Abstract
The primitive streak establishes the antero-posterior body axis in all amniote species. It is thought to be the conduit through which mesoderm and endoderm progenitors ingress and migrate to their ultimate destinations. Despite its importance, the streak remains poorly defined and one of the most enigmatic structures of the animal kingdom. In particular, the posterior end of the primitive streak has not been satisfactorily identified in any species. Unexpectedly, and contrary to prevailing notions, recent evidence suggests that the murine posterior primitive streak extends beyond the embryo proper. In its extraembryonic site, the streak creates a node-like cell reservoir from which the allantois, a universal caudal appendage of all amniotes and the future umbilical cord of placental mammals, emerges. This new insight into the fetal/umbilical relationship may explain the etiology of a large number of umbilical-associated birth defects, many of which are correlated with abnormalities of the embryonic midline.
Keywords: allantois, antero-posterior, axis, embryo, exocoelom, extraembryonic mesoderm, niche, node, notochord, placenta, posterior, primitive streak, stem cell niche, stem cells
Introduction
For more than a century, developmental biologists have been fascinated by the mechanisms through which the amniote body plan is deployed. The primitive streak, a key structure in this process, provides unambiguous physical evidence of bilateral symmetry in the developing organism. During gastrulation, the streak extends through the embryonic midline, creating the anterior-posterior (A-P) body axis, and transforming primitive ectoderm (“epiblast”) into mesoderm and endoderm. Yet, the streak's cellular nature, and the mechanisms by which the streak it forms, elongates, transforms epiblast into two of the primary germ layers, regresses and ultimately disappears, are far from clear. Moreover, while it is well established that the primitive streak and its anterior derivatives, the node and notochord, extend the body axis anteriorly,(1) the streak's posterior end has not been defined in any species.
It is little appreciated that the allantois, a caudal appendage of the embryo, is a universal feature of all vertebrate amniote species. The allantois extends, finger-like, into the exocoelom and establishes blood vessels that, whatever the species, interact with the environment to collect and deliver vital gases and nutrients to the developing fetus. Ultimately, in placental mammals, the allantois creates the umbilical cord. At the cord's distal end, its umbilical blood vessels are secured to the chorion to establish the chorioallantoic placenta. At the cord's proximal end, they form a vascular continuum with the yolk sac and fetus to ensure exchange with the mother during gestation.
The vital relationship between the umbilical cord and the fetus is one of the most understudied areas of human development and disease. Of 43 umbilical associated birth defects and diseases,(2) 20, or nearly 50%, are considered rare diseases.(3) In the mouse, a large number of genes affecting development of the nascent umbilical cord have been identified.(4–6) Most encompass blood vessels, and include defects in urogenital and gastrointestinal systems that are sometimes associated with axial misalignment. Yet, the mechanisms by which the allantois is built and organized with respect to the embryonic body plan are not understood in any species.
From historical and recent experimental perspectives, I examine the posterior region of the murine primitive streak and its relation to the allantois. Results of recent studies are presented which suggest that the posterior primitive streak reaches beyond the embryo, and into the exocoelomic cavity. There, in collaboration with overlying visceral endoderm, the extraembryonic component of the primitive streak creates the allantoic bud and a germinal center, the allantoic core domain (ACD). The ACD is essential for allantoic elongation to the chorion and thus, placentation. Not only does the ACD contain progenitors of the umbilical blood vessels, but it might also be a source of primordial germ cells (PGCs)(7) and progenitors of gut endoderm,(8) thereby contributing to vital fetal lineages.
Together, anterior node and posterior ACD are stem cell reservoirs that deploy a variety of cell types and lengthen the A-P axis in rostral and caudal directions, uniting the embryo and its chorio-allantoic placenta through a common midline.
Morphological relationship between the primitive streak and the allantois
Throughout its history, developmental biology has been shaped and guided by the identification of novel architectural elements. Direct observation, often of the most detailed kind, has created essential blueprints for the design of functional studies. Duval(9) indicated that the rodent allantois was wholly mesodermal. Several decades later, Sobotta(10) suggested that allantoic mesoderm issued from the posterior end of the primitive streak, whose limits were confined to the embryo (p. 316 of that study):
“Andererseits zeigt sich ein kleiner, solider, mesodermaler Vorsprung in die Exocoelomhöhle, der von der Stelle des Hinterendes des Primitivstreifens ausgeht und als kurzer stumpfer Kegel in das Exocoelom hineinragt. Es ist die erste Anlage der auch später solid und rein mesodermal bleibenden Allantois der Maus. Sie entsteht an der Stelle, wo sich das allererste Mesoderm zeigte, wo die Schwanzfalte des Amnions sich bildete, im Bereiche des zylindrischen, hämoglobinresorbierenden Dottersackepithels, der Stelle, die wir bereits früher (S. 305) als Hinterende des Primitivstreifens gedeutet haben. Um diese Zeit ist die Anlage der Allantois, die gegen Ende des achten bis Anfang des neunten Tages nach der Befruchtung durch das Exocoelom hindurch zum Chorion vorwächst, noch sehr klein, denn Fig. 10 stellt den fast genau median geführten Sagittalschnitt dar, auf dem die Allantoisanlage der Keimblase ihre stärkste Ausbildung zeigte.”
“A small, solid mesodermal outpocketing into the exocoelomic cavity from the hind end of the primitive streak is the first anlage of the solid and purely mesodermal allantois of the mouse. It forms at the place where the very first mesoderm showed up, where the tailfold of the amnion forms, in the domain of the cylindrical hemoglobin-resorbing yolk sac epithelium, the place which we previously already had described as the hind end of the primitive streak. The anlage of the allantois, which can be found at the end of the 8th to the beginning the 9th day after fertilization, is still small.”
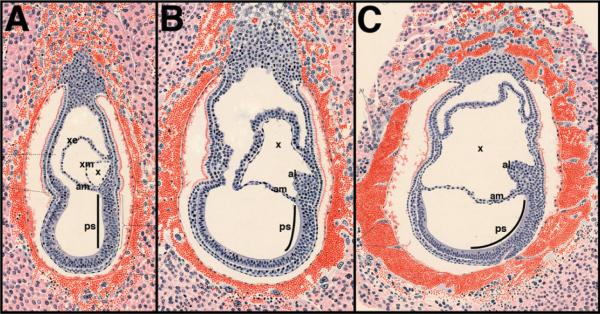
Sobotta's data are presented in Fig. 1. The streak's “hind” or posterior end is situated beneath the nascent amnion (Fig. 1A), which, by convention, delineates the mammalian embryonic and extraembryonic regions. From the intraembryonic primitive streak, the allantois emerges (Fig. 1B) and extends into the exocoelom (Fig. 1C). Ultimately, the allantois fuses with the chorion.
Figure 1.
The posterior primitive streak is confined to the embryo proper and gives rise to the allantois. Modified from Sobotta (1911)(10), Figs. 9, 10, and 12, with kind permission of Springer Science + Business Media. Staging of Sobotta's sections was according to Ref. 14 with recent further refinements at the headfold stages in Ref. 8. A: Presumptive late streak (LS) stage (~7.0 dpc) showing the formation of the exocoelom (x) and the extent of the primitive streak (ps) within the embryonic compartment of the conceptus. The nascent amnion (am) is forming. B: Presumptive very early headfold (vEHF) stage (~7.75 dpc) depicts the allantoic bud (al) in association with the intraembryonic primitive streak, whose posterior limit is within the embryo proper, just beneath the amnion. C: Presumptive early headfold (EHF) stage (~7.75–8.0 dpc) shows an enlarging allantois. The primitive streak, black line within the nascent amniotic cavity, traced from Sobotta's original figures, is limited to the embryo proper.
Sobotta's conclusions have become a central tenet of mouse embryology. One possible implication of his view is that the intraembryonic primitive streak contains the information required for allantoic elongation. Although the allantois appears to be a posterior extension of the embryonic body axis and, in this regard, is similar to the anterior-reaching notochord, a posterior stem cell reservoir with node-like properties has not been identified during the period of allantoic ontogeny.
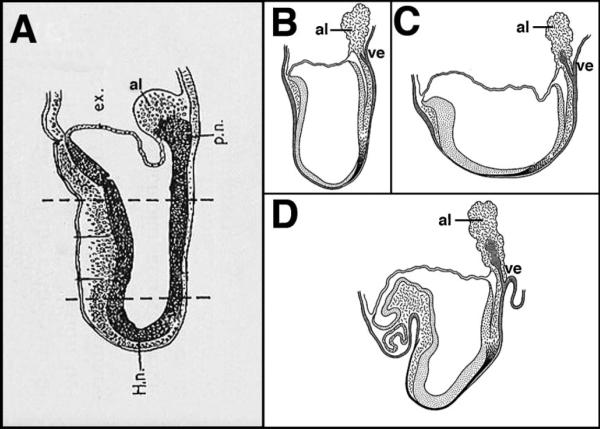
Later investigators embellished the allantois with tantalizing architectural features (Fig. 2). Dalcq(11) noted from biochemical observations(12) that the rodent allantois contains a dense proximal region contiguous with the intraembryonic primitive streak; he referred to this structure as the “posterior node” (Fig. 2A). Unfortunately, whole-embryo culture systems were not yet available for the post-implantation conceptus, making it impossible to investigate this intriguing possibility. In a series of schematic diagrams, Jurand(13) depicted visceral endoderm as diverticulating from the outer endodermal surface of the posterior egg cylinder into the base of the allantois; the experimental source of these figures was, however, unattributed. While I am not aware of formal evidence supporting the latter claims, results of recent studies – morphological, genetic, and experimental – presented here, may bear out Dalcq's view that the densely packed cells of the base of the allantois constitute a posterior node.
Figure 2.
The allantois as depicted by other investigators. A: Modified from Dalcq (p. 125, Fig. 51)(11), after Mulnard(12), Fig. 43a, with permission from the Oxford University Press and Informa Clinical Medicine-Journals. The allantois contains a “posterior node” (p.n.) contiguous with the intraembryonic primitive streak. Estimated stage: early headfold (EHF, ~7.75 dpc). B–D: Figs. 18–20 reproduced and adapted with permission from the Company of Biologists,(13) showing an increasingly elongated bifurcation of the outer visceral endoderm into the base of the allantois. Estimated stages: B: late headfold (LHF, ~8.0 dpc); C: 1–2-somite pairs (1–2-s, ~8.0–8.25 dpc); D: 6-s (~8.5 dpc). al, allantois; ex, exocoelom; H.n., Henson's node; ve, visceral endoderm.
Evidence for an extraembryonic extension of the posterior primitive streak and stem cell reservoir within the allantois
Over the past several decades, the gastrula's developmental stages have been refined.(14) The interval between Sobotta's Fig. 1A and B represents the late streak [LS, ~6.75–7.0 days post-coitum (dpc)] through neural plate/late bud (LB, ~7.5 dpc) stages, and encompasses about 18 h. During this time, the allantoic bud emerges. As developmental processes, particularly for short-gestation species like the mouse, are dynamic, rapid and transient, critical details concerning the relationship of the primitive streak to the allantois could have been missed by Sobotta and others.
Recently, we systematically investigated the emergence of the allantoic bud during this critical period, the neural plate through headfold stages (~7.0–8.0 dpc) just prior to the appearance of the allantoic bud through its elongation to the chorion. Unexpectedly, the posterior primitive streak, located within the midline of the conceptus, appeared to extend its reach some distance into the exocoelom(15) (Fig. 3A); its cells were both continuous with and similar in polarity to those of the intraembryonic primitive streak.(15) The extraembryonic primitive streak (henceforth referred to as the “XPS”) contained Brachyury (T), which has been used extensively to confirm the streak's presence in mutant embryos in studies too numerous to cite here. At least two homeobox genes, Hoxb1 and Hoxb8, which are involved in axial genesis, localized to the presumptive XPS just prior to and during formation of the allantoic bud.(16) In this context, it is also worth noting that Huber(17) presented a camera lucida tracing of a similar posterior extraembryonic extension at the same stage in the guinea pig gastrula (Fig. 4). However, he did not remark upon its presence or possible significance.
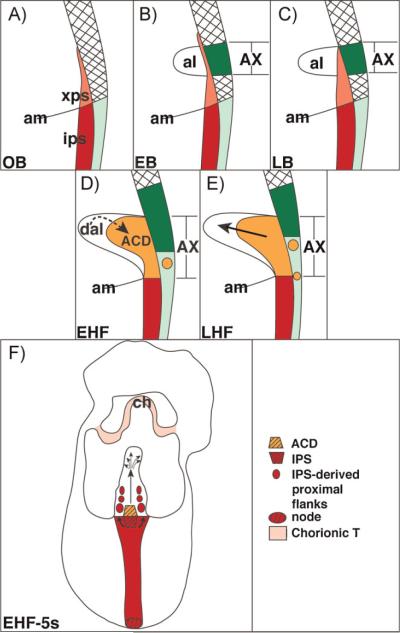
Figure 3.
The posterior primitive streak extends into the extraembryonic region where it establishes the ACD. Reprinted from Ref. 15 with permission from John Wiley and Sons, Inc. Key features of this model: A: The posterior primitive streak establishes the extraembryonic primitive streak (xps). B: The allantoic bud emerges from the distal part of the xps, over which lies allantois-associated extraembryonic visceral endoderm (AX).(8,15) C: In collaboration with the AX, the xps begins to expand. D: By the early headfold (EHF) stage, the ACD (orange) is visible, and enlarging. Together with the distal region of the allantois, the ACD may regulate allantoic length. The AX is becoming transformed and contains T- and Oct-3/4-positive cells (represented by the orange circle). E: The ACD is fully mature, while the AX continues to be transformed into a squamous cell type containing T- and Oct-3/4 cells. F: Arrows indicate the contributions of the ACD and intraembryonic primitive streak (IPS) to the allantois.
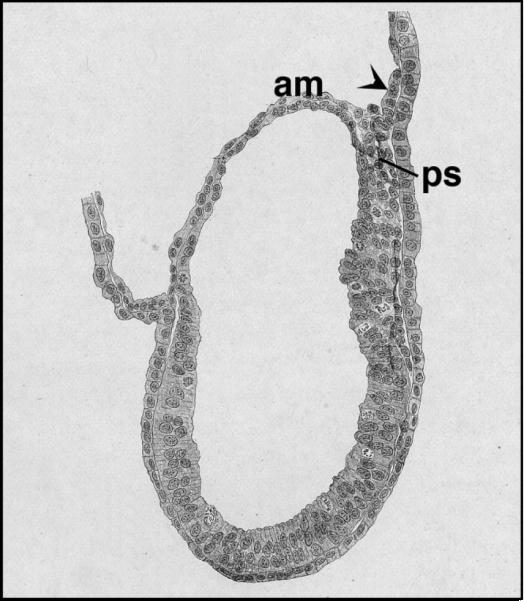
Figure 4.
The posterior primitive streak of the guinea pig may extend its reach into the exocoelom. Modified from Huber, 1918(17) with permission from John Wiley and Sons, Inc. The arrowhead was not in the original figure; it indicates what appears morphologically to be a similar extension of the posterior primitive streak into the exocoelom in this embryonic specimen of the guinea pig. Stage estimated to be neural plate/no bud (OB; ~7.0 dpc) stage. am, amnion; ps, intraembryonic primitive streak.
From this apparent extraembryonic extension of the primitive streak, the allantoic bud emerged (Fig. 3B). At first, the bud had no connection to the embryo. But, by the late bud stage (Fig. 3C), the cells of the XPS loosened their contacts and began to expand into the ventral midline core of the allantois, forming the T-defined ACD (Fig. 3D). As the ACD matured, the allantois enlarged and merged with the embryo proper (Fig. 3D, E), as depicted by Sobotta (Fig. 1). Ectopic grafting experiments suggested that the ACD consists of relatively undifferentiated cells.(18) While distal and midallantoic cells were developmentally restricted at headfold stages (~7.75–8.0 dpc; Fig. 3D, E), some proximal cells within the presumptive ACD were pluripotent and contributed to derivatives of all three primary germ layers.(18) Molecular analyses revealed that the proximal allantoic region contains c-myc mRNA,(19) as well as overlapping Oct-3/4 protein and T protein,(8,15) all of which are found in pluripotent cells.(20–22) Some allantoic Oct-3/4- and T-positive cells contained Flk-1,(8) required for blood vessel formation,(23) and suggesting that some ACD cells are deployed into elements of the umbilical vasculature.
Intriguingly, PGCs have been thought for a long time to reside within the base of the allantois.(24–26) Re-examination of alkaline phosphatase activity in early and recent studies suggests that the PGCs are initially localized within the tip of the primitive streak as it extends into the exocoelomic cavity and creates the allantoic bud.(24–27) PGCs also contain both Oct-3/4 and Brachyury,(20,28) both of which, as described above, are found in the base of the allantois. Interferon-inducible transmembrane signals, localized to the allantois, are thought to direct allantoic PGCs into the hindgut.(29) Steel factor also localizes to the base of the allantois, where it surrounds presumptive PGCs prior to and during their colonization of the hindgut.(7)
How the hindgut forms is not known, but several lines of evidence suggest that the visceral endoderm overlying the allantois may be involved. First, the allantois and hindgut are anatomically physically juxtaposed by 4-somite pairs (4-s), when the hindgut invagination appears just beneath the base of the allantois.(30) The hindgut endoderm may be derived in part from “allantois-associated extraembryonic visceral endoderm” (AX) (Fig. 3B–E), a dynamic structure that becomes transformed into hindgut-like epithelium between the neural plate and headfold stages.(15) Initially, the AX is a columnar/cuboidal and highly vesiculated epithelium whose apical vesicles contain T;(15) Oct-3/4 was not observed in the AX at this time.(8) Then, at the start of the headfold stages, the proximal portion of the AX becomes more squamous, and less vesiculated. T, when present, is no longer found in the cytoplasm but localizes to the nucleus. Further, at this time, Oct-3/4-positive cells also begin to appear in the squamous AX.(8) Thereafter, numbers of T- and Oct-3/4-positive cells ebb and flow in synchrony within the visceral endoderm. How the AX comes to contain T- and Oct-3/4-positive cells is not clear, but they may be derived from the ACD.(8,15) Perhaps they are PGCs leaving the allantois and moving into the developing hindgut, and/or they contribute to the hindgut endoderm.(8)
Finally, a critical vascular structure, the vessel of confluence, or site of amalgamation between the umbilical, yolk sac and embryonic circulatory systems, invariably arises within the base of the allantois at 4-s (~8.25–8.5 dpc).(30) The allantois also contains definitive hematopoietic potential,(31,32) but the spatiotemporal whereabouts of allantoic hematopoietic stem cells has not yet been identified.
Together, these observations provide compelling evidence for an emerging stem cell niche within the ACD. Given the well-established properties of the embryonic primitive streak in organizing the A-P axis and organ anlagen, it makes sense that an extraembryonic component of the primitive streak would provide spatial coordinates to align the nascent umbilical vasculature with those of the fetus and yolk sac. It would also ensure correct spatial deployment of its stem cells with respect to the body axis.
Function of the ACD: a posterior node?
Although the presumptive extraembryonic extension of the primitive streak is found in the conceptus's midline and contains T,(15) declaring that it is truly part of the primitive streak is anything but trivial. Results of previous and expanded recent expression studies have clearly demonstrated that T is not unique to the primitive streak.(33–37) Thus, T expression alone is not conclusive proof of the streak's extraembryonic extension. Moreover, because morphologically similar primitive streak cells express different markers depending upon their position within the streak,(38) the task of defining the streak by molecular markers is not an easy one. Given these difficulties, the nature of the T-defined ACD was investigated by genetic and experimental approaches. These are summarized in Fig. 5.
Figure 5.
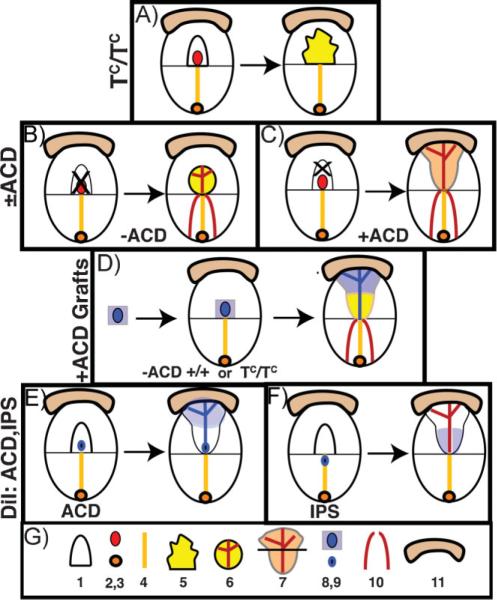
Experimental strategies to discover ACD function. A: TC/TC mutants, In the TC/TC mutant, left, the ACD dies, resulting in a highly blebbed remnant allantois that has a tendency to spread over the amnion and does not elongate to fuse with the chorion. Data from Ref. 42. B: −ACD allantoic regenerates. Left, the entire allantois and its ACD are removed by aspiration. Right, a short −ACD allantoic regenerate forms that does not elongate far enough to fuse with the chorion but displays a properly patterned vasculature that is fused with the paired embryonic dorsal aortae. Data from Refs. 15, 43. C: +ACD regenerates. Left, all but the proximal ACD-containing region was removed by aspiration. Right, the ACD-containing allantoic regenerate elongates, fuses with the chorion, and displays an appropriately patterned vasculature that is fused with the embryonic dorsal aortae. Data from Ref. 15. D: Center, the allantois (“−ACD”) is removed from wildtype (+/+) or TC/TC mutant hosts, and replaced by a graft (left, blue) containing the ACD. Right, the regenerated allantois contains a patterned donor midline that fans out and contributes largely to the distal region, while the flanks are composed of host cells emanating from the intraembryonic primitive streak. From Ref. 15. E: In situ fate mapping of the ACD. Left, DiI (blue) is applied to the center of the ACD. Right, ACD-labeled cells remain in place and form a midline file through the length of the allantois, fanning throughout the distal region. Data from Ref. 15. F: In situ fate mapping of the intraembryonic primitive streak. Left, DiI (blue) is applied to the posterior midline of the intraembryonic primitive streak. Right, labeled descendants, when they contributed to the allantois (only 66% of the time), were found in the allantoic flanks. The original labeled cells did not appear to remain in place. Data from Ref. 15. G: Legend: 1, allantois prior to genetic analysis or manipulation; 2, ACD; 3, node; 4, intraembryonic primitive streak; 5, blebbed TC/TC allantois; 6, −ACD regenerate allantois exhibiting correctly patterned vasculature; 7, elongated fused allantois with correctly patterned vasculature; horizontal bar separates distal (top) and proximal (bottom) regions; 8, ACD-containing donor graft; 9, DiI-labeled cell cluster; 10, paired embryonic dorsal aortae; 11, chorion.
For decades, it had been recognized that embryos lacking T exhibited foreshortening of both the embryonic body axis(39,40) and the allantois.(41) Consequently, T/T embryos did not establish a chorio-allantoic placenta and ultimately died during mid-gestation. Systematic examination of the allantois of TCurtailed (TC/TC) mutants revealed that the T-defined allantoic core,(42) which we now know as the ACD, above, may have formed, but subsequently underwent apoptosis.(42) Consequently, TC/TC allantoises failed to elongate far enough to fuse with the chorion (Fig. 5A). By contrast, the TC/TC intraembryonic primitive streak was unaffected during this early time period, contributing mesoderm to what remained of the mutant allantois. Thus, the allantoic T defect is not secondary to a primary one in the intraembryonic primitive streak; rather, T activity is intrinsic to the allantois.
Prior to discovery of the ACD, the role of the intraembryonic primitive streak in the differentiation of allantoic mesoderm was investigated.(43) Whole allantoises were removed by micro-surgery (Fig. 5B). Allantoic regenerates formed after culture, but most of these failed to elongate far enough to fuse with the chorion.(43) We concluded that the intraembryonic primitive streak transformed only a finite amount of primitive ectoderm, or epiblast, into allantoic mesoderm; alternatively, we entertained the possibility that allantoic regenerates lacked an element normally present in the allantois and critical for elongation (discussed by Downs, Hellman, McHugh, Barrick-mann and Inman, 2004).(43) Once the existence of the ACD was known and shown to be missing in the regenerates, the presence of an intrinsic allantoic element was confirmed.(15)
Removal of all but the ACD-containing region (Fig. 5C) strengthened this conclusion. Most “+ACD” regenerates elongated and fused with the chorion, no matter how late in allantoic development they were created. The latter observations suggest that cross-talk between the distal and proximal allantoic regions controls allantoic length. Grafting a donor ACD-bearing region to host wildtype or TC/TC mutant embryos whose allantois had been removed restored elongation to most of the resulting chimeric allantoises (Fig. 5D).
Because proximal flanking cells remained associated with the ACD in the ±ACD regenerate studies and grafting experiments (Fig. 5B–D), fate mapping in situ was used to discover the respective contributions of the ACD, proximal allantoic flanks, and intraembryonic primitive streak to the allantois. Similar to the node at the anterior end of the streak, which retained cells as it extended a file through the notochord,(44,45) labeled descendants of the ACD remained in place as others assembled into the allantoic midline, appearing as a posterior extension of the embryonic body axis (Fig. 5E).(15) When they reached the distal region, ACD descendants fanned out into an extensive cellular network. By contrast, descendants of the intraembryonic primitive streak inconsistently contributed to the allantois; when they did, they were assimilated into the proximal allantoic flanks without any apparent patterning(15) with respect to the body axis (Fig. 5F).
Thus, the posterior primitive streak spans the embryonic and extraembryonic compartments of the conceptus. The streak's extraembryonic component establishes an intrinsic germinal center, the ACD. The ACD is mature by head-fold stages, and exhibits the properties of a stable domain, maintaining a relatively constant length until chorio-allantoic fusion, when it no longer contains T and may be depleted of its stem cells (6–8-s, ~8.5 dpc(46,47)).(15) Its contribution to the allantois is extensive and patterned. By contrast, the intraembryonic primitive streak contributes sporadically to the allantois, possibly under feedback control from the ACD.(15) Moreover, its contribution is not patterned (Fig. 3F).
Thus, while the anterior primitive streak creates the Henson's node, a conspicuous architectural feature that lengthens the embryonic body axis anteriorly via the notochord,(44) the posterior primitive streak creates the ACD, a less conspicuous feature that extends the body axis posteriorly via the allantois.(15)
But are the XPS and ACD truly part of the primitive streak? In their recent review, Rossant and Tam(48) defined the primitive streak as
“The structure that appears in the posterior region of the gastrulating embryo where the epiblast cells undergo epithelio-mesenchymal transition and ingressional movement to form the germ layers”.
Ectoderm, mesoderm, and endoderm are embryonic germ layers, but formation of ectoderm is not thought to involve the primitive streak.(49) Moreover, Rossant and Tam's definition ignores the fact that the primitive streak is also thought to be the source of extraembryonic mesoderm in the mouse (see below).(45,50,51) These problems notwithstanding, their definition would exclude the XPS as part of the streak. However, as the ACD has not yet been exhaustively fate-mapped, it may, as described above, contribute to embryonic germ layer derivatives such as the PGCs and hindgut endoderm. Given the lack of a clear definition of the primitive streak, our understanding of the anterior node, and properties of the ACD and its relation to the embryonic body axis, the tentative suggestion that the ACD is a posterior node (Fig. 2A) created by the XPS is not unreasonable.
Origin of the extraembryonic primitive streak
From where does the extraembryonic extension of the primitive streak originate? Lawson, Meneses, and Pedersen(45) presented a schematic diagram showing that proximal allantoic cells come from anterior proximal epiblast at the onset of gastrulation. In a later summary of this study,(52) Lawson presented a figure containing the raw data upon which her conclusion was based. If Lawson's results are placed within the context of this essay, it appears that descendants of a single 6.5-dpc anterior proximal epiblast cell ended up as a file of cells at the base of the allantois, juxtaposed to the visceral endoderm, similar to the XPS shown here and continuous with the intraembryonic primitive streak (Fig. 3A–C). Then, over time, the XPS descendants expanded into the ACD,(52) as shown here (Fig. 3D).
Given that an epithelial-to-mesenchymal transition creates the primitive streak,(53) perhaps anterior epiblast cells rapidly translocate to the posterior region and become integrated into ectoderm that spans both the extraembryonic and embryonic compartments. Within both compartments, extra and intraembryonic components of the streak delaminate at the onset of gastrulation. This idea is supported by T expression in the pregastrula mouse conceptus.(36) At about 5.5 dpc, T was expressed radially in the proximal extraembryonic ectoderm. By 6.0 dpc, its domain extended to the proximal epiblast, but to only one side of the egg cylinder. At 6.5 dpc, immediately prior to the onset of gastrulation, T became asymmetrically restricted to the extraembryonic ectoderm and the underlying proximal epiblast. Intriguingly, Wnt3, required for axis formation,(54) localized to embryonic visceral endoderm just before T's restriction.(36) While the authors placed Wnt3's limit to the embryonic compartment of the egg cylinder, it is possible that the expression spanned the posterior embryonic/extraembryonic boundary, and created a broader ectodermal region from which the primitive streak delaminated. Huber(55) had remarked that the boundary between the embryonic and extraembryonic regions of mouse gastrulae at these early stages is impossible to ascertain.
Origin of the allantoic bud and mesoderm, both embryonic and extraembryonic
Although Lawson's results fit our model for an extraembyronic extension of the primitive streak and its expansion into the node, it is still possible that the labeled descendants she described are little more than extraembryonic allantoic mesoderm. Mesoderm is classified as embryonic and extraembryonic, depending upon where it is found in the conceptus. The current conventional view is that all mesoderm is derived from the primitive ectoderm, or epiblast,(56) which is transformed via passage through the intraembryonic primitive streak.(57) Yet, in the mouse, a number of tissues unrelated to the primitive streak have been proposed as mesodermal sources. As it is difficult to envision how epiblast might be transformed within the streak's extraembryonic extension, it is worthwhile to consider the evidence for alternative sources of allantoic mesoderm.
Duval, whose monographs on the histological anatomy of Rodentia, Lagomorpha, Chiroptera, and Carnivora are still authoritative today,(58) concluded that, quite apart from the primitive streak, which arises in the posterior proximal region of the egg cylinder, a thickened part of proximal anterior endoderm gives rise to mesoderm(9):
“Au contraire, sur la partie droite de cette même figure [Fig. 94], et à ce même niveau, l'entoderme proximal présente un simple épaississement; il est formé par deux rangs de cellules, dont les profondes sont sans connexion avec l'ectoderm. Quoi qu'il en soit, l'évolution ultérieure de ces épaississements entodermiques montre qu'ils représentent les premiers rudiments du feuillet moyen, de sorte que l'étude du blastoderme de la souris, malgré ses dispositions si aberrantes, vient confirmer la loi générale que nous nous sommes éfforcé d'établir dans d'autres études [i.e., those carried out on the primitive streak in the chick], à savoir que le mésoderme provient de l'entoderm primitif.”
“By contrast, on the right part of this same figure [Fig. 94], and at the same level [as the primitive streak, opposite], the proximal endoderm is thickening; it consists of two rows of cells, in which the deepest lack connection to the ectoderm. Whatever these are, ultimately they represent the beginnings of mesoderm, and confirm that, despite the dissimilar topography of the mouse to the chick, as in the chick, mesoderm comes from primitive endoderm.”
A source of mesoderm distinct from the primitive streak is supported by observations in non-rodent species, notably the armadillo,(59) the macaque,(60) and humans.(61) All of these exhibit extraembryonic mesoderm prior to the formation of the primitive streak. In the macaque, it was postulated that visceral endoderm gives rise to extraembryonic mesoderm;(62) consequently, the allantois originates from the latter. In addition, some human extravillous cytotrophoblast undergoes an epithelial-to-mesenchymal transition during differentiation, infiltrating deeply into the maternal decidual stroma and blood vessels.(63)
Given that the allantois issues from the posterior region of the egg cylinder, anterior visceral endoderm may not be directly relevant to the origin of the murine allantois. On the other hand, Bonnevie's(64) observations may be. She put forth that murine embryonic mesoderm is derived from the intraembryonic primitive streak, while extraembryonic mesoderm delaminates from the “inner layer” of the mesometrial portion of the pro-amniotic cavity. Based on the material presented, this appears to be trophoblast-derived extraembryonic ectoderm(65,66) (Fig. 11 of that study). Intriguingly, T is expressed in extraembryonic ectoderm and its derivative chorionic ectoderm.(36) The delaminated extraembryonic mesoderm both “dissolves” and becomes infiltrated by fluid, so that many small cavities gradually unite into larger, laterally situated sacs, i.e. the walls of the exocoelom. The allantois, composed of extraembryonic mesoderm, then forms at what Bonnevie suggested to be a posterior survival site where extraembryonic mesoderm – derived from extraembryonic ectoderm – is spared from dissolution. Unfortunately, Bonnevie's study was published posthumously, and her conclusions have not been rigorously re-examined.
The current notion that all mesoderm is derived from epiblast undoubtedly comes from a combination of morphological analyses and fate mapping. In the rat, appearance of the mesoderm and the primitive streak, located within the embryonic compartment of the egg cylinder, appeared simultaneously.(55) Sobotta reached similar conclusions in the mouse. Jolly and Férester-Tadié(67) examined both mouse and rat conceptuses and reported that, although mesoderm appeared slightly in advance of the primitive streak, the latter was nevertheless the source of embryonic and extraembryonic mesoderm, including exocoelomic mesoderm and the allantois. However, their material was not comprehensive and did not represent a finely divided series of morphological stages encompassing ontogeny of extraembryonic mesoderm. Finally, Poelmann(68) concluded that, while some mesoderm arose from mesoderm-organizing centers within the neural crest, most mesoderm was derived from the primitive streak. However, the extraembryonic compartment of the conceptus was not examined.
Fate mapping reinforced an epiblast-derived source of mesoderm, but the results were not unequivocal. For example, blastocyst reconstitution experiments, which followed the approximate fate of the inner cell mass and trophectoderm, provided little information concerning the origin of chorionic mesoderm and the allantois.(69,71) While fate mapping of the intraembryonic primitive streak at a variety of time points indicated mesodermal contribution to the exocoelom and allantois,(45,50,51) the amount was modest and the criteria used to identify the boundary between primitive and extraembryonic ectoderm, particularly at the pre-streak stage,(45) were not well described. This omission leaves open the possibility that, in some cases, more distal extraembryonic sites, including extraembryonic ectoderm, were mapped. Again, in this regard, it is intriguing that extraembryonic ectoderm and chorion express T.(34,36)
Together, these data provide no definitive evidence that transformation of epiblast via the intraembryonic primitive streak is the sole mechanism of mesoderm formation, either in the mouse or in Mammalia as a whole. With the exception of Bonnevie's studies, the origin of extraembryonic mesoderm has not been singularly investigated. Even Wnt3 knockout mouse embryos, which do not form a primitive streak and do not exhibit mesoderm, are not entirely helpful, for Wnt3 protein was not comprehensively localized to all tissues, embryonic and extraembryonic, of the gastrula.(54)
In accord with Bonnevie, the XPS may form a survival site in which extraembryonic mesoderm, spared from cavitation, produces the allantoic bud. Alternatively, the XPS gives rise to the allantois de novo. The nascent allantois exhibited round T-positive cells throughout its length, which may have delaminated from the XPS at the base of the bud. Then, by influence from the overlying visceral endoderm, the extraembryonic streak became transformed into the ACD which, by proliferation, sustained contribution and built the core of the allantois.(15)
The ACD: a universally conserved feature of Mammalia?
Is the ACD a universal feature of mammals? Unfortunately, with the exception of the mouse, no systematic morphological and functional studies have been carried out on the allantois in any other animal group. Nevertheless, some insight may be gleaned from the allantois's mature form in other Mammalia.
Mammals have taken steps to compensate for a reduced yolk supply. In Metatheria (marsupials), the lining of the uterus has become specialized to supply nutrients, and the yolk sac absorbs secretions and other nutritive substances present in the uterine exudate. In all marsupials, the yolk sac is very large while the allantois is always relatively small. Although the allantois elongates far enough to fuse with the chorion in a few groups, e.g. Perameles (long-nosed bandicoot), Phascolarctos (koala), and Phascolomis (wombat), it is small and unattached in most.(72) In the latter, the allantois nourishes the embryos just long enough to allow the differentiation of their main organs and systems, after which they translocate to the pouch where they are nourished by mammary secretions until birth. Whether such short allantoises lack the ACD is not known. Alternatively, the ACD may be present, but its activity is attenuated relative to species whose allantoises fuse with the chorion.
Thus, while extension of the embryonic body plan via the node and notochord is a universally conserved feature of mammalian embryos, their posterior connections to the environment vary greatly.(73) Depending upon the placental needs of each species, the posterior end of the primitive streak may be an evolutionarily unconstrained and labile region, adapted to each species' particular survival strategy. It remains to be determined whether the ACD is a universal feature of Mammalia.
Conclusions
The posterior region of the mouse is far more complex than has been appreciated. Intimate linkage between the primitive streak and the allantois undoubtedly plays a major role in fetal survival and even posterior fetal development. With the exception of Brachyury,(15,42) not a single mutation affecting allantoic development has been examined in any systematic detail.
Given the weaknesses in the case for a definition of the primitive streak,(48) important tasks ahead will be to acknowledge that the streak remains an enigmatic and poorly defined structure whose properties are far from being understood. Moreover, embryologists must cease the practice of confining studies of mammalian development to the embryo proper. The mammalian conceptus is a composite of embryonic and extraembryonic tissues that share their origin within the fertilized egg, and are deployed in intimate juxtaposition to each other. Finally,
“…non seulement nous décrivons ce qui est, mais….nous devons encore le décrire de façon à bien montrer ce qui n'est pas, c'est-à-dire le mal fondé d'interprétations diverses, qui ont pour origine de pures hypothèses, rendues sans doute nécessaires par l'absence de séries suffisantes de préparations.”
“…….not only must we describe what there is, but…also what there is not, e.g., the poorly documented interpretations that originate from pure hypotheses, undoubtedly necessitated by the lack of an insufficient series of préparation.” (from Duval, as cited in Ref. 59).
Over the course of several centuries, embryologists have examined and re-examined eggs and their cleavage products. And always new information is forthcoming. This does not mean that earlier studies were irrelevant. Rather, it demonstrates the sheer developmental complexity of the conceptus. Omission or misinterpretation of the slightest detail may change completely the significance of a result,(74) and drive future generations to look in the wrong places for an explanation of biological processes, especially those that have gone wrong.
Acknowledgments
K. M. D. is grateful to Professor Robert Auerbach for translating the relevant passages of Sobotta (1911), to Professors Allen Enders and Anthony Carter for valuable discussions, to members of her laboratory and especially, to the anonymous reviewers who provided positive and constructive suggestions on the manuscript. This essay was supported by funds from The National Institutes of Child Health and Development (RO1 HD042706) and the March of Dimes.
References
- 1.Cambray N, Wilson V. Two distinct sources for a population of maturing axial progenitors. Development. 2007;134:2829–2840. doi: 10.1242/dev.02877. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson RE, Hall JG, editors. Human malformations and related anomalies. Oxford University Press; Oxford: 2006. [Google Scholar]
- 3. http://rarediseases.info.nih.gov/
- 4.Inman KE, Downs KM. The murine allantois: emerging paradigms in formation and development of the mammalian umbilical cord and its relation to the fetus. Genesis. 2007;45:237–258. doi: 10.1002/dvg.20281. [DOI] [PubMed] [Google Scholar]
- 5.Naiche LA, Papaioannou VE. Loss of Tbx4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development. 2003;130:2681–2693. doi: 10.1242/dev.00504. [DOI] [PubMed] [Google Scholar]
- 6.Schreiner CA, Hoornbeek FK. Developmental aspects of sirenomelia in the mouse. J Morphol. 1973;141:345–358. doi: 10.1002/jmor.1051410308. [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Runyan C, Shoemaker A, Surani MA, Wylie C. Steel factor controls primordial germ cell survival and motility from the time of their specification in the allantois, and provides a continuous niche throughout their migration. Development. 2009;136:1295–1303. doi: 10.1242/dev.030619. [DOI] [PubMed] [Google Scholar]
- 8.Downs KM. Systematic localization of Oct-3/4 to the gastrulating mouse conceptus suggests manifold roles in mammalian development. Dev Dyn. 2008;237:464–475. doi: 10.1002/dvdy.21438. [DOI] [PubMed] [Google Scholar]
- 9.Duval M. Le placenta des rongeurs. Troisiéme partie. Le placenta de la souris et du rat. J Anat. 1891;27:515–612. [Google Scholar]
- 10.Sobotta J. Die Entwicklung des Eies der Maus vom ersten Auftreten des Mesoderms an bis zur Ausbildung der Embryonalanlage und dem Auftreten der Allantois. I. Teil: Die Keimblase. Archiv fur mikroskopische Anatomie. 1911;78:271–352. [Google Scholar]
- 11.Dalcq AM. Introduction to general embryology. Oxford University Press; Oxford: 1957. [Google Scholar]
- 12.Mulnard J. Contribution à la connaissance des enzymes dans l'ontogénèse. Les Phosphomonoestérases acide and alcaline dans le développement du rat et de la souris. Arch Biol. 1955;66:525–685. [PubMed] [Google Scholar]
- 13.Jurand A. Some aspects of the development of the notochord in mouse embryos. J Embryol Exp Morphol. 1974;32:1–33. [PubMed] [Google Scholar]
- 14.Downs KM, Davies T. Staging of gastrulation in mouse embryos by morphological landmarks in the dissection microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- 15.Downs KM, Inman KE, Jin DX, Enders AC. The Allantoic Core Domain (ACD): New insights into development of the murine allantois and its relation to the primitive streak. Dev Dyn. 2009;238:532–553. doi: 10.1002/dvdy.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deschamps J, van den Akker E, Forlani S, de Graaff W, Oosterveen T, et al. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int J Dev Biol. 1999;43:635–650. [PubMed] [Google Scholar]
- 17.Huber GC. On the anlage and morphogenesis of the chorda dorsalis in mammalia in particular the guinea pig (Cavia cobaya) Anat Rec. 1918;14:217–264. [Google Scholar]
- 18.Downs KM, Harmann C. Developmental potency of the murine allantois. Development. 1997;124:2769–2780. doi: 10.1242/dev.124.14.2769. [DOI] [PubMed] [Google Scholar]
- 19.Downs KM, Martin GM, Bishop JM. Contrasting patterns of myc and N-myc expression during gastrulation of the mouse embryo. Genes Dev. 1989;3:860–869. doi: 10.1101/gad.3.6.860. [DOI] [PubMed] [Google Scholar]
- 20.Scholer HR. Octamania: the POU factors in murine development. Trends Genet. 1991;7:1–5. doi: 10.1016/0168-9525(91)90422-m. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki A, Raya A, Kawakami Y, Morita M, Matsui T, et al. Nanog binds to Smad1 and blocks bone morphogenetic protein-induced differentiation of embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:10294–10299. doi: 10.1073/pnas.0506945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu X-F, et al. Failure of blood-island formation and vasculogenesis in Flk-1 deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 24.Chiquoine AD. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat Rec. 1954;118:135–146. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- 25.Ginsburg M, Snow MHL, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- 26.Ozdzenski W. Observations on the origin of primordial germ cells in the mouse. Zool Pol. 1967;17:367–381. [Google Scholar]
- 27.Chuva de. Sousa., Lopes SM, Roelen BAJ. Primordial germ cell specification: the importance of being “blimped”. Histol Histopathol. 2008;23:1553–1561. doi: 10.14670/HH-23.1553. [DOI] [PubMed] [Google Scholar]
- 28.Yabuta Y, Kurimoto K, Ohinata Y, Seki Y, Saitou M. Gene expression dynamics during germline specification in mice identified by quantitative single-cell gene expression profiling. Biol Reprod. 2006;75:705–716. doi: 10.1095/biolreprod.106.053686. [DOI] [PubMed] [Google Scholar]
- 29.Lange UC, Adams DJ, Lee C, Barton S, Schneider R, et al. Normal germ line establishment in mice carrying a deletion of the Ifitm/Fragilis gene family cluster. Mol Cell Biol. 2008;28:4688–4696. doi: 10.1128/MCB.00272-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downs KM, Gifford S, Blahnik M, Gardner RL. The murine allantois undergoes vasculogenesis that is not accompanied by erythropoiesis. Development. 1998;125:4507–4521. doi: 10.1242/dev.125.22.4507. [DOI] [PubMed] [Google Scholar]
- 31.Corbel C, Salaun J, Belo-Diabangouaya P, Dieterlen-Lievre F. Hematopoietic potential of the pre-fusion allantois. Dev Biol. 2007;301:478–488. doi: 10.1016/j.ydbio.2006.08.069. [DOI] [PubMed] [Google Scholar]
- 32.Zeigler BM, Sugiyama D, Chen M, Guo Y, Downs KM, Speck NA. The allantois and chorion, which are isolated before circulation or chorio-allantoic fusion, have hematopoietic potential. Development. 2006;133:4183–4192. doi: 10.1242/dev.02596. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann BG. Expression pattern of the Brachyury gene in wholemount TWis/TWis mutant embryos. Development. 1991;113:913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- 34.Inman K, Downs KM. Localization of Brachyury (T) in embryonic and extraembryonic tissues during mouse gastrulation. Gene Expr Patterns. 2006;6:783–793. doi: 10.1016/j.modgep.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Perea-Gomez A, Camus A, Moreau A, Grieve K, Moneron G, et al. Initiation of gastrulation in the mouse embryo is preceded by an apparent shift in the orientation of the anterior-posterior axis. Curr Biol. 2004;14:197–207. doi: 10.1016/j.cub.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 36.Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 38.Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–3819. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- 39.Chesley P. Development of the short-tailed mutant in the house mouse. J Exp Zool. 1935;70:429–459. [Google Scholar]
- 40.Dobrovolskaia-Zavadskaia N. Sur la mortification spontanee de la queue chez la souris nouveau-nee et sur l'existence d'un charactere heriditaire “non-viable”. CR Soc Biol. 1927;97:114–116. [Google Scholar]
- 41.Gluecksohn-Schoenheimer S. The development of normal and homozygous brachy (T/T) mouse embryos in the extraembryonic coelom of the chick. Proc Natl Acad Sci USA. 1944;30:134–140. doi: 10.1073/pnas.30.6.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inman KE, Downs KM. Brachyury is required for elongation and vasculogenesis in the murine allantois development. 2006;133:2947–2959. doi: 10.1242/dev.02454. [DOI] [PubMed] [Google Scholar]
- 43.Downs KM, Hellman ER, McHugh J, Barrickman K, Inman K. Investigation into a role for the primitive streak in development of the murine allantois. Development. 2004;131:37–55. doi: 10.1242/dev.00906. [DOI] [PubMed] [Google Scholar]
- 44.Beddington RSP. Induction of a second neural axis by the mouse node. Development. 1994;120:613–620. doi: 10.1242/dev.120.3.613. [DOI] [PubMed] [Google Scholar]
- 45.Lawson KA, Meneses J, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 46.Downs KM. Early placentation in the mouse. Placenta. 2002;23:116–131. doi: 10.1053/plac.2001.0763. [DOI] [PubMed] [Google Scholar]
- 47.Downs KM, Gardner RL. An investigation into early placental ontogeny: allantoic attachment to the chorion is selective and developmentally regulated. Development. 1995;121:407–416. doi: 10.1242/dev.121.2.407. [DOI] [PubMed] [Google Scholar]
- 48.Rossant J, Tam PP. Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development. 2009;136:701–713. doi: 10.1242/dev.017178. [DOI] [PubMed] [Google Scholar]
- 49.Beddington RSP. An autoradiographic analysis of tissue potency in different regions of the embryonic ectoderm during gastrulation in the mouse. J Embryol Exp Morphol. 1982;69:265–285. [PubMed] [Google Scholar]
- 50.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis A-K, Nagy A, Tam PPL. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 51.Tam PPL, Beddington RSP. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987;99:109–126. doi: 10.1242/dev.99.1.109. [DOI] [PubMed] [Google Scholar]
- 52.Lawson KA. Fate mapping the mouse embryo. Int J Dev Biol. 1999;43:773–775. [PubMed] [Google Scholar]
- 53.Hashimoto K, Nakatsuji N. Formation of the primitive streak and mesoderm cells in mouse embryos – detailed scanning electron microscopic study. Dev Growth Differ. 1989;31:209–218. doi: 10.1111/j.1440-169X.1989.00209.x. [DOI] [PubMed] [Google Scholar]
- 54.Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 55.Huber GC. The development of the albino rat, mus norvegicus albinus. I. From the pronuclear stage to the stage of mesoderm anlage; end of the first to the end of the ninth day. J Morphol. 1915;26:247–358. [Google Scholar]
- 56.Gardner RL. Contributions of blastocyst micromanipulation to the study of mammalian development. Bioessays. 1998;20:168–180. doi: 10.1002/(SICI)1521-1878(199802)20:2<168::AID-BIES9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 57.Beddington RSP. The origin of the foetal tissues during gastrulation in the rodent. In: Johnson MH, editor. Development in mammals. Vol. 5. Elsevier Science Publishers; Amsterdam: 1983. pp. 1–32. [Google Scholar]
- 58.Pijnenborg R, Vercruysse L. Classics revisited: Mathias Duval on placental development in mice and rats. Placenta. 2005;27:109–118. doi: 10.1016/j.placenta.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Enders AC. Implantation in the nine-banded armadillo: how does a single blastocyst form four embryos? Placenta. 2002;23:71–85. doi: 10.1053/plac.2001.0753. [DOI] [PubMed] [Google Scholar]
- 60.Enders AC. Implantation in the macaque: expansion of the implantation site during the first week of implantation. Placenta. 2007;28:794–802. doi: 10.1016/j.placenta.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Bianchi DW, Wilkins-Haug LE, Enders AC, Hay ED. Origin of extraembryonic mesoderm in experimental animals: Relevance to chorionic mosaicism in humans. Am J Med Genet. 1993;46:542–550. doi: 10.1002/ajmg.1320460517. [DOI] [PubMed] [Google Scholar]
- 62.Enders AC, King BF. Formation and differentiation of extraembryonic mesoderm in the rhesus monkey. Am J Anat. 1988;181:327–340. doi: 10.1002/aja.1001810402. [DOI] [PubMed] [Google Scholar]
- 63.Vicovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat. 1996;156:202–216. doi: 10.1159/000147847. [DOI] [PubMed] [Google Scholar]
- 64.Bonnevie K. New facts on mesoderm formation and proamnion derivatives in the normal mouse embryo. J Morphol. 1950;86:495–546. doi: 10.1002/jmor.1050860303. [DOI] [PubMed] [Google Scholar]
- 65.Gardner RL, Papaioannou VE, Barton SC. Origin of the ectoplacental cone and secondary giant cells in mouse blastocysts reconstituted from isolated trophoblast and inner cell mass. J Embryol Exp Morphol. 1973;30:561–572. [PubMed] [Google Scholar]
- 66.Papioannou VE. Lineage analysis of inner cell mass and trophectoderm using microsurgically reconstituted mouse blastocysts. J Embryol Exp Morphol. 1982;68:199–209. [PubMed] [Google Scholar]
- 67.Jolly J, Ferester-Tadié M. Recherches sur l'œuf du rat et de la souris. Arch d'Anat Microsc. 1936;32:322–390. [Google Scholar]
- 68.Poelmann RE. The formation of the embryonic mesoderm in the early post-implantation mouse embryo. Anat Embryol. 1981;162:29–40. doi: 10.1007/BF00318092. [DOI] [PubMed] [Google Scholar]
- 69.Gardner RL. An investigation of inner cell mass and trophoblast tissue following their isolation from the mouse blastocyst. J Embryol Exp Morphol. 1972;28:279–312. [PubMed] [Google Scholar]
- 70.Gardner RL. Origin and differentiation of extraembryonic tissues in the mouse. Int Rev Exp Pathol. 1983;24:63–143. [PubMed] [Google Scholar]
- 71.Papaioannou VE. Lineage analysis of inner cell mass and trophectoderm using microsurgically reconstituted mouse blastocysts. J Embryol Exp Morphol. 1982;68:199–209. [PubMed] [Google Scholar]
- 72.Amoroso EC. Placentation. In: Parkes EC, editor. Marshall's physiology of reproduction. 3rd ed. Vol. II. Longmans, Green and Co.; London: 1952. pp. 127–311. [Google Scholar]
- 73.Enders AC. Reasons for diversity of placental structure. Placenta. 2008 doi: 10.1016/j.placenta.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 74.Dalcq AM. In: Form and causality in early development. Waddington CH, editor. Cambridge University Press; Cambridge: 1938. [Google Scholar]