Abstract
BACKGROUND
Gram-negative bacilli (GNB) cause as many as 20% of episodes of late-onset sepsis among very low birth weight (VLBW, birth weight < or =1500 g) infants in the neonatal intensive care unit. As the gastrointestinal (GI) tract can serve as a reservoir for GNB, we hypothesized that VLBW infants with prior GI tract colonization with gentamicin-susceptible GNB who developed bloodstream infections (BSI) would do so with gentamicin-susceptible GNB.
METHODS
A prospective cohort study of VLBW infants was performed in 2 level III neonatal intensive care units from September 2004 to October 2007. GI tract surveillance cultures were obtained weekly. Risk factors for GNB BSI and for GI tract colonization with GNB were assessed.
RESULTS
Fifty-one (7.3%) of 698 subjects experienced 59 GNB BSIs of which 34 occurred by 6 weeks of life and 625 (90%) of 698 subjects were colonized with GNB. Overall, 25% of BSI and 16% of GI tract isolates were nonsusceptible to gentamicin and colonization with the same species and same gentamicin susceptibility profile preceded 98% of GNB BSIs. Vaginal delivery, birth weight < or =750 g, GI tract pathology, increased use of central venous catheters, use of vancomycin, mechanical ventilation, and H2 blockers/proton pump inhibitors were associated with GNB BSI. Vaginal delivery, birth weight >1000 g, and treatment with carbapenem agents were associated with GNB colonization.
CONCLUSIONS
These data support the use of empiric gentamicin to treat late-onset sepsis in infants colonized with gentamicin-susceptible GNB. Targeted GI tract surveillance cultures of infants with specific risk factors during weeks 2 to 6 of life could be used to guide empiric therapy for late-onset sepsis.
Keywords: NICU, neonatal infections, gram-negative bloodstream infection, gastrointestinal tract, colonization, late-onset sepsis
Introduction
Infants hospitalized in the neonatal intensive care unit (NICU) are at increased risk of late-onset sepsis due to both innate and acquired risk factors [1-7]. Risk factors for late-onset sepsis in this population have included relative immunodeficiency [1, 2], low birthweight [4, 5, 7], use of central venous catheters (CVC) [4-7], surgical procedures [6], delays in enteral feeding [5], delivery by Cesarean section [8], and colonization with potential pathogens [9-11]. More recently, we demonstrated that risk factors for late-onset sepsis caused by gram-negative bacilli (GNB) included use of a CVC for >10 days, nasal cannula continuous positive airway pressure (NC-CPAP), and/or H2 blockers/proton pump inhibitor (PPI) use as well as gastrointestinal (GI) tract pathology [3, 12]. In addition, the GI tract of hospitalized infants has been shown to serve as a reservoir for infections caused by Candida species [6, 13, 14], Klebsiella pneumonia [11, 15], Escherichia coli [11] and Pseudomonas aeruginosa [16].
As many as 20% of episodes of late-onset sepsis are caused by GNB [5, 17]. Thus, empiric therapy for such infections includes antimicrobial agents for both gram-negative and gram-positive pathogens. In the United States, the most commonly used therapy for late-onset sepsis includes vancomycin and gentamicin [18], although sepsis caused by GNB resistant to gentamicin is well documented [19].
We previously performed a pilot study and showed, using pulsed-field gel electrophoresis that 18 (95%) of 19 endemic GNB BSIs were caused by the same strain with the same antimicrobial susceptibility pattern as previously cultured from these infants' GI tracts [20]. In the current study, we hypothesized that very low birthweight (VLBW birthweight ≤ 1500 grams) infants with prior GI tract colonization with gentamicin-susceptible GNB who developed blood stream infections (BSI) would do so with gentamicin-susceptible GNB and thus be safely treated with gentamicin as empiric therapy for episodes of late-onset sepsis. The aims of this study were to determine if the same strain of GNB associated with antecedent GI tract colonization caused subsequent BSIs, to characterize the epidemiology of GI tract colonization with GNB, and to assess risk factors for GNB colonization and for GNB BSI in VLBW infants. The ultimate goal of this study is to improve the care of infants in the NICU by modifying risk factors to reduce their risk of GNB BSI and using targeted surveillance cultures to reduce the use of unnecessary broad spectrum antibiotics for empiric therapy for late-onset sepsis.
Methods
Study Design
This was a prospective cohort study of VLBW infants admitted to NICU 1 from September 10, 2004 to October 11, 2007 and to NICU 2 from January 6, 2005 to October 11, 2007. The institutional review boards of Columbia University and Weill Cornell Medical College approved the study protocol and waived the requirement for documentation of written informed consent. An information sheet was given to parents of eligible infants that described the study, provided the study team's contact information, and offered parents the option to decline participation in the study.
Study Sites and Study Subjects
The study sites have been described previously [18]. These two level III NICUs are within NewYork-Presbyterian/Morgan Stanley Children's Hospital (58 beds) and the Komansky Center for Children's Health at New York Presbyterian Hospital/Weill Cornell Medical Center (50 beds) and have 1,500 annual discharges combined. The NICUs are geographically separated by 9 miles and only occasionally share staff or patients. Gentamicin is used as empiric therapy for late-onset sepsis caused by GNB in both study NICUs.
Eligible subjects were VLBW infants admitted to the study sites during the study period. Exclusion criteria were (1) parental refusal; (2) congenital anomalies that made obtaining rectal surveillance cultures impossible or unsafe to obtain, e.g., an imperforate anus; (3) age >14 days upon admission to the study sites; and (4) death within 48 hours of admission. A subset of the subjects in this study with GNB BSI was previously described [20].
Microbiology Studies
Surveillance cultures of the GI tract were obtained weekly until NICU discharge. The rectal verge of eligible infants was swabbed with a rayon-tipped swab (CultureSwab Collection and Transport System, BD Diagnostic Systems, Sparks, MD). Surveillance cultures were processed by the Clinical Microbiology Laboratory at Columbia University using selective MacConkey agar plates (BD) which were inoculated, incubated at 35° C, and examined for growth at 24 and 48 hours. Up to two predominant bacterial morphotypes were selected for identification and susceptibility testing using the MicroScan WalkAway® SI System (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY). The categorical interpretations of susceptible (≤4 μg/ml), intermediate (8 μg/ml) or resistant (≥ 16 μg/ml) were derived from established minimal inhibitory concentration (MIC) breakpoints for gentamicin against GNB [21]. In this study, isolates were considered to be non-susceptible to gentamicin if the MIC was ≥ 8 μg/ml.
Blood cultures were obtained by the NICU staff as clinically indicated and processed in each site's Clinical Microbiology Laboratory using BACTEC Peds Plus® bottles in the BACTEC FX system (BD) at site 1 and the BacT/ALERT system (bioMérieux, Durham, NC) at site 2, as per the standard of care.
Case Definitions
The study's primary outcome was a BSI caused by a GNB that occurred in a VLBW infant ≥ 72 hours after birth. If an infant had ≥ 2 BSIs caused by GNB, only the first was included in the analysis of risk factors. However, if an infant had ≥ 2 BSIs caused by different species of GNB, all were included in the analysis of concordance between colonizing and infecting isolates. GI tract surveillance culture isolates were presumed concordant with subsequent BSI isolates if the GNB species and gentamicin susceptibility profile were the same.
Assessing Risk Factors for BSIs and GI Tract Colonization with GNB
Potential risk factors for GNB BSIs and GI tract colonization were collected by reviewing subjects' electronic medical records. Demographic and clinical characteristics, device use and medical treatments, and age (in days) at first enteral feeding (breast milk and/or formula) were assessed as risk factors.
Statistical Analysis
Logistic regression was used to estimate the effects of hypothesized risk factors for GNB BSI. For potential risk factors, bivariate analyses were performed using the Chi-squared test or Fisher's exact test, when appropriate and logistic regression was performed for continuous variables. In multivariate analysis, variables with p<0.25 in the bivariate analysis were entered into the initial logistic regression model. In addition, NC-CPAP use was forced into the model, due to its significance as a risk factor in a previous study [4]. As birthweight and gestational age were highly correlated (r=0.746, p<0.001) only birthweight was used to avoid multicollinearity between predictors. Backward elimination was used to build a multivariate model that included all variables with p<0.05 and that controlled for the number of surveillance cultures performed for each infant. Only infants colonized with a GNB during their NICU stay who had data for their first enteral feeding were included in the multivariate model. Manual model selection tested the robustness of the findings.
Cox Proportional Hazards Models were used to evaluate factors contributing to GNB colonization because colonization was measured as time-to-event (days to colonization). Infants who were not colonized were considered censored at the day of the last surveillance culture result or NICU discharge, whichever occurred first. Hazards ratios and 95% confidence intervals (CI95) were calculated for each significant factor. All statistical analyses were performed using SAS® 9.1 for Windows.
Results
Characteristics of Participating Subjects
During the study period, 698 VLWB infants were enrolled; no parents refused to participate in the study. The characteristics of the subjects are shown in Table 1. Approximately half (49%, 341/698) were extremely low birthweight (ELBW, birthweight ≤ 1,000 grams). Infants in NICU 1 had lower birthweights (p<0.001) and shorter lengths of stay (p=0.049) than infants in NICU 2.
Table 1. Demographic and clinical characteristics of infants by study site.
| Characteristic | NICU 1 (N=453) n (%) |
NICU 2 (N=245) n (%) |
Total (N=698) n (%) |
P-value |
|---|---|---|---|---|
| Male | 231(51.0) | 105(42.9) | 336(48.1) | 0.040 |
| Gestational age (weeks) | ||||
| Mean(sd) | 27.9 (2.6) | 28.8 (2.5) | 28.2 (2.6) | <0.001 |
| Birthweight (grams) | ||||
| ≤750 | 113 (24.9) | 45 (18.4) | 158 (22.6) | <0.001 |
| 751-1,000 | 133 (29.4) | 50 (20.4) | 183 (26.1) | |
| 1,001-1,250 | 110 (24.3) | 69 (28.2) | 179 (25.6) | |
| 1,251-1,500 | 97 (21.4) | 81 (33.0) | 178 (25.5) | |
| GNB BSI | 32 (7.1) | 19 (7.8) | 51 (7.3) | 0.738 |
| Length of Stay (days) | ||||
| Mean (sd) | 48.6 (37.9) | 54.6 (38.5) | 50.7 (38.2) | 0.049 |
| Number of Surveillance Cultures | ||||
| Mean (sd) | 7.1 (5.0) | 8.0 (4.6) | 7.4 (4.8) | 0.021 |
| Age at first enteral feeding (days)a | ||||
| Mean (sd) | 7.6 (10.6) | 8.5 (11.7) | 7.9 (11.0) | 0.312 |
NOTE. GNB BSI, gram-negative bacilli bloodstream infection. Data are presented as number (%), unless otherwise specified. Analysis was performed using the chi-squared test for categorical variables and logistic regression for continuous variables.
Excludes 18 infants with missing data for age at enteral feeding.
BSIs caused by GNB
Fifty one (7.3%) of the 698 subjects had 59 GNB BSIs including 7 who experienced ≥ 2 GNB BSIs. The proportion of infants who developed a GNB BSI was similar at both study sites (Table 1). The ages at presentation and rate of GNB BSIs for the first 18 weeks of life are shown in the Figure. Most (88%, 45/51) subjects with GNB BSI were ELBW infants and over half (58%, 34/59) of the episodes of GNB BSIs occurred within the first 6 weeks of life. However, among the 7 infants with ≥ 2 GNB BSIs, the second episode occurred after the 11th week of life and 5 had ≥ one gentamicin non-susceptible isolate causing their BSIs.
Figure. Gram-negative bacilli colonization and blood stream infection by week of life.
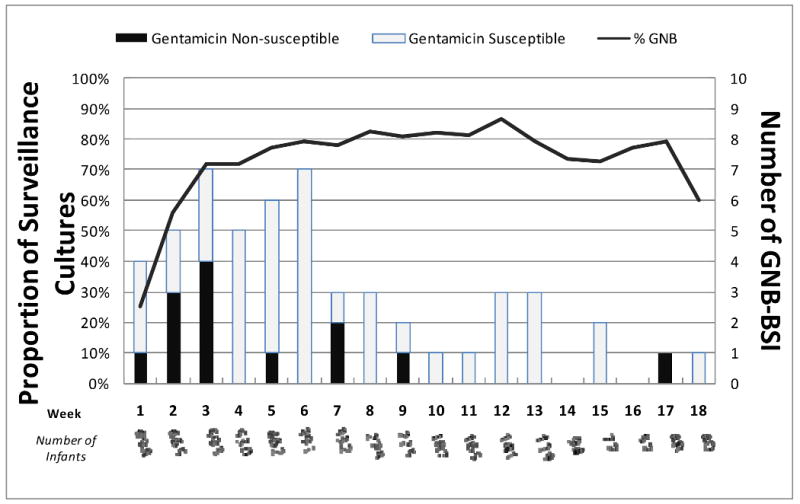
The left hand Y-axis shows the proportion of infants colonized with GNB and the right hand Y-axis shows the number of GNB BSIs during the first 18 weeks of life. The X-axis represents the week of life and shows the number of infants remaining in the study NICUs each week. Gentamicin-susceptible and gentamicin-non-susceptible BSIs are shown. Five BSIs occurred after the 18th week of life and are not shown in the Figure.
Concordance of GNB from GI tract and BSI
All 59 episodes of GNB BSIs were preceded by a surveillance culture with the same strain and 58 (98%) had the same gentamicin susceptibility profile; the one discordant case had a gentamicin-non-susceptible surveillance isolate and gentamicin-susceptible blood isolate. Of the strains causing BSIs, 25%, 5% and 0% were non-susceptible to gentamicin, third generation cephalosporin agents, and carbapenem agents, respectively. Concordant surveillance cultures were obtained a mean of 6 days (median 6, range 1-17 days) prior to the onset of GNB BSI. The sensitivity, specificity, and positive predictive value of GI tract surveillance cultures to predict gentamicin-non-susceptible GNB BSI were 100%, 98%, and 94% respectively.
Risk factors Associated with GNB BSI
Risk factors significantly associated with GNB BSI as assessed by univariate analysis are shown in Table 2. While longer duration of treatment with gentamicin or ampicillin were risk factors for GNB BSI, the duration of treatment with other agents was not, although few infants were treated with piperacillin-tazobactam (n=33), imipenem or meropenem (n=42) or cefazolin (n=14). The week of life when GNB colonization was first detected was not associated with BSI. No factors were significantly associated with having gentamicin-non-susceptible GNB BSI compared with gentamicin-susceptible GNB BSI.
Table 2. Univariate analysis of factors associated with bloodstream infections caused by gram-negative bacilli among infants in the NICU.
| Risk Factor | GNB BSI (n=51) |
No GNB BSI (n=647) |
P-value |
|---|---|---|---|
| Male | 32 (63%) | 304 (47%) | 0.030 |
| Gestational Age weeks (sd) | 26 (2) | 28 (3) | <0.001 |
| Birthweight (grams) | 760 (173) | 1032 (280) | <0.001 |
| Birthweight (grams) | |||
| ≤750 | 27 (53%) | 135 (21%) | <0.001 |
| 751-1,000 | 18 (35%) | 161 (25%) | <0.001 |
| 1,001-1500 | 6 (12%) | 351 (54%) | Ref |
| Vaginal delivery | 21 (41%) | 137 (21%) | 0.001 |
| GI Tract Pathology | 17 (33%) | 28 (4%) | <0.001 |
| CVC (days) | 31 (24) | 18 (22) | <0.001 |
| Mechanical ventilation use | 41 (80%) | 233 (36%) | <0.001 |
| Mechanical ventilation (days) | 16 (23) | 6(20) | 0.021 |
| Gentamicin (days) | 10 (10) | 6(8) | <0.001 |
| Vancomycin use | 43 (84%) | 285 (44%) | <0.001 |
| Ampicillin (days) | 5 (4) | 3 (4) | 0.037 |
| H2 blockers/PPI use | 23 (45%) | 128 (20%) | <0.001 |
| H2 blockers/PPI (days) | 33 (61) | 8 (28) | <0.001 |
| Age at first enteral feeding (days) | 17 (19) | 7 (10) | <0.001 |
| Number of surveillance cultures | 5 (4) | 8 (5) | 0.002 |
| NC-CPAP use | 41 (80%) | 549 (85%) | 0.396 |
NOTE. NICU, neonatal intensive care unit; GI, gastrointestinal; CVC, central venous catheter; and NC-CPAP, nasal cannula continuous positive airway pressure. This table presents results of univariate analysis using the chi-squared test for categorical variables and logistic regression for continuous variables. Data are presented as number (%) of infants for categorical variables and mean (standard deviation) for continuous variables.
Multivariate analysis was performed on 619 infants colonized with GNB and with complete data for enteral feeding. Vaginal delivery, longer duration of CVC use, birthweight ≤750 grams, and use of mechanical ventilation, vancomycin, and H2 blockers/PPIs, as well as GI tract pathology remained significantly associated with GNB BSI (Table 3). The Hosmer and Lemeshow Test of Goodness of Fit indicated that the model was a good fit (p=0.761).
Table 3. Multivariate analysis of risk factors associated with bloodstream infections caused by gram-negative bacilli among infants in the NICU.
| Risk Factor | Odds Ratio(CI95) | P-value |
|---|---|---|
| Vaginal Delivery | 4.36 (1.60, 11.92) | 0.004 |
| Birthweight (grams) | ||
| ≤750 g | 5.85 (1.63, 21.0) | 0.007 |
| 751-1,000 | 4.33 (1.20,15.62) | 0.025 |
| 1,001-1500 | -- | -- |
| GI Tract Pathology | 6.08 (1.90,19.50) | 0.002 |
| Vancomycin use | 6.10 (1.85, 20.10) | 0.003 |
| H2 blockers/PPI use | 7.64 (2.76, 21.17) | <0.001 |
| Mechanical ventilation | 4.18 (1.41, 12.38) | 0.010 |
| CVC days | 1.07 (1.04, 1.10) | <0.001 |
NOTE. NICU, neonatal intensive care unit; GI, gastrointestinal; CVC, central venous catheter.
GI Tract Colonization with GNB
During the study period, 5,931 surveillance cultures were performed and 90% (625/698) of subjects had ≥ one GNB positive culture. The proportion of infants colonized with GNB increased with age (Figure); 25%, 56%, 72%, 72%, 77%, and 79% of infants had positive surveillance cultures in the 1st, 2nd, 3rd, 4th, 5th and 6th week of life, respectively. The three most commonly isolated GNB from both surveillance and blood cultures were Escherichia coli, Klebsiella oxytoca, and K. pneumoniae. Overall, 16%, 4%, and 1% of isolates colonizing the GI tract were non-susceptible to gentamicin, third generation cephalosporin agents, and carbapenem agents, respectively. The proportion of gentamicin non-susceptible isolates was higher among Klebsiella species compared with E. coli and other species (p<0.001).
Factors Associated with GNB Colonization
The probability of GNB colonization was increased in infants delivered vaginally, with birthweight >1000 grams, and with longer duration of carbapenem use. For example, the risk of being colonized with GNB at any given time was 52% higher for infants delivered vaginally compared with infants delivered by Cesarean section, adjusting for other risk factors. In contrast, infants with delayed enteral feeding and/or longer duration of CVC use had a decreased probability of GNB colonization (Table 4). No factors were significantly associated with time to colonization with a gentamicin-non-susceptible strain.
Table 4. Hazard ratios of risk factors for colonization with gram-negative bacilli among infants in the NICU.
| Risk Factors | Hazard Ratio (CI95) | P-value |
|---|---|---|
| Vaginal Delivery | 1.52 (1.25, 1.84) | <0.001 |
| Birthweight 751-1,000 grams | 1.15 (0.90, 1.47) | <0.254 |
| Birthweight 1,001-1,500 grams | 1.56 (1.21, 2.00) | <0.001 |
| Use of CVCa | 0.99 (0.99, 1.00) | 0.035 |
| Use of carbapenem agenta | 1.04 (1.01, 1.07) | 0.002 |
| Delay to first enteral feedinga | 0.98 (0.97, 0.99) | <0.001 |
NOTE. GNB, Gram-negative bacilli; CI95, 95% confidence intervals; CVC, central venous catheter. Hazard ratios were estimated using a multivariate Cox Proportional Hazards Model. Eighteen infants missing enteral feeding data were excluded from the analysis.
Hazard ratio for each additional day of use of a central venous catheter or day of use of imipenem and/or meropenem, or day of delay to first enteral feeding.
Discussion
To our knowledge, this is the largest recent study prospectively evaluating the potential use of GI tract surveillance cultures among VLBW infants. We focused on gentamicin susceptibility of GNB in surveillance cultures of the GI tract with the goal of providing safe empiric treatment for late-onset sepsis in this population. We found that 98% of BSIs caused by GNB were preceded by GI tract colonization with the same species and same gentamicin susceptibility profile. In addition, we found that several risk factors (Table 4), some of which are modifiable, e.g., prolonged use of CVCs and use of H2 blockers and PPIs, were significantly associated with endemic GNB BSI. These data suggest that targeted surveillance cultures of the GI tract could be used in selected high risk infants to guide treatment of late-onset sepsis and to reduce the inappropriate use of broad spectrum agents.
Gentamicin-non-susceptible strains caused approximately 25% of GNB BSIs and colonized 16% of infants in this study. Colonization with gentamicin-non-susceptible strains occurred as early as the first week of life. However, no risk factors, including antibiotic use or vaginal delivery, were found for either colonization or infection with gentamicin-non-susceptible strains. In the late 1990's, Toltzis et al. found that 101 (8.6%) of 1180 infants (mean birth weight 2512 grams) became colonized with GNB resistant to gentamicin, piperacillin-tazobactam, or ceftazidime and that colonization occurred as early as the first day of life. Several factors were found to be associated with colonization with resistant GNB, using univariate analysis, including low birthweight and antibiotic exposure. When compared with our study, differences in population, including birthweight, as well as differences in data analysis could account for these findings. [22]
There has been previous interest in the utility of surveillance cultures in the NICU population to predict the pathogens associated with sepsis. In 1978, Sprunt et al. reported that infants with “abnormal” pharyngeal colonization, defined as ≥ 104 colonies/ml of bacteria other than α-hemolytic streptococci, had an estimated 15-fold greater risk of bacteremia caused by these bacteria compared with normally colonized infants [9]. In contrast, in 1988-1989, Finelli et al. did not find an association between prior pharyngeal colonization and subsequent BSIs or meningitis [23]. Slagle et al. found no association between routine surveillance cultures of endotracheal tube aspirates and subsequent episodes of sepsis [24]. In previous studies conducted in the NICU, the sensitivity, specificity, and positive predictive value (PPV) of surveillance cultures from different body sites for developing BSIs were 56%, 82%, and 8%, respectively [25], however, these studies did not assess the predictive value of the antimicrobial susceptibility of colonizing flora. In the current study, we found higher sensitivity (100%), specificity (98%), and PPV (94%) of GI tract surveillance cultures to predict gentamicin-non-susceptible GNB BSI, suggesting that targeted use of such surveillance cultures could be useful in choosing empiric therapy for late-onset sepsis.
The risk factors associated with GNB BSI that were demonstrated in this study have biological plausibility. Vaginal delivery was associated with both GNB BSI and a higher rate of colonization with GNB suggesting acquisition of maternal flora during delivery. However, vaginal delivery was not demonstrated to be a risk factor for either infection or colonization with gentamicin-non-susceptible GNB. As shown previously, ELBW as confounded by intrinsic immuno- and barrier- deficiencies was a risk factor [1, 2]. Similarly, increased duration of CVC use and mechanical ventilation were confirmed as risk factors, presumably due to disruption of normal barrier functions [3-5, 7].
Enteral feeding can provide immunological protection to infants by promoting intestinal acquisition of commensal flora. Stoll et al. have reported an increased risk of late-onset sepsis among infants with a delay in first enteral feeding [5]. Similarly, Terrin et al. found that infants who initially received trophic feeds in addition to total parenteral nutrition (TPN) had reduced risk of sepsis compared to infants who received TPN alone [26], and Perlman et al. found that infants receiving TPN (with CVCs) were 4-fold more likely to have a BSI compared with those not receiving TPN (with CVCs) [7]. Consistent with these previous studies, we found a delay in enteral feeding was associated with GNB BSI in univariate analysis; however, this association was not found in the multivariate model and was most likely confounded by use of CVCs.
We have previously reported that GI tract pathology (i.e., necrotizing enterocolitis, congenital anomalies such as intestinal atresia, or functional abnormalities such as Hirschsprung's disease) and the use of H2 blockers/PPIs were associated with candidemia in the NICU population [6, 12]. In the current study, we found that these same risk factors were also associated with GNB BSI likely due to loss of normal barrier functions, i.e., reduced gastric acidity and disruption of the integrity of the bowel mucosa which could result in translocation of GNB from the GI tract to the bloodstream. This is the first study to demonstrate that vancomycin use was significantly associated with GNB BSI, suggesting that this agent can alter GI tract flora, but this observation needs to be confirmed.
This study had potential limitations. We did not perform molecular typing on the GNB colonizing the GI tracts of all infants with BSI as we had previously shown 95% concordance between BSI and GI tract isolates in a subset of the infants (n=15) in this study [20]. Race and ethnicity could not be assessed as possible risk factors for colonization and infection as these data were not systematically collected at the study sites. While the timing of first enteral feeding was studied, the volume and types of feeds and the impact of trophic feeds were not determined. Additionally, the generalizability of this study may be limited due to the local ecology of the study NICUs. The timing and use of GI surveillance cultures may not easily transfer into clinical practice and their cost effectiveness was not determined.
In summary, we demonstrated a high rate of presumed concordance between GNB isolated from surveillance cultures and subsequent blood culture isolates of which 25% were gentamicin-non-susceptible. These data support the empiric use of gentamicin for treatment of late-onset sepsis among VLBW infants colonized with gentamicin-susceptible GNB and suggest that infants harboring gentamicin-non-susceptible GNB require alternative empiric therapy. Our data further suggest that GNB BSIs could be reduced in the NICU population by minimizing exposure to certain risk factors such as vancomycin and H2 blockers/PPIs and by prompt removal of CVCs when no longer necessary. While universal use of surveillance cultures is not feasible in clinical practice, targeted surveillance of ELBW infants with specific risk factors during the 2-6th week of life could be useful to guide empiric therapy of late-onset sepsis. Future studies should assess the most effective strategy for targeted surveillance cultures for GNB in the NICU population.
Acknowledgments
Financial support. This study was supported by NIH Mentored Clinical Research Scholar Grant K12-RR017648.
Footnotes
No reprints available
References
- 1.Hall SL. Coagulase-negative staphyloccocal infections in neonates. Pediatr Infect Dis J. 1991;10:57–67. doi: 10.1097/00006454-199101000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Jason JM. Infectious disease-related deaths of low birth weight infants, United States, 1968 to 1982. Pediatrics. 1989;84(2):296–303. [PubMed] [Google Scholar]
- 3.Graham PL, III, Begg MD, Larson E, Della-Latta P, Allen A, Saiman L. Risk factors for late onset gram-negative sepsis in low birth weight infants hospitalized in the neonatal intensive care unit. Pediatr Infect Dis J. 2006;25(2):113–7. doi: 10.1097/01.inf.0000199310.52875.10. [DOI] [PubMed] [Google Scholar]
- 4.Beck-Sague CM, Azimi P, Fonseca SN, et al. Bloodstream infections in neonatal intensive care unit patients: results of a multicenter study. Pediatr Infect Dis J. 1994;13(12):1110–6. [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110(2):285–91. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 6.Saiman L, Ludington E, Pfaller M, et al. Risk factors for candidemia in neonatal intensive care unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19(4):319–24. doi: 10.1097/00006454-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Perlman SE, Saiman L, Larson EL. Risk factors for late-onset health care-associated bloodstream infections in patients in neonatal intensive care units. Am J Infect Control. 2007;35(3):177–82. doi: 10.1016/j.ajic.2006.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: a national survey. Pediatrics. 2002;109(1):34–9. doi: 10.1542/peds.109.1.34. [DOI] [PubMed] [Google Scholar]
- 9.Sprunt K, Leidy G, Redman W. Abnormal colonization of neonates in an intensive care unit: means of identifying neonates at risk of infection. Pediatr Res. 1978;12(10):998–1002. doi: 10.1203/00006450-197810000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Goldmann DA, Leclair J, Macone A. Bacterial colonization of neonates admitted to an intensive care environment. J Pediatr. 1978;93(2):288–93. doi: 10.1016/s0022-3476(78)80523-x. [DOI] [PubMed] [Google Scholar]
- 11.Almuneef MA, Baltimore RS, Farrel PA, Reagan-Cirincione P, Dembry LM. Molecular typing demonstrating transmission of gram-negative rods in a neonatal intensive care unit in the absence of a recognized epidemic. Clin Infect Dis. 2001;32(2):220–7. doi: 10.1086/318477. [DOI] [PubMed] [Google Scholar]
- 12.Feja KN, Wu F, Roberts K, et al. Risk factors for candidemia in critically ill infants: a matched case-control study. J Pediatr. 2005;147(2):156–61. doi: 10.1016/j.jpeds.2005.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saiman L, Ludington E, Dawson JD, et al. Risk factors for Candida species colonization of neonatal intensive care unit patients. Pediatr Infect Dis J. 2001;20(12):1119–24. doi: 10.1097/00006454-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Vendettuoli V, Tana M, Tirone C, et al. The role of Candida surveillance cultures for identification of a preterm subpopulation at highest risk for invasive fungal infection. Pediatr Infect Dis J. 2008;27(12):1114–6. doi: 10.1097/INF.0b013e31817fce78. [DOI] [PubMed] [Google Scholar]
- 15.Gupta A, Della-Latta P, Todd B, et al. Outbreak of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal intensive care unit linked to artificial nails. Infect Control Hosp Epidemiol. 2004;25(3):210–5. doi: 10.1086/502380. [DOI] [PubMed] [Google Scholar]
- 16.Foca M, Jakob K, Whittier S, et al. Endemic Pseudomonas aeruginosa infection in a neonatal intensive care unit. N Engl J Med. 2000;343(10):695–700. doi: 10.1056/NEJM200009073431004. [DOI] [PubMed] [Google Scholar]
- 17.Cordero L, Rau R, Taylor D, Ayers LW. Enteric gram-negative bacilli bloodstream infections: 17 years' experience in a neonatal intensive care unit. Am J Infect Control. 2004;32(4):189–95. doi: 10.1016/j.ajic.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Grohskopf LA, Huskins WC, Sinkowitz-Cochran RL, et al. Use of antimicrobial agents in United States neonatal and pediatric intensive care patients. Pediatr Infect Dis J. 2005;24(9):766–73. doi: 10.1097/01.inf.0000178064.55193.1c. [DOI] [PubMed] [Google Scholar]
- 19.Friedman S, Shah V, Ohlsson A, Matlow AG. Neonatal Escherichia coli infections: concerns regarding resistance to current therapy. Acta Paediatr. 2000;89(6):686–9. doi: 10.1080/080352500750044007. [DOI] [PubMed] [Google Scholar]
- 20.Graham PL, 3rd, Della-Latta P, Wu F, Zhou J, Saiman L. The gastrointestinal tract serves as the reservoir for Gram-negative pathogens in very low birth weight infants. Pediatr Infect Dis J. 2007;26(12):1153–6. doi: 10.1097/INF.0b013e31814619d4. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute (CLSI) 19th informational supplement. Vol. 29. Wayne, PA: Clinical and Laboratory Standards Institute; 2009. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 22.Toltzis P, Dul MJ, Hoyen C, et al. Molecular epidemiology of antibiotic-resistant gram-negative bacilli in a neonatal intensive care unit during a nonoutbreak period. Pediatrics. 2001;108:1143. doi: 10.1542/peds.108.5.1143. [DOI] [PubMed] [Google Scholar]
- 23.Finelli L, Livengood JR, Saiman L. Surveillance of pharyngeal colonization: detection and control of serious bacterial illness in low birth weight infants. Pediatr Infect Dis J. 1994;13(10):854–9. [PubMed] [Google Scholar]
- 24.Slagle TA, Bifano EM, Wolf JW, Gross SJ. Routine endotracheal cultures for the prediction of sepsis in ventilated babies. Arch Dis Child. 1989;64:34–8. doi: 10.1136/adc.64.1_spec_no.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans ME, Schaffner W, Federspiel CF, Cotton RB, McKee KT, Stratton CW. Sensitivity, specificity, and predictive value of body surface cultures in a neonatal intensive care unit. JAMA. 1988;259(2):248–52. [PubMed] [Google Scholar]
- 26.Terrin G, Passariello A, Canani RB, Manguso F, Paludetto R, Cascioli C. Minimal enteral feeding reduces the risk of sepsis in feed-intolerant very low birth weight newborns. Acta Paediatr. 2009;98(1):31–5. doi: 10.1111/j.1651-2227.2008.00987.x. [DOI] [PubMed] [Google Scholar]


