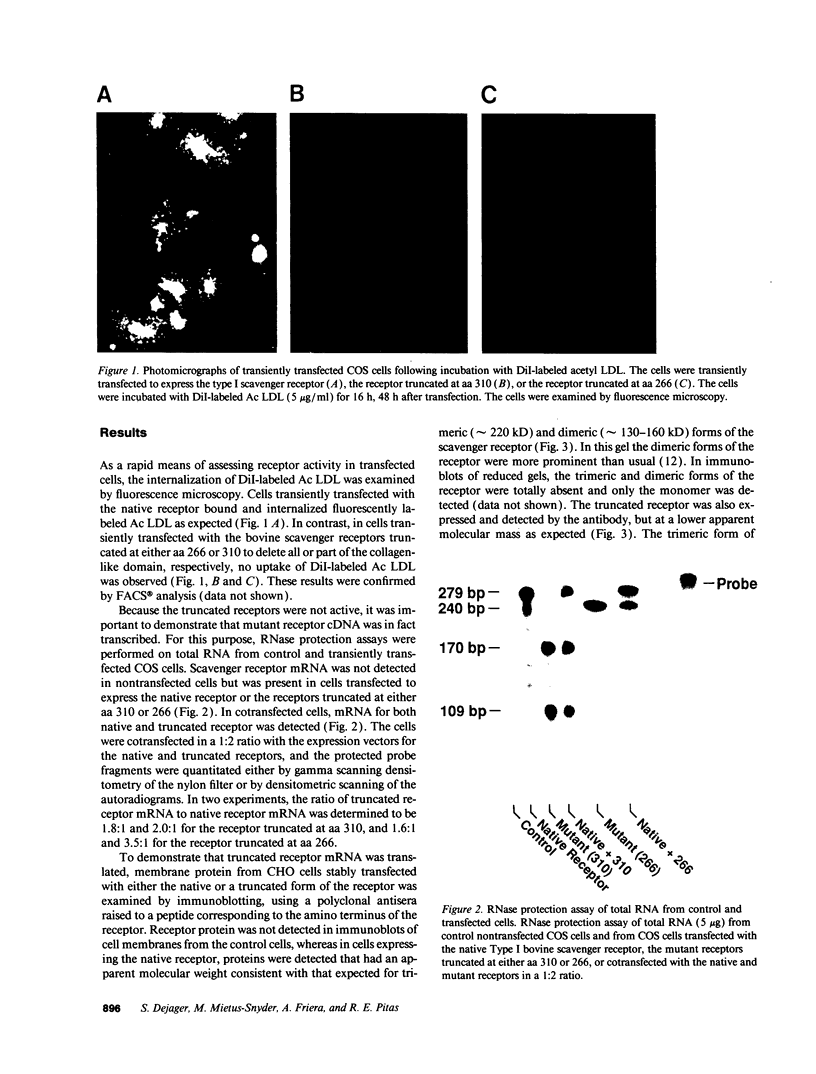
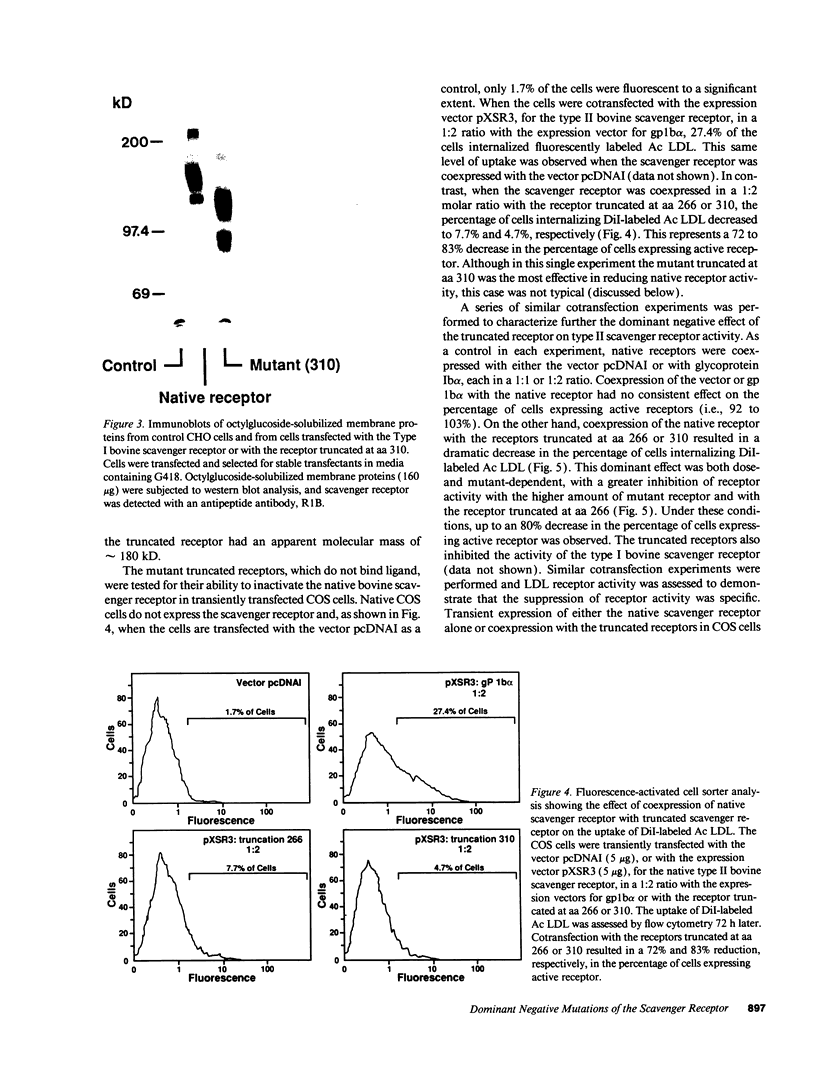
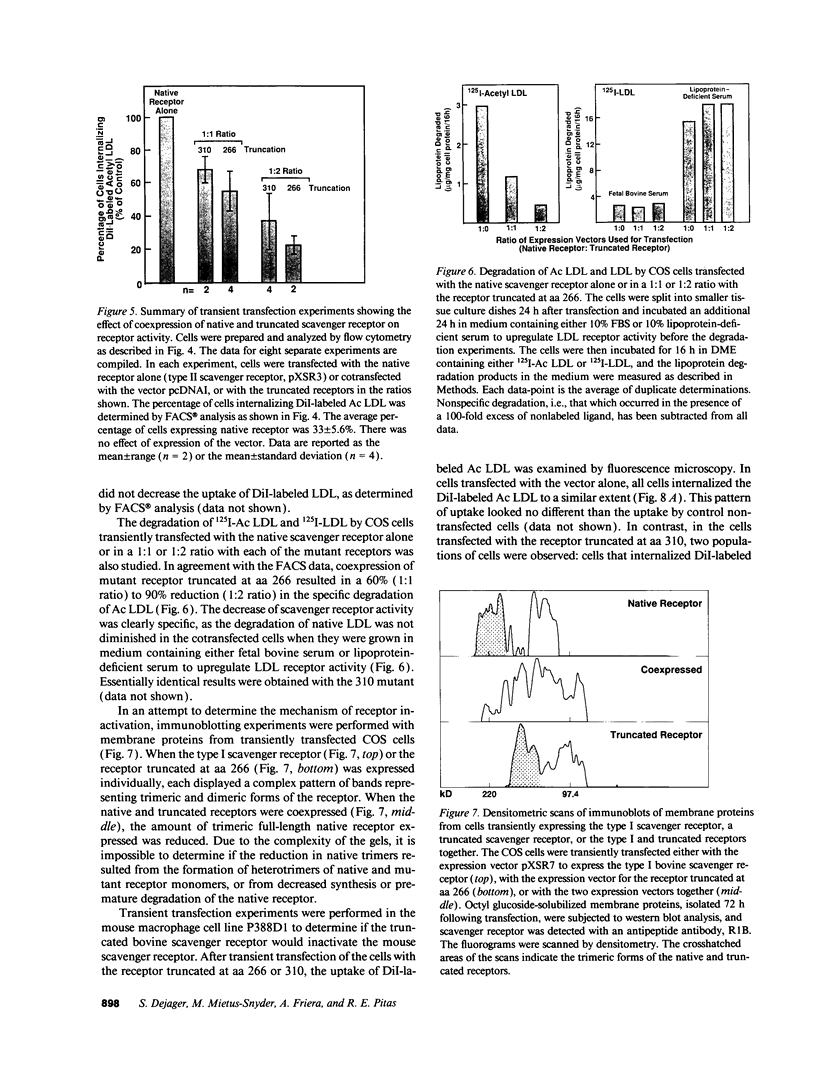
Abstract
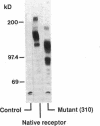
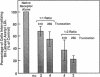
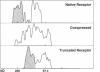
The bovine scavenger receptor was truncated at amino acid 266 or 310 to delete either all or part, respectively, of the collagen-like domain. The truncated receptors were inactive in the binding and internalization of acetyl (Ac) low density lipoprotein (LDL). Coexpression of truncated receptor with the native receptor dramatically reduced the percentage of cells internalizing fluorescently labeled Ac LDL, compared with cells expressing the native receptor alone. The mutant truncated at amino acid 266 was most effective in receptor inactivation, resulting in a 42% or 80% decrease in the percentage of cells expressing active receptor when transfected in a 1:1 or 1:2 molar ratio (native:mutant), respectively, with native receptor. Degradation of 125I-Ac LDL was reduced up to 90% when the native and truncated mutant receptors were coexpressed. Scavenger receptor inhibition was specific because the activity of the LDL receptor was not altered. Transient transfection of the mouse macrophage cell line P388D1 with truncated scavenger receptor resulted in a 65% decrease in the uptake and degradation of Ac LDL but did not decrease the degradation of beta-migrating very low density lipoprotein, which is LDL receptor-mediated. These results demonstrate that expression of truncated bovine scavenger receptor inactivates both the native bovine and murine scavenger receptors, producing a dominant negative phenotype in vitro.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton S., Resnick D., Freeman M., Ekkel Y., Ashkenas J., Krieger M. The collagenous domains of macrophage scavenger receptors and complement component C1q mediate their similar, but not identical, binding specificities for polyanionic ligands. J Biol Chem. 1993 Feb 15;268(5):3530–3537. [PubMed] [Google Scholar]
- Basu S. K., Goldstein J. L., Anderson G. W., Brown M. S. Degradation of cationized low density lipoprotein and regulation of cholesterol metabolism in homozygous familial hypercholesterolemia fibroblasts. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3178–3182. doi: 10.1073/pnas.73.9.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilheimer D. W., Eisenberg S., Levy R. I. The metabolism of very low density lipoprotein proteins. I. Preliminary in vitro and in vivo observations. Biochim Biophys Acta. 1972 Feb 21;260(2):212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Dejager S., Mietus-Synder M., Pitas R. E. Oxidized low density lipoproteins bind to the scavenger receptor expressed by rabbit smooth muscle cells and macrophages. Arterioscler Thromb. 1993 Mar;13(3):371–378. doi: 10.1161/01.atv.13.3.371. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M., Ashkenas J., Rees D. J., Kingsley D. M., Copeland N. G., Jenkins N. A., Krieger M. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8810–8814. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M., Ekkel Y., Rohrer L., Penman M., Freedman N. J., Chisolm G. M., Krieger M. Expression of type I and type II bovine scavenger receptors in Chinese hamster ovary cells: lipid droplet accumulation and nonreciprocal cross competition by acetylated and oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4931–4935. doi: 10.1073/pnas.88.11.4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brown M. S. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M. The role of altered lipoproteins in the pathogenesis of atherosclerosis. Am Heart J. 1987 Feb;113(2 Pt 2):573–577. doi: 10.1016/0002-8703(87)90635-1. [DOI] [PubMed] [Google Scholar]
- Hampton R. Y., Golenbock D. T., Penman M., Krieger M., Raetz C. R. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991 Jul 25;352(6333):342–344. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Lipoprotein-receptor interactions. Methods Enzymol. 1986;129:542–565. doi: 10.1016/0076-6879(86)29091-6. [DOI] [PubMed] [Google Scholar]
- Kashles O., Yarden Y., Fischer R., Ullrich A., Schlessinger J. A dominant negative mutation suppresses the function of normal epidermal growth factor receptors by heterodimerization. Mol Cell Biol. 1991 Mar;11(3):1454–1463. doi: 10.1128/mcb.11.3.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T., Freeman M., Rohrer L., Zabrecky J., Matsudaira P., Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990 Feb 8;343(6258):531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- Kodama T., Reddy P., Kishimoto C., Krieger M. Purification and characterization of a bovine acetyl low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9238–9242. doi: 10.1073/pnas.85.23.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo C., Wernette-Hammond M. E., Innerarity T. L. Uptake of canine beta-very low density lipoproteins by mouse peritoneal macrophages is mediated by a low density lipoprotein receptor. J Biol Chem. 1986 Aug 25;261(24):11194–11201. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. B., Oh S. Y. Altered metabolism (in vivo and in vitro) of plasma lipoproteins after selective chemical modification of lysine residues of the apoproteins. J Clin Invest. 1979 Sep;64(3):743–750. doi: 10.1172/JCI109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Innerarity T. L., Windmueller H. G. Accelerated clearance of low-density and high-density lipoproteins and retarded clearance of E apoprotein-containing lipoproteins from the plasma of rats after modification of lysine residues. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1746–1750. doi: 10.1073/pnas.76.4.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcel Y. L., Innerarity T. L., Spilman C., Mahley R. W., Protter A. A., Milne R. W. Mapping of human apolipoprotein B antigenic determinants. Arteriosclerosis. 1987 Mar-Apr;7(2):166–175. doi: 10.1161/01.atv.7.2.166. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Naito M., Itakura H., Ikemoto S., Asaoka H., Hayakawa I., Kanamori H., Aburatani H., Takaku F., Suzuki H. Human macrophage scavenger receptors: primary structure, expression, and localization in atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9133–9137. doi: 10.1073/pnas.87.23.9133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamaye K. L., Eckstein F. Inhibition of restriction endonuclease Nci I cleavage by phosphorothioate groups and its application to oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1986 Dec 22;14(24):9679–9698. doi: 10.1093/nar/14.24.9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odawara M., Kadowaki T., Yamamoto R., Shibasaki Y., Tobe K., Accili D., Bevins C., Mikami Y., Matsuura N., Akanuma Y. Human diabetes associated with a mutation in the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):66–68. doi: 10.1126/science.2544998. [DOI] [PubMed] [Google Scholar]
- Penman M., Lux A., Freedman N. J., Rohrer L., Ekkel Y., McKinstry H., Resnick D., Krieger M. The type I and type II bovine scavenger receptors expressed in Chinese hamster ovary cells are trimeric proteins with collagenous triple helical domains comprising noncovalently associated monomers and Cys83-disulfide-linked dimers. J Biol Chem. 1991 Dec 15;266(35):23985–23993. [PubMed] [Google Scholar]
- Pitas R. E. Expression of the acetyl low density lipoprotein receptor by rabbit fibroblasts and smooth muscle cells. Up-regulation by phorbol esters. J Biol Chem. 1990 Jul 25;265(21):12722–12727. [PubMed] [Google Scholar]
- Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. Acetoacetylated lipoproteins used to distinguish fibroblasts from macrophages in vitro by fluorescence microscopy. Arteriosclerosis. 1981 May-Jun;1(3):177–185. doi: 10.1161/01.atv.1.3.177. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Constantinou C. D., Dombrowski K. E., Hojima Y., Kadler K. E., Kuivaniemi H., Tromp G., Vogel B. E. Type I procollagen: the gene-protein system that harbors most of the mutations causing osteogenesis imperfecta and probably more common heritable disorders of connective tissue. Am J Med Genet. 1989 Sep;34(1):60–67. doi: 10.1002/ajmg.1320340112. [DOI] [PubMed] [Google Scholar]
- Rohrer L., Freeman M., Kodama T., Penman M., Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990 Feb 8;343(6258):570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Tan J. C., Nocka K., Ray P., Traktman P., Besmer P. The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science. 1990 Jan 12;247(4939):209–212. doi: 10.1126/science.1688471. [DOI] [PubMed] [Google Scholar]
- Taylor J. W., Ott J., Eckstein F. The rapid generation of oligonucleotide-directed mutations at high frequency using phosphorothioate-modified DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8765–8785. doi: 10.1093/nar/13.24.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. W., Schmidt W., Cosstick R., Okruszek A., Eckstein F. The use of phosphorothioate-modified DNA in restriction enzyme reactions to prepare nicked DNA. Nucleic Acids Res. 1985 Dec 20;13(24):8749–8764. doi: 10.1093/nar/13.24.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Colbert H., Escobedo J. A., Williams L. T. Inhibition of PDGF beta receptor signal transduction by coexpression of a truncated receptor. Science. 1991 May 10;252(5007):844–848. doi: 10.1126/science.1851331. [DOI] [PubMed] [Google Scholar]
- Vandenberg P., Khillan J. S., Prockop D. J., Helminen H., Kontusaari S., Ala-Kokko L. Expression of a partially deleted gene of human type II procollagen (COL2A1) in transgenic mice produces a chondrodysplasia. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7640–7644. doi: 10.1073/pnas.88.17.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via D. P., Dresel H. A., Cheng S. L., Gotto A. M., Jr Murine macrophage tumors are a source of a 260,000-dalton acetyl-low density lipoprotein receptor. J Biol Chem. 1985 Jun 25;260(12):7379–7386. [PubMed] [Google Scholar]
- Via D. P., Kempner E. S., Pons L., Fanslow A. E., Vignale S., Smith L. C., Gotto A. M., Jr, Dresel H. A. Mouse macrophage receptor for acetylated low density lipoprotein: demonstration of a fully functional subunit in the membrane and with purified receptor. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6780–6784. doi: 10.1073/pnas.89.15.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker J., Soos M. A., Siddle K. Hybrid insulin receptors. Molecular mechanisms of negative-dominant mutations in receptor-mediated insulin resistance. Diabetes Care. 1990 Jun;13(6):576–581. doi: 10.2337/diacare.13.6.576. [DOI] [PubMed] [Google Scholar]