured arteries. Nature. 1977;265:625. doi: 10.1038/265625a0. [DOI] [PubMed] [Google Scholar]
- 37.Pukac L.A. Castellot J.J., Jr. Wright T.C., Jr. Caleb B.L. Karnovsky M.J. Heparin inhibits c-fos and c-myc mRNA expression in vascular smooth muscle cells. Cell Regul. 1990;1:435. doi: 10.1091/mbc.1.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adachi H. Tsujimoto M. Arai H. Inoue K. Expression cloning of a novel scavenger receptor from human endothelial cells. J Biol Chem. 1997;272:31217. doi: 10.1074/jbc.272.50.31217. [DOI] [PubMed] [Google Scholar]
- 39.Ong A.T.L. McFadden E.P. Regar E. de Jaegere P.P.T. van Domburg R.T. Serruys P.W. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol. 2005;45:2088. doi: 10.1016/j.jacc.2005.02.086. [DOI] [PubMed] [Google Scholar]
- 40.Shirotani M. Yui Y. Kawai C. Restenosis after coronary angioplasty: pathogenesis of neointimal thickening initiated by endothelial loss. Endothelium. 1993;1:5. [Google Scholar]
- 41.Reidy M.A. Schwartz S.M. Endothelial regeneration. III. Time course of intimal changes after small defined injury to rat aortic endothelium. Lab Invest. 1981;44:301. [PubMed] [Google Scholar]
- 42.Bjorkerud S. Bondjers G. Arterial repair and atherosclerosis after mechanical injury. 5. Tissue response after induction of a large superficial transverse injury. Atherosclerosis. 1973;18:235. doi: 10.1016/0021-9150(73)90103-2. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki Y. Suehiro S. Becker A.E. Kinoshita H. Ueda M. Role of endothelial cell denudation and smooth muscle cell dedifferentiation in neointimal formation of human vein grafts after coronary artery bypass grafting: therapeutic implications. Heart. 2000;83:69. doi: 10.1136/heart.83.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kipshidze N. Dangas G. Tsapenko M. Moses J. Leon M.B. Kutryk M. Serruys P. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 2004;44:733. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 45.Raines E.W. The extracellular matrix can regulate vascular cell migration, proliferation, and survival: relationships to vascular disease. Int J Exp Pathol. 2000;81:173. doi: 10.1046/j.1365-2613.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kubota Y. Kleinman H.K. Martin G.R. Lawley T.J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yurchenco P.D. Schittny J.C. Molecular architecture of basement membranes. FASEB J. 1990;4:1577. doi: 10.1096/fasebj.4.6.2180767. [DOI] [PubMed] [Google Scholar]
- 48.Asahara T. Murohara T. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 49.Hristov M. Erl W. Weber P.C. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol. 2003;23:1185. doi: 10.1161/01.ATV.0000073832.49290.B5. [DOI] [PubMed] [Google Scholar]
- 50.Xu Q. The impact of progenitor cells in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2006;3:94. doi: 10.1038/ncpcardio0396. [DOI] [PubMed] [Google Scholar]
- 51.Dejana E. Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5:261. doi: 10.1038/nrm1357. [DOI] [PubMed] [Google Scholar]
- 52.Hirschi K.K. Burt J.M. Hirschi K.D. Dai C. Gap junction communication mediates transforming growth factor-{beta} activation and endothelial-induced mural cell differentiation. Circ Res. 2003;93:429. doi: 10.1161/01.RES.0000091259.84556.D5. [DOI] [PubMed] [Google Scholar]
- 53.Lindner V. Reidy M.A. Platelet-derived growth factor ligand and receptor expression by large vessel endothelium in vivo. Am J Pathol. 1995;146:1488. [PMC free article] [PubMed] [Google Scholar]
- 54.McNeil P.L. Muthukrishnan L. Warder E. D'Amore P.A. Growth factors are released by mechanically wounded endothelial cells. J Cell Biol. 1989;109:811. doi: 10.1083/jcb.109.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nathan C. Xie Q.W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 56.Venugopal S.K. Devaraj S. Yuhanna I. Shaul P. Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 57.Lip G.Y.H. Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34:255. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 58.Bonetti P.O. Lerman L.O. Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 59.Saunders K.B. D'Amore P.A. An in vitro model for cell-cell interactions. In Vitro Cell Dev Biol Anim. 1992;28:521. doi: 10.1007/BF02634136. [DOI] [PubMed] [Google Scholar]
- 60.Duband J.L. Gimona M. Scatena M. Sartore S. Small J.V. Calponin and SM22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55:1. doi: 10.1111/j.1432-0436.1993.tb00027.x. [DOI] [PubMed] [Google Scholar]
- 61.Axel D.I. Riessen R. Athanasiadis A. Runge H. Koveker G. Karsch K.R. Growth factor expression of human arterial smooth muscle cells and endothelial cells in a transfilter coculture system. J Mol Cell Cardiol. 1997;29:2967. doi: 10.1006/jmcc.1997.0541. [DOI] [PubMed] [Google Scholar]
- 62.Rose S.L. Babensee J.E. Smooth muscle cell phenotype alters cocultured endothelial cell response to biomaterial-pretreated leukocytes. J Biomed Mater Res. 2007;84A:661. doi: 10.1002/jbm.a.31305. [DOI] [PubMed] [Google Scholar]
- 63.Davies P.F. Truskey G.A. Warren H.B. O'Connor S.E. Eisenhaure B.H. Metabolic cooperation between vascular endothelial cells and smooth muscle cells in co-culture: changes in low density lipoprotein metabolism. J Cell Biol. 1985;101:871. doi: 10.1083/jcb.101.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Korff T. Kimmina S. Martiny-Baron G. Augustin H.G. Blood vessel maturation in a 3-dimensional spheroidal coculture model: direct contact with smooth muscle cells regulates endothelial cell quiescence and abrogates VEGF responsiveness. FASEB J. 2001;15:447. doi: 10.1096/fj.00-0139com. [DOI] [PubMed] [Google Scholar]
- 65.van Buul-Wortelboer M.F. Brinkman H.J.M. Dingemans K.P. De Groot P.G. van Aken W.G. van Mourik J.A. Reconstitution of the vascular wall in vitro: a novel model to study interactions between endothelial and smooth muscle cells. Exp Cell Res. 1986;162:151. doi: 10.1016/0014-4827(86)90433-7. [DOI] [PubMed] [Google Scholar]
- 66.Ziegler T. Alexander R. Nerem R. An endothelial cell-smooth muscle cell co-culture model for use in the investigation of flow effects on vascular biology. Ann Biomed Eng. 1995;23:216. doi: 10.1007/BF02584424. [DOI] [PubMed] [Google Scholar]
- 67.Niwa K. Kado T. Sakai J. Karino T. The effects of a shear flow on the uptake of LDL and acetylated LDL by an EC monoculture and an EC–SMC coculture. Ann Biomed Eng. 2004;32:537. doi: 10.1023/b:abme.0000019173.79939.54. [DOI] [PubMed] [Google Scholar]
- 68.Wada Y. Sugiyama A. Kohro T. Kobayashi M. Takeya M. Naito M. Kodama T. In vitro model of atherosclerosis using coculture of arterial wall cells and macrophage. Yonsei Med J. 2000;41:740. doi: 10.3349/ymj.2000.41.6.740. [DOI] [PubMed] [Google Scholar]
- 69.Takaku M. Wada Y. Jinnouchi K. Takeya M. Takahashi K. Usuda H. Naito M. Kurihara H. Yazaki Y. Kumazawa Y. Okimoto Y. Umetani M. Noguchi N. Niki E. Hamakubo T. Kodama T. An in vitro coculture model of transmigrant monocytes and foam cell formation. Arterioscler Thromb Vasc Biol. 1999;19:2330. doi: 10.1161/01.atv.19.10.2330. [DOI] [PubMed] [Google Scholar]