Abstract
Magnetic resonance (MRI) and diffusion tensor imaging (DTI) data were acquired in 13 AD patients, 15 elderly alcoholics, and 32 elderly controls. Midsagittal area, length, dorsoventral height, fractional anisotropy (FA), and mean diffusivity (MD) of the total corpus callosum and volume of the lateral ventricles were measured; area, FA, and MD were also determined for the callosal genu, body, and splenium. On DTI, both patient groups had lower FA and higher MD than controls in all callosal regions. On MRI, both patient groups had smaller genu than controls; additional size deficits were presents in the alcoholic's callosal body and the AD splenium. The callosal arch was higher in the AD but not the alcoholic group compared with controls. The two patient groups had larger ventricles than controls, and the AD had larger ventricles than alcoholics. Callosal area correlated with its height, and callosal FA and MD correlated with ventricular volume in AD, whereas callosal area correlated only with FA in alcoholics. In AD, the disruption of the callosal integrity, which was associated with distorted callosal shape, was related to ventricular dilation, which has been shown in twin studies to be under a multitude of genetic, polygenetic, and environmental influences. Conversely, in alcoholism, disruption of callosal microstructural integrity is related to shrinkage of the corpus callosum itself.
Keywords: Alcoholism, Alzheimer disease, corpus callosum, MRI, DTI, ventricle
1. Introduction
Diffusion tensor imaging (DTI) provides in vivo assessment of integrity of tissue microstructure, quantified as fractional anisotropy (FA) and mean diffusivity (MD). DTI studies have identified microstructural abnormalities (low FA and high MD) in the corpus callosum in a number of neurological and psychiatric conditions, including Alzheimer's disease (AD, Wang et al., 2009), multiple sclerosis (Roosendaal et al., 2009), alcohol dependence (Pfefferbaum and Sullivan, 2005), schizophrenia (Rotarska-Jagiela et al., 2009), and bipolar disorders (Bellani et al., 2009). Although diffusion parameters can index regional microstructural tissue integrity, the coarse resolution of DTI prevents specifying the underlying structural neuropathology. Because DTI is typically used to study a single diagnostic group, the lack of comparison groups precludes determination of selectivity of observed abnormalities. Even when common DTI abnormalities of the corpus callosum are observed in different groups, they may reflect different pathological processes. The combined use of DTI and structural magnetic resonance imaging (MRI) used to characterize multiple diagnoses within a single study has the potential of identifying different radiologically-evident neuropathological causes of shared phenotypes.
Recent DTI investigations reported lower FA (Cho et al., 2008; Chua et al., 2008; Parente et al., 2008; Ukmar et al., 2008; Wang et al., 2009) and higher MD (Stahl et al., 2007; Wang et al., 2009) in the corpus callosum of patients with Alzheimer’s disease than in controls, indicative of callosal microstructural compromise. Although neuropathological studies have shown that AD broadly results in loss of myelinated axons (Scheltens et al., 1995), microglia inflammation (Xiang et al., 2006), oligodendrocytic reduction and astrocytosis (Sjobeck and Englund, 2003), the specific effects of AD on callosal macrostructure and microstructure detectable neuroradiologically remain unclear. From a macrostructural perspective, MRI studies have reported callosal shrinkage notably in the genu and the splenium of AD patients (Chaim et al., 2007; Li et al., 2008; Tomaiuolo et al., 2007; Wang et al., 2006), whereas others have noted a smaller callosal arch angle indicative of a more convex callosal shape in AD patients than controls (Shuyu et al., 2007; Thompson et al., 1998; Tomaiuolo et al., 2007). Twin studies have shown that callosal shape and size have different determinants, the size being mainly determined by genetic factors shown to be stable in longitudinal study, whereas the dorsoventral height, which reflects its inflection, is influenced by mutiple genetic, polygenetic, and environment factors and is influenced by lateral ventricular volume dilatation (Pfefferbaum et al., 2004; Pfefferbaum et al., 2000). Ventricular enlargement, classically reported in AD (Creasey et al., 1986; DeCarli et al., 1992; Forstl et al., 1995), has been considered an objective and sensitive measure of AD-related neuropathological change (Nestor et al., 2008), especially because ventricular enlargement is highly correlated with senile plaque and neurofibrillary tangle counts (Silbert et al., 2003). Therefore, ventricular enlargement could distort callosal shape (Pfefferbaum et al., 2000) rather than lead to axonal deletion or myelin degradation and could result in altered callosal microstructure.
Microstructural abnormalities of the corpus callosum also occur in chronic alcoholism (Pfefferbaum and Sullivan, 2005) and are exacerbated with advancing age in alcoholics (Pfefferbaum et al., 2006a). DTI combined with postmortem studies suggest that callosal fiber degradation includes demyelination (Lewohl et al., 2000; Tarnowska-Dziduszko et al., 1995) and microtubule disruption (de la Monte, 1988; Mayfield et al., 2002; Paula-Barbosa and Tavares, 1985; Putzke et al., 1998; Wiggins et al., 1988). Shrinkage of the corpus callosum has been reported in structural MRI studies of alcoholics, with the genu being predominantly affected (Hommer et al., 1996; Pfefferbaum et al., 1996). White matter volume deficits in alcoholics may be explained by fewer white matter fibers in alcoholics than controls, as is reported in non-callosal white matter bundles (Chanraud et al., 2009). Therefore, microstructural abnormalities in this brain region may directly reflect structural damage of corpus callosum per se in alcoholism and not simply from distortion due to ventricular expansion.
Callosal macrostructure and microstructure are altered in both AD and alcohol dependence. However, shrinkage and low FA with high MD in the corpus callosum can result from at least two different processes specific to each diagnosis and possibly discernable through neuroimaging. To examine these potential interrelations, we tested the following hypotheses: 1) both elderly alcoholics and AD patients would have macrostructural and microstructural abnormalities indicated by smaller callosal areas, lower FA and higher MD than controls; and 2) in AD, callosal DTI metrics would be related to ventricular enlargement and not to corpus callosum shrinkage, whereas in elderly alcoholics, callosal DTI metrics would be related to corpus callosum shrinkage and not to lateral ventricular dilatation.
2. Methods
2.1. Participants
The 60 participants were 13 patients with AD, 15 detoxified patients with alcohol dependence, and 32 healthy control subjects. Alcoholics and controls were selected from prior analyses (Pfefferbaum et al., 2007). All subjects were volunteers, gave written informed consent obtained according to institutional review board guidelines of SRI International and Stanford University School of Medicine to participate in this study, and were paid a modest stipend for participation.
The alcoholic group comprised 10 men and 5 women (age 60–76 years), who were recruited from local rehabilitation centers, met DSM-IV criteria for alcohol substance dependence, and had refrained from drinking alcohol for 2 weeks to 1 year, except for one man who was sober for 2 years (median=117 days). The AD group comprised 8 men and 5 women (age 64–93 years), who were recruited from the neighboring neurology and psychiatry clinics, and met the National Institute of Neurological and Communicative Diseases and Stroke–Alzheimer's Disease and Related Disorders Association criteria for probable AD (Khachaturian, 1985; McKhann et al., 1984). The healthy control group comprised 13 men and 19 women (age 61–82 years) and were recruited by referral from patient participants, Internet posting, newspaper advertisements, flyers, and word of mouth. All potential study subjects were examined to identify the following exclusionary criteria: presence of DSM-IV Axis I diagnoses of Bipolar Disorder or Schizophrenia, history of nonalcohol substance dependence, alcohol-related amnestic disorder, CNS trauma (such as loss of consciousness for greater than 30 min, seizures not related to alcohol withdrawal, degenerative disease), or serious medical condition (such as, insulin-dependent diabetes, hepatic disorder).
Table 1 presents demographic data for each subject group. One-way analyses of variance (ANOVA) with follow-up comparisons based on least significant difference tests indicated that the groups did not differ significantly in education, estimated premorbid intelligence quotient (IQ, Nelson, 1982), socioeconomic status (Hollingshead and Redlich, 1958), or body mass index. The groups did differ in age, with alcoholics being younger than controls, who were younger than AD subjects. AD subjects had significantly lower scores than the two other groups on global measures of cognitive functioning, the Mini-Mental State Examination (MMSE, Folstein et al., 1975) and the Dementia Rating Scale (DRS, Mattis, 1988). The alcoholics consumed significantly more alcohol over their lifetime than did the two other groups (about 10 times more than controls and 5 times more than AD subjects).
Table 1.
Demographic and clinical data in the three groups (Mean ± standard deviation)
| Group | Control N=32 |
Alzheimer N=13 |
Alcoholic N=15 |
F value | Comparisons |
|---|---|---|---|---|---|
| Age (years) | 69.38 ± 5.62 | 78.21 ± 9.51 | 65.16 ± 5.43 | F(2,57)=14.22 | Alc<Ctrl<AD |
| Education (years) | 16.31 ± 2.40 | 17.38 ± 1.89 | 15.87 ± 3.78 | F(2,57)=1.15 | NS |
| BMI | 33.33c ± 22.42 | 37.96b ± 28.13 | 26.47 ± 5.93 | F(2,52)=0.004 | NS |
| SES | 42.47 ± 15.61 | 45.23 ± 10.44 | 44.87b ± 25.33 | F(2,55)=1.50 | NS |
| NART IQ | 114.63a ± 7.02 | 114.92 ± 4.89 | 111.27a ± 9.41 | F(2,55)=5.75 | NS |
| MMSE | 27.83b ± 1.62 | 22.15 ± 2.41 | 28.00a ± 1.29 | F(2,54)=28.60 | Ctrl=Alc>AD |
| DRS | 140.47b ± 3.27 | 110.84 ± 3.87 | 137.08b ± 4.29 | F(2,53)=51.81 | Ctrl=Alc>AD |
| Alcohol history | |||||
| Sobriety (days) | – | – | 172 ± 190 | – | |
| Lifetime alcohol intake (Kg) | 80.87 ± 92.46 | 165.35d ± 215.40 | 881.62a ± 533.25 | F(2,52)=38.62 | Alc>Ctrl=AD |
BMI: Body Mass Index
SES: socioeconomic status
NART IQ: National Adult Reading Test Intelligence Quotient
MMSE: Mini Mental State Examination
DRS: Mattis Dementia Rating Scale
Ctrl: control subjects; Alc: alcoholic subjects; AD: Alzheimer subjects
NS: no significant differences between the three groups
value for one subject is missing
value for two subject is missing
value for 3 subjects are missing
value for 4 subjects are missing
2.2. MRI and DTI acquisition protocols
All scans were acquired on a 1.5 T GE Signa (General Electric, Milwaukee, WI) with Horizon EchoSpeed gradients using the following acquisition protocol. Two coronal structural sequences were used for this analysis: (1) a SPoiled Gradient Recalled Echo (SPGR) sequence (94, 2-mm-thick slices; TR/TE = 25/5 ms, flip angle = 30°, matrix = 256 * 192; FOV=24cm), (2) a thin-slice, late-echo fast spin echo (FSE) sequence prescribed at the same slice locations as the SPGR (94, 2-mm-thick slices; TR/TE = 11050/98 ms, matrix = 256 * 192; FOV=24cm).
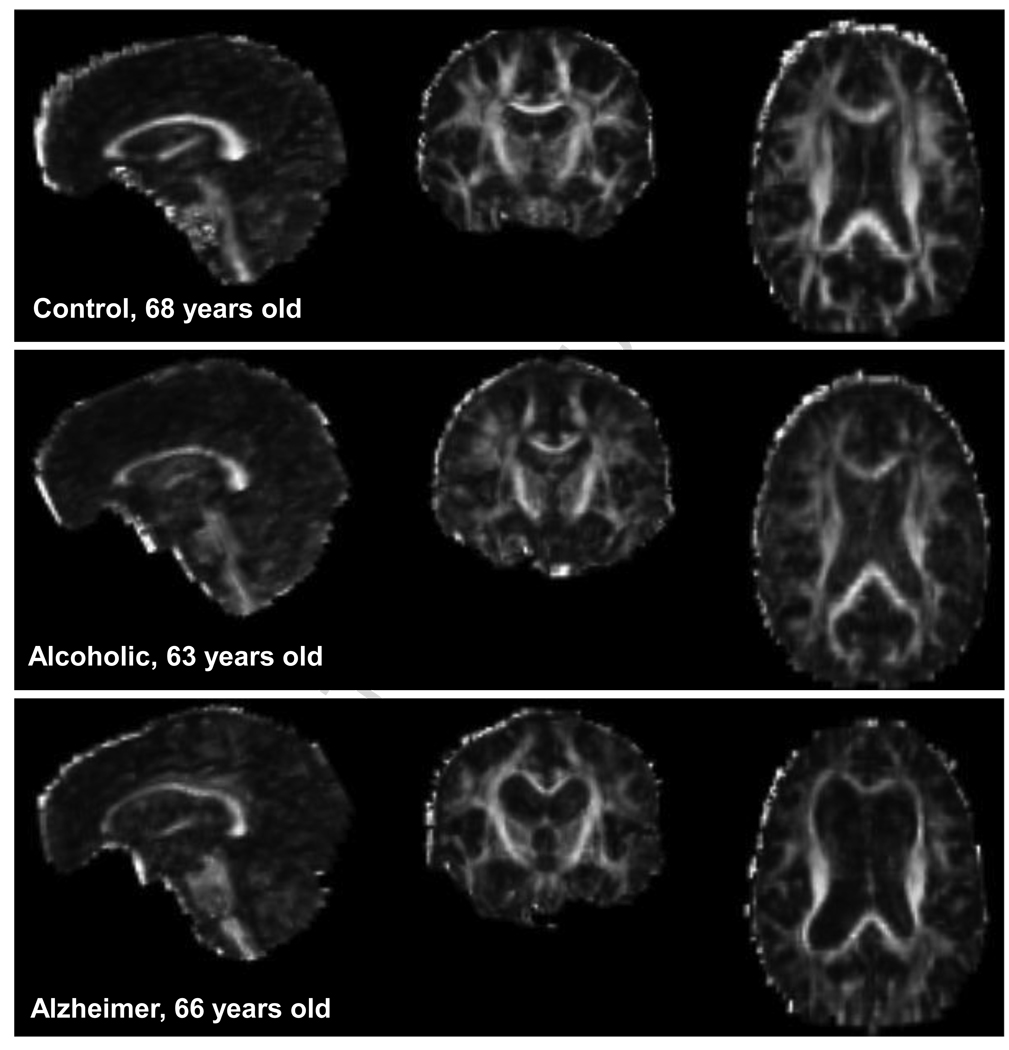
DTI was performed in the coronal plane with the same slice location parameters as the FSE, using a single shot spin-echo echo-planar imaging technique (47 contiguous, 4 mm thick slices, TR/TE = 10 000/103 ms, matrix = 128 * 128, in-plane resolution = 1.875 mm2, b-value=860 s/mm2; FOV=24cm). Diffusion was measured along six non-collinear directions (for further details on acquisition parameters, see Pfefferbaum et al., 2007). Examples of FA images of each diagnosis are presented in Figure 1.
Figure 1.
Examples of DTI FA images in the sagittal (left), coronal (middle), and axial (right) planes.
2.3. Atlas-based parcellation of supratentorial brain tissue and cerebrospinal fluid (CSF) spaces
The FSE sequence produced high contrast CSF/tissue conspicuity and is particularly suited for identification of the intracranial volume because of the high signal sulcal CSF adjacent to the low signal of dura and skull. A template was created using the FSE data of a control subject (52-year-old man) and was skull stripped with FSL-BET. The supratentorial volume was identified manually and excluded the posterior fossa, pons, and brain stem.
The lateral ventricles and corpus callosum were manually identified in our laboratory on a high-resolution, low noise template brain. The corpus callosum was identified on the midsagittal and 5 bilateral, 1-mm-thick parasagittal slices. This measure reflected the midsagittal portion of the corpus callosum rather than the entire corpus callosum and its cortical projections. Herein, “total corpus callosum area” refers to the midsagittal portion including the genu, body and, splenium. The SPGR data from each subject were aligned with a template brain in a two-step process (for further details see Pfefferbaum et al., 2006b), which produced subject-specific labeled brain structures. The same two-step registration process used for the SPGR data was used for the FSE data and FSE template. The final registration applied the parcellated FSE template brain to each subject, producing an intracranial volume for each subject.
2.4. Corpus callosum shape
The corpus callosum silhouette was rotated to a plane parallel to the inferior extremes of the rostrum anteriorly and splenium posteriorly. The midpoint along this plane between the anterior extreme of the genu and posterior extreme of the splenium was used as the center of a circle, and radii were projected at +30° and +150° angles relative to the x-axis from the plane, thus dividing the corpus callosum into genu+rostrum, body, and splenium. From this rotated image, the dorsoventral height and length of the callosal silhouette were determined (Sullivan et al., 2002).
2.5. DTI processing and quantification
After eddy-current correction,the DTI data were aligned with the FSE data applying a non-linear 3D warp (3rd order polynomial), which provided in-plane and through-plane alignment. Based on the eigenvalues from the tensor, FA and MD were calculated on a voxel-by-voxel basis. FA was expressed as a percent, and MD was expressed in units of 10−6 mm2/s.
Using a region of interest method, the corpus callosum was identified on the midsagittal slice extracted from the aligned FA data with a semi-automated edge identification procedure providing a division of the corpus callosum into genu+rostrum, body, and splenium with the same geometric divisions as the structural data. FA and MD were expressed as the average values for all the voxels included in the five, 1 mm thick, mid- and para-sagittal slices for each of the three callosal sectors.
To examine the possibility that observed results were due to effects from partial voluming, FA and MD were also measured in 3×3×3 mm3 volumes of interest placed in the genu and splenium at the locus of highest FA on a subject-by-subject basis, thus creating rarefied focal samples of white matter (Pfefferbaum et al., 2007).
2.6. Statistical analysis
Z-scores for each MRI brain measure were calculated to adjust for normal variation in intracranial volume (ICV) and age using a two-step regression approach (Pfefferbaum et al., 1992). For DTI metrics, FA and MD were adjusted for age only and expressed as age-corrected Z-scores (Pfefferbaum et al., 2006b). Tissue and FA measures were best fit with linear regression models, and the ventricular and MD measures were best fit with quadratic models.
The patient groups had more men than women and the control group more women than men. Nonetheless, comparison between men and women did not reveal any significant difference, allowing us to pool men and women together within each group.
We compared the standardized regional callosal area and DTI Z-scores in the three groups by conducting repeated measures ANOVA (3 groups × 3 regions). Group differences were tested for the callosal height and the ventricular volume with one-way ANOVA and follow- up t- tests. In addition to the primary analyses based on age-corrected Z-scores, we conducted confirmatory analyses to compare the two groups of patients with age-matched control subgroups derived from the total group of 32 controls. Accordingly, we divided the controls into a younger control group matching the alcoholic group (N=17, <70 year old; t=0.05, p=0.96) and an older control group matching the AD group (N=15, >70 year old; t=1.43, p=0.16).
Relations between MRI and DTI metrics were tested with nonparametric Spearman rank (Rho) correlations and multiple regression analyses. Using family-wise Bonferroni corrections for multiple comparisons, correlations were considered significant for P<0.017 for MRI metrics (0.05 divided by 3 variables: height, length and total corpus callosum area) and p<0.0125 for DTI metrics (0.05 divided by 4 variables: total corpus callosum, genu, body and splenium areas).
One man with AD had an MD Z-score = −1.98, which was ~4 SD from the remaining AD group mean. To retain this subject's values in analysis and to reduce the effect of this outlying value (Erceg-Hurn and Mirosevich, 2008), we transformed MD by using the variance of the AD distribution to Winsorize the measures (Keselman et al., 2008). The Winsorized values of MD of this subject were used in ANOVAs, but these diffusivity values were excluded from the correlation and regression analyses.
3. Results
Descriptive statistics for MRI and DTI data uncorrected and, also corrected for age and/or ICV are presented in Table 2.
Table 2.
MRI and DTI metrics (raw data) in the three groups
| Control | Alzheimer | Alcoholic | |||
|---|---|---|---|---|---|
| MRI | corpus callosum height (mm) | 26 ± 3 | 29 ± 5 | 26 ± 4 | |
| corpus callosum length (mm) | 74 ± 4 | 76 ± 7 | 73 ± 5 | ||
| Total corpus callosum area (mm2) | 406 ± 43 | 364 ± 38 | 370 ± 28 | ||
| Genu area (mm2) | 84 ± 14 | 68 ± 13 | 69 ± 9 | ||
| Body area (mm2) | 194 ± 27 | 186 ± 36 | 177 ± 22 | ||
| Splenium area (mm2) | 129 ± 16 | 110 ± 10 | 124 ± 17 | ||
| Lateral ventricles volume (cm3) | 25 ± 9 | 60 ± 27 | 35 ± 18 | ||
| DTI | Total corpus callosum | FA | 0.48 ± 0.03 | 0.41 ± 0.04 | 0.44 ± 0.04 |
| MD | 0.60 ± 0.05 | 0.70 ± 0.06 | 0.66 ± 0.06 | ||
| Genu | FA | 0.44 ± 0.05 | 0.36 ± 0.05 | 0.39 ± 0.04 | |
| FA: rarefied sample | 0.64 ± 0.09 | 0.49 ± 0.07 | 0.53 ± 0.09 | ||
| MD (10−3 mm2 /s) | 0.60 ± 0.07 | 0.72 ± 0.09 | 0.66 ± 0.07 | ||
| MD (10−3mm2 /s): rarefied sample | 0.58 ± 0.09 | 0.77 ± 0.10 | 0.68 ± 0.09 | ||
| Body | FA | 0.46 ± 0.04 | 0.38 ± 0.04 | 0.41 ± 0.04 | |
| MD (10−3 mm2/s) | 0.62 ± 0.56 | 0.73 ± 0.07 | 0.70 ± 0.06 | ||
| Splenium | FA | 0.55 ± 0.04 | 0.49 ± 0.05 | 0.51 ± 0.05 | |
| FA: rarefied sample | 0.80 ± 0.07 | 0.80 ± 0.06 | 0.80 ± 0.07 | ||
| MD (10−3mm2/ s) | 0.56 ± 0.05 | 0.62 ± 0.06 | 0.59 ± 0.07 | ||
| MD (10−3mm2 /s): rarefied sample | 0.48 ± 0.05 | 0.49 ± 0.05 | 0.48 ± 0.06 | ||
3.1. DTI callosal measures
FA
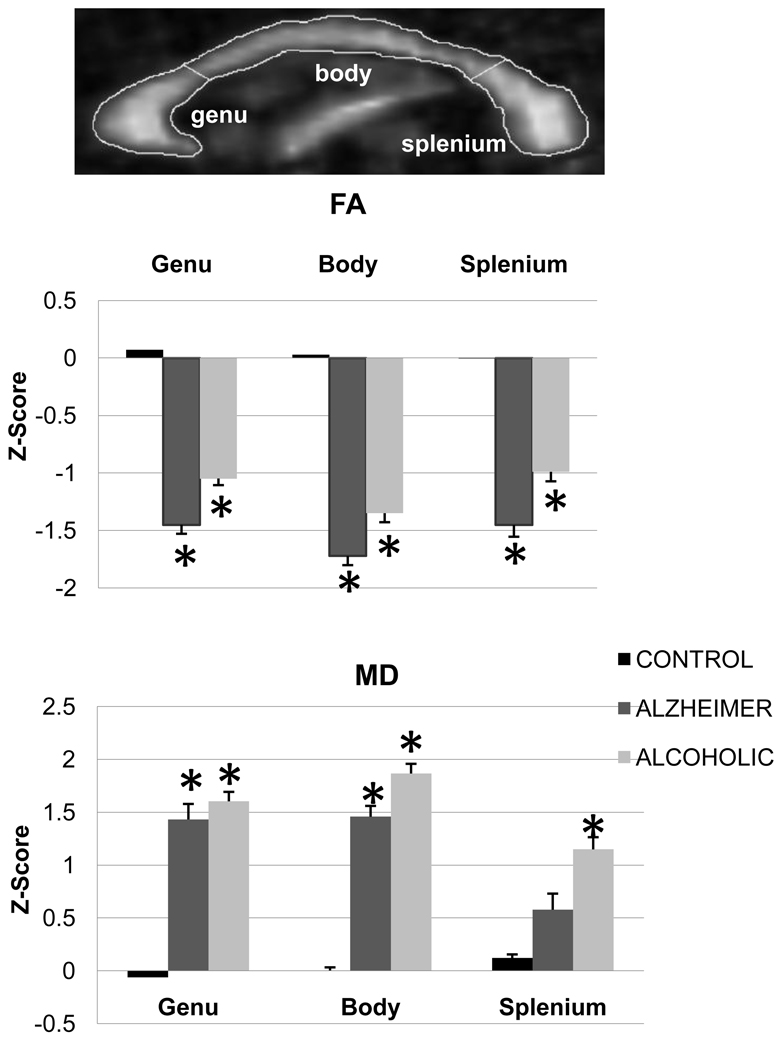
The three group-by-three callosal region ANOVA conducted on callosal FA revealed a group effect [F(2,57)=17.18, p<0.001] but neither a region effect [F(2,114)=1.73, p=0.18] nor an interaction [F(4,114)=0.61, p=0.65]. Follow-up tests revealed that both patient groups had lower FA than the control group (p<0.01 in all cases) but did not differ from each other (p>0.10 in each case) in any region (Figure 2).
Figure 2.
DTI image of FA and MD and bar graphs of mean±SE of FA and MD (z-scores) for each group before correction for partial voluming.
*: significant differences compared with the control group
MD
The three group-by-three callosal region ANOVA conducted on callosal MD revealed effects of group [F(2,57)=11.31, p<0.001] and region [F(2,114)=4.15, p=0.02] and an interaction [F(4,114)=2.57, p=0.04]. Both patient groups had higher MD than controls in genu and body (p<0.05 in each case), but only the alcoholic group had higher MD than controls (p<0.02) in splenium. The two patient groups did not differ significantly from each other in MD in any of these regions (Figure 2).
Correction for partial volume effects
When using the rarefied focal samples, FA measures were higher than without this correction in all three groups. With this correction, the groups did not differ from each other in FA or MD in the splenium (p>0.05 in each case), but the group differences endured in the genu (P<0.001 in each case).
Comparisons between the patient groups and their age-matched control subgroups
The DTI results endured when comparing the patient groups with their age-matched controls on the raw FA and MD measures.
3.2. MRI size and morphology measures
Callosal area
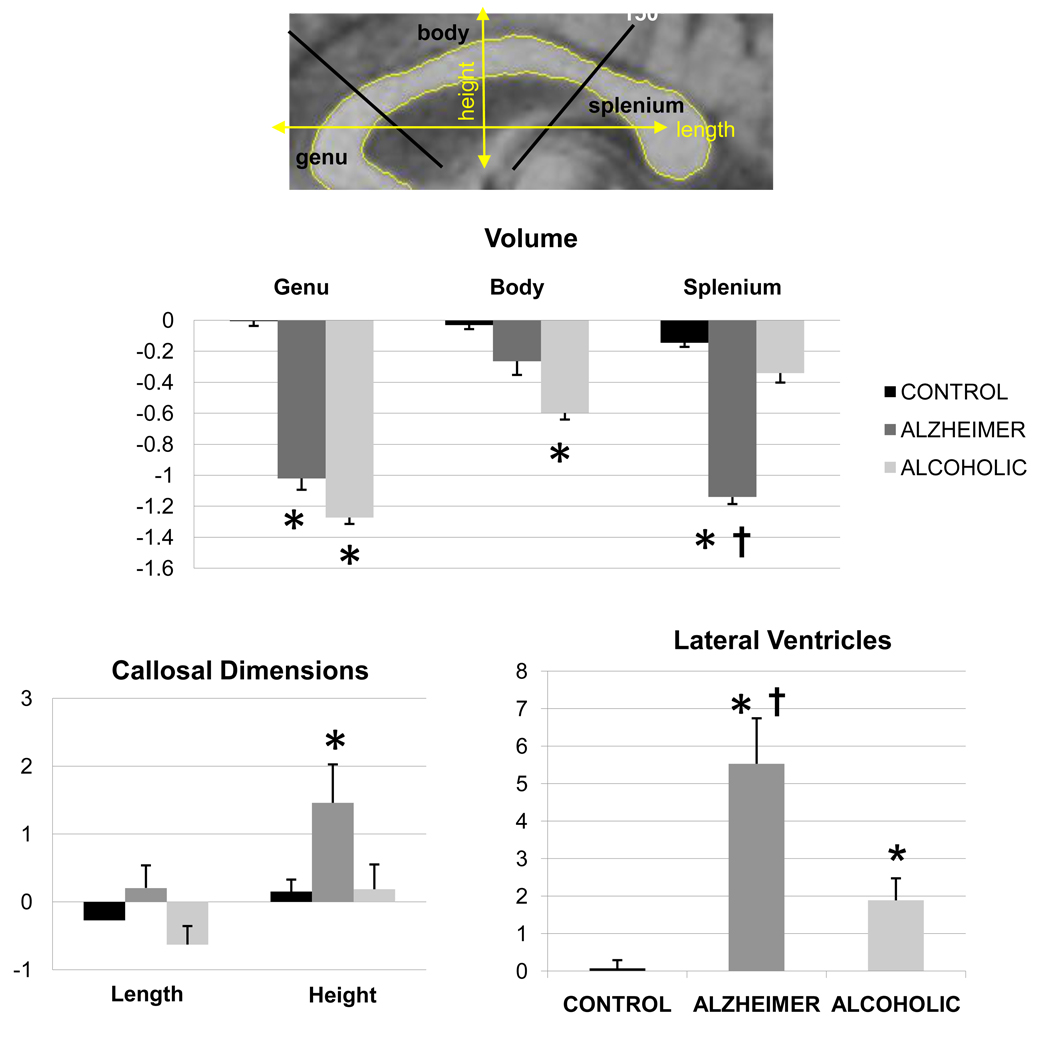
The three group-by-three callosal region (genu, body, splenium) ANOVA revealed effects of group [F(2,57)=10.16, p<0.001] and region [F(2,114)=4.66, p=0.01] and an interaction [F(4,114)=4.16, p=0.004] in callosal area. Both patient groups had smaller genu areas than controls (p<0.01 in each case). Only the AD group had smaller splenium (p<0.0001) and only the alcoholic group had smaller callosal body (p=0.03) than controls. The two patient groups differed in splenium size (p<0.01), which was smaller in the AD group than the alcoholic group (Figure 3).
Figure 3.
MRI images showing ventricular and corpus callosum parcellations and metrics. Also presented are the mean±SE of the MRI callosal and ventricular volumes (z-scores).
*: significant differences compared with the control group
†: significant differences between the alcoholic and Alzheimer groups
Callosal shape
Callosal height [F(2,57)=4.48, p=0.02] but not length [F(2,57)=2.27, p=0.11] differed among the three groups. The arch of the corpus callosum was higher in the AD group than in the alcoholic (p=0.02) or control (p=0.01) groups, which did not differ from each other (p=0.94; Figure 3).
Lateral ventricular volume
A group effect for lateral ventricular volume [F(2,57)=21.39, p<0.001] indicated a step-wise enlargement, where the alcoholic group had larger ventricles than controls (p=0.009), and the AD group had larger ventricles than the two other groups (p<0.01 in both cases; Figure 3).
Comparisons between the patient groups and their age-matched control subgroups
Recognizing the loss of power due to the reduced sample size of the control groups, the MRI results endured or were significant with one-tailed tests (for callosal height in the AD group, p=0.044 one-tailed). Only the splenium area (ICV-corrected Z-score) was not significantly smaller in the AD group than in its age-matched control subgroup (p=0.29 one-tailed).
3.3. Relations between MRI and DTI metrics
Nonparametric correlations and p-values between regional MRI and DTI metrics are presented for each patient group in Table 3.
Table 3.
Non-parametric (Spearman’s rho) correlations between DTI and MRI metrics in the two patients groups
| MRI | ||||||
|---|---|---|---|---|---|---|
| Alzheimer group | Alcoholic group | |||||
| Total corpus callosum area |
Lateral ventricles volume |
Total corpus callosum area |
Lateral ventricles volume |
|||
| MRI | Height | Rho=0.66/p=0.01 | Rho=0.84/p=0.0001 | Rho=0.25/p=0.37 | Rho=0.92/p=0.0001 | |
| Length | Rho=0.21/p=0.48 | Rho=0.02/p=0.96 | Rho=0/p=1 | Rho=−0.03/p=0.93 | ||
| Total corpus callos um area | / | Rho=0.46/p=0.11 | / | Rho=0.20/p=0.47 | ||
| DTI |
Total corpus callosum |
FA | Rho=0.0 5/p=0.87 | Rho=−0.70/p=0.008 | Rho=0.50/p=0.06 | Rho=−0.10/p=0.73 |
| MD | Rho=−0.25/p=0.42 | Rho=0.60/p=0.03 | Rho=−0.18/p=0.52 | Rho=0.11/p=0.69 | ||
| Genu | FA | Rho=0.04/p=0.89 | Rho=−0.71/p=0.006 | Rho=0.68/p=0.006 | Rho=0.08/p=0.77 | |
| MD | Rho=−0.30/p=0.32 | Rho=0.41/p=0.17 | Rho=−0.46/p=0.08 | Rho=0.24/p=0.39 | ||
| Body | FA | Rho=0.22/p=0.47 | Rho=−0.53 /p=0.06 | Rho=0.16/p=0.56 | Rho=0.16/p=0.56 | |
| MD | Rho=−0.35/p=0 25 | Rho=0.50/p=0.0 8 | Rho=−0.90/p=0.74 | Rho=−0.09/p=0.74 | ||
| Splenium | FA | Rho=−0.13/p=0.68 | Rho=−0.85/p=0.0001 | Rho=0.17/p=0.54 | Rho=−0.43/p=0.11 | |
| MD | Rho=−0.18/p=0.57 | Rho=0.41/p=0.19 | Rho=−0.10/p=0.73 | Rho=0.20/p=0.47 | ||
Significant correlations are reported in bold front
Callosal area relations with callosal height and DTI metrics
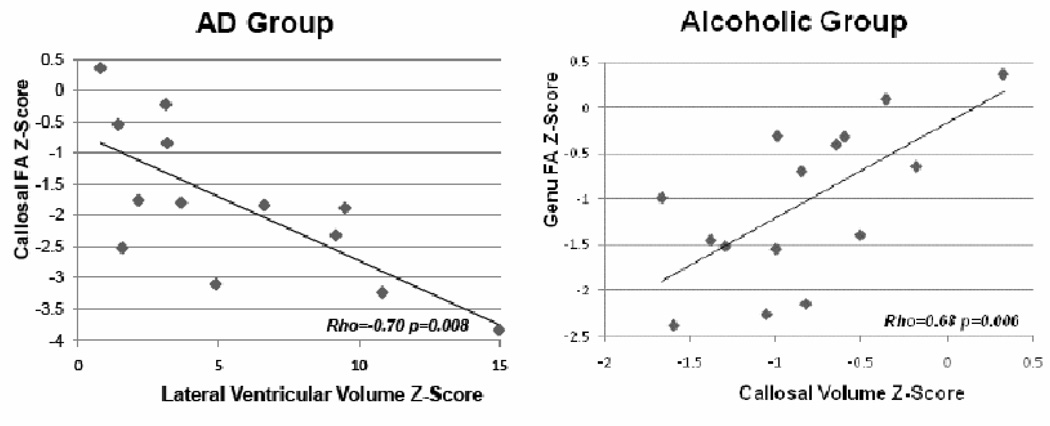
Bivariate correlations revealed significant relations between total callosal area and height in the AD but not the alcoholic groups (Table 2 and Figure 4).
Figure 4.
Correlations between corpus callosum area and height in the Alzheimer’s Disease (AD) group and between callosal area and FA in genu in the alcoholic group (z-scores).
Multiple regression analysis entering total callosal FA, MD, and callosal height as predictors of total callosal area identified FA as a unique predictor over height and MD in the alcoholic group (34.3% of the variance explained, p=0.02). By contrast, callosal height, after accounting for FA and MD, was the best predictor of total callosal area (46.0%, p=0.01) in the AD group (Figure 4).
Using the regional callosal DTI metrics and the callosal height as predictors of total callosal area, FA in the genu endured as a unique predictor of its area (Figure 4) over height and MD in the alcoholic group (43.8% of the variance explained, p=0.007), whereas callosal height remained the best predictor in the AD group (46.0% of variance, p=0.01).
Ventricular volume relations with callosal height and DTI metrics
Ventricular volume was highly correlated with callosal height but not length in each patient group (Table 2). However, ventricular volume was related to callosal FA in the AD patients but not the alcoholics. Multiple regression analyses entering total callosal metrics as predictors of ventricular volume revealed FA as a unique predictor (50.5% of the variance, p=0.006) in the AD group.
Next, we tested the bivariate correlations between ventricular volume and regional callosal FA and MD. Ventricular volume correlated significantly with FA in the genu and splenium in the AD and not the alcoholic group (Table 2).
4. Discussion
Despite an overlap in the macrostructural and microstructural abnormalities of the AD and alcoholic groups, concurrent analysis of MRI and DTI data indicated different underlying radiological sources of callosal abnormalities. Similar to recent reports, we observed that, compared with controls, AD patients had smaller genu and splenium (Chaim et al., 2007) and lower FA and higher MD (Wang et al., 2009) in the three regions of the corpus callosum, significant in the genu and body. Also consistent with previous studies (Thompson et al., 1998), the AD group had exaggerated callosal arching, related to extensive lateral ventricular enlargement and callosal area. Despite callosal microstructural abnormalities, neither FA nor MD in the AD group related to callosal area but did to ventricular enlargement. Thus, the severe lateral ventricular enlargement observed in the AD group influenced the midsagittal silhouette of the corpus callosum and led to its distortion, resulting in dysmorphology of callosal macrostructure and disruption of callosal microstructure. That FA correlated with ventricular but not callosal size suggests that FA is a nonspecific, secondary index of dementia severity rather than a specific index of regional callosal fiber integrity.
Like the AD patients, the elderly alcoholics had smaller callosal areas with the genu being predominantly damaged, suggesting an antero-posterior gradient in the macrostructural abnormalities. Even though microstructural damage inferred from low FA and high MD was detected in the three callosal regions relative to controls, an antero-posterior gradient in the macrostructural abnormalities was also observed after correction for partial volume effects. In the alcoholic group, callosal macrostructure and microstructure were significantly and specifically related: the thinner the corpus callosum, the lower the FA, especially in the genu. The microstructural white matter abnormalities in alcoholism may result from a loss of white matter fibers rather than from a distortion caused by moderate lateral ventricular enlargement also present in these subjects.
Neither the macrostructural nor microstructural markers of callosal or ventricular condition quantified herein are specific markers of diagnosis. Taken together, however, these patterns of normality and abnormality go beyond distinguishing the diseases, which would be readily done clinically, to provide leads for determining which abnormalities have greater genetic or environmental regulation. In elderly individuals with probable AD or alcoholism, microstructure of the corpus callosum is damaged, but this shared observation does not necessarily reflect the same underlying mechanisms. Indeed, the present study revealed a double dissociation, with abnormalities in FA being specifically correlated with ventricular but not callosal area in AD, and being correlated with callosal but not ventricular volume in alcoholism. The disruption of callosal white matter integrity and its size in AD appear to be consequence and, therefore, an epiphenomenon of the ventricular enlargement, which twin studies have shown to be under a multitude of influences from genes, polygenes, and environment (Pfefferbaum et al., 2000). Conversely, because of the concomitant macrostructural and microstructural damage of this brain region, elderly individuals with alcoholism may be characterized by genuine corpus callosum pathology, radiological markers of which have been shown to be under strong stable genetic influences (Pfefferbaum et al., 2004). Thus, in addition to the obvious differences in neuropathogenesis defining AD and alcoholism, the dissociable sets of neuroradiological signs distinguishing these diagnoses may present leads to differential genetic and environmental contributions to disruption of callosal integrity. The present findings suggest that microstructural abnormalities in corpus callosum may be influenced by polygenetic factors in AD (Persson et al., 2006) and by the genetically-linked vulnerability to the environmental exposure to toxic in alcoholism (Li and Burmeister, 2009).
The present study has certain limitations. First, the three groups were not matched for age, with the AD group being significantly older than the two other groups and the alcoholic group being significantly younger than the two other ones. However, the use of age-corrected z-scores for brain metrics allows us to compare the brain abnormalities in the three groups. Further, our subanalysis using age-matched patient and control samples comported with the analysis based on age-corrected z-scores. Second, lifetime alcohol intake was 5 times higher in the AD group than in the control group (though non significantly different). Most AD studies do not document this measure but this high alcohol consumption in AD patients may have influenced the findings. Finally, the correlational results have to be interpreted with caution and without causal implications.
Acknowledgment
This work was supported by NIH grants AA012388, AA017168, AA017923, and AG017919. The authors would like to thank Andrea Spadoni, Ph.D., for her part in subject scheduling and data acquisition of the patients with AD and the elderly controls, and Margaret J. Rosenbloom, M.A. for her helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of interest
None of the authors has any involvement, financial or otherwise, that might potentially bias their work.
References
- Bellani M, Yeh PH, Tansella M, Balestrieri M, Soares JC, Brambilla P. DTI studies of corpus callosum in bipolar disorder. Biochemical Society Transactions. 2009;37:1096–1098. doi: 10.1042/BST0371096. [DOI] [PubMed] [Google Scholar]
- Chaim TM, Duran FL, Uchida RR, Perico CA, de Castro CC, Busatto GF. Volumetric reduction of the corpus callosum in Alzheimer's disease in vivo as assessed with voxel-based morphometry. Psychiatry Research. 2007;154:59–68. doi: 10.1016/j.pscychresns.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Reynaud M, Wessa M, Penttila J, Kostogianni N, Cachia A, Artiges E, Delain F, Perrin M, Aubin HJ, Cointepas Y, Martelli C, Martinot JL. Diffusion Tensor Tractography in Mesencephalic Bundles: Relation to Mental Flexibility in Detoxified Alcohol-Dependent Subjects. Neuropsychopharmacology. 2009;34:1223–1232. doi: 10.1038/npp.2008.101. [DOI] [PubMed] [Google Scholar]
- Cho H, Yang DW, Shon YM, Kim BS, Kim YI, Choi YB, Lee KS, Shim YS, Yoon B, Kim W, Ahn KJ. Abnormal integrity of corticocortical tracts in mild cognitive impairment: a diffusion tensor imaging study. Journal of Korean Medical Science. 2008;23:477–483. doi: 10.3346/jkms.2008.23.3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Current Opinion in Neurology. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Creasey H, Schwartz M, Frederickson H, Haxby JV, Rapoport SI. Quantitative computed tomography in dementia of the Alzheimer type. Neurology. 1986;36:1563–1568. doi: 10.1212/wnl.36.12.1563. [DOI] [PubMed] [Google Scholar]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Archives of Neurology. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- DeCarli C, Haxby JV, Gillette JA, Teichberg D, Rapoport SI, Schapiro MB. Longitudinal changes in lateral ventricular volume in patients with dementia of the Alzheimer type. Neurology. 1992;42:2029–2036. doi: 10.1212/wnl.42.10.2029. [DOI] [PubMed] [Google Scholar]
- Erceg-Hurn DM, Mirosevich VM. Modern robust statistical methods: an easy way to maximize the accuracy and power of your research. The American Psychologist. 2008;63:591–601. doi: 10.1037/0003-066X.63.7.591. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forstl H, Zerfass R, Geiger-Kabisch C, Sattel H, Besthorn C, Hentschel F. Brain atrophy in normal ageing and Alzheimer's disease. Volumetric discrimination and clinical correlations. British Journal of Psychiatry. 1995;167:739–746. doi: 10.1192/bjp.167.6.739. [DOI] [PubMed] [Google Scholar]
- Hollingshead A, Redlich F. Social Class and Mental Illness. New York: John Wiley and Sons; 1958. [Google Scholar]
- Hommer D, Momenan R, Rawlings R, Ragan P, Williams W, Rio D, Eckardt M. Decreased corpus callosum size among alcoholic women. Archives of Neurology. 1996;53:359–363. doi: 10.1001/archneur.1996.00550040099019. [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Algina J, Lix LM, Wilcox RR, Deering KN. A generally robust approach for testing hypotheses and setting confidence intervals for effect sizes. Psychological Methods. 2008;13:110–129. doi: 10.1037/1082-989X.13.2.110. [DOI] [PubMed] [Google Scholar]
- Khachaturian ZS. Diagnosis of Alzheimer's disease. Archives of Neurology. 1985;42:1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcoholism, Clinical and Experimental Research. 2000;24:1873–1882. [PubMed] [Google Scholar]
- Li MD, Burmeister M. New insights into the genetics of addiction. Nature Reviews. Genetics. 2009;10:225–231. doi: 10.1038/nrg2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Pu F, Shi F, Xie S, Wang Y, Jiang T. Regional white matter decreases in Alzheimer's disease using optimized voxel-based morphometry. Acta Radiologica. 2008;49:84–90. doi: 10.1080/02841850701627181. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale (DRS) Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1988. [Google Scholar]
- Mayfield RD, Lewohl JM, Dodd PR, Herlihy A, Liu J, Harris RA. Patterns of gene expression are altered in the frontal and motor cortices of human alcoholics. J Neurochemistry. 2002;81:802–813. doi: 10.1046/j.1471-4159.2002.00860.x. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Nelson HE. The National Adult Reading Test (NART) Windsor, Canada: Nelson Publishing Company; 1982. [Google Scholar]
- Nestor SM, Rupsingh R, Borrie M, Smith M, Accomazzi V, Wells JL, Fogarty J, Bartha R. Ventricular enlargement as a possible measure of Alzheimer's disease progression validated using the Alzheimer's disease neuroimaging initiative database. Brain. 2008;131:2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente DB, Gasparetto EL, da Cruz LC, Jr, Domingues RC, Baptista AC, Carvalho AC, Domingues RC. Potential role of diffusion tensor MRI in the differential diagnosis of mild cognitive impairment and Alzheimer's disease. American Journal of Roentgenology. 2008;190:1369–1374. doi: 10.2214/AJR.07.2617. [DOI] [PubMed] [Google Scholar]
- Paula-Barbosa MM, Tavares MA. Long term alcohol consumption induces microtubular changes in the adult rat cerebellar cortex. Brain Research. 1985;339:195–199. doi: 10.1016/0006-8993(85)90645-6. [DOI] [PubMed] [Google Scholar]
- Persson J, Lind J, Larsson A, Ingvar M, Cruts M, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Altered brain white matter integrity in healthy carriers of the APOE epsilon4 allele: a risk for AD? Neurology. 2006;66:1029–1033. doi: 10.1212/01.wnl.0000204180.25361.48. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging. 2006a;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Desmond JE, Sullivan EV. Thinning of the corpus callosum in older alcoholic men: a magnetic resonance imaging study. Alcoholism, Clinical and Experimental Research. 1996;20:752–757. doi: 10.1111/j.1530-0277.1996.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Rosenbloom MJ, Lane B, Ha CN, Sullivan EV. Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcoholism, Clinical and Experimental Research. 1992;16:1078–1089. doi: 10.1111/j.1530-0277.1992.tb00702.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64. doi: 10.1093/brain/awl242. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Adalsteinsson E, Kemper CA, Deresinski S, Sullivan EV. Contribution of alcoholism to brain dysmorphology in HIV infection: effects on the ventricles and corpus callosum. Neuroimage. 2006b;33:239–251. doi: 10.1016/j.neuroimage.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiology of Aging. 2004;25:175–183. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Swan GE, Carmelli D. Brain structure in men remains highly heritable in the seventh and eighth decades of life. Neurobiology of Aging. 2000;21:63–74. doi: 10.1016/s0197-4580(00)00086-5. [DOI] [PubMed] [Google Scholar]
- Putzke J, De Beun R, Schreiber R, De Vry J, Tolle TR, Zieglgansberger W, Spanagel R. Long-term alcohol self-administration and alcohol withdrawal differentially modulate microtubule-associated protein 2 (MAP2) gene expression in the rat brain. Brain Research. Molecular Brain Research. 1998;62:196–205. doi: 10.1016/s0169-328x(98)00253-8. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Geurts JJ, Vrenken H, Hulst HE, Cover KS, Castelijns JA, Pouwels PJ, Barkhof F. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009;44:1397–1403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Rotarska-Jagiela A, Oertel-Knoechel V, DeMartino F, van de Ven V, Formisano E, Roebroeck A, Rami A, Schoenmeyer R, Haenschel C, Hendler T, Maurer K, Vogeley K, Linden DE. Anatomical brain connectivity and positive symptoms of schizophrenia: a diffusion tensor imaging study. Psychiatry Research. 2009;174:9–16. doi: 10.1016/j.pscychresns.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Barkhof F, Leys D, Wolters EC, Ravid R, Kamphorst W. Histopathologic correlates of white matter changes on MRI in Alzheimer's disease and normal aging. Neurology. 1995;45:883–888. doi: 10.1212/wnl.45.5.883. [DOI] [PubMed] [Google Scholar]
- Shuyu L, Fang P, Xiangqi D, Li D, Tianzi J. Shape analysis of the corpus callosum in Alzheimer's disease. Bioinformatics and Biomedical Engineering ICBBE 2007; The 1st International Conference; 2007. pp. 1095–1098. [Google Scholar]
- Silbert LC, Quinn JF, Moore MM, Corbridge E, Ball MJ, Murdoch G, Sexton G, Kaye JA. Changes in premorbid brain volume predict Alzheimer's disease pathology. Neurology. 2003;61:487–492. doi: 10.1212/01.wnl.0000079053.77227.14. [DOI] [PubMed] [Google Scholar]
- Sjobeck M, Englund E. Glial levels determine severity of white matter disease in Alzheimer's disease: a neuropathological study of glial changes. Neuropathology and Applied Neurobiology. 2003;29:159–169. doi: 10.1046/j.1365-2990.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007;243:483–492. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Adalsteinsson E, Swan GE, Carmelli D. Differential rates of regional brain change in callosal and ventricular size: a 4-year longitudinal MRI study of elderly men. Cerebral Cortex. 2002;12:438–445. doi: 10.1093/cercor/12.4.438. [DOI] [PubMed] [Google Scholar]
- Tarnowska-Dziduszko E, Bertrand E, Szpak GM. Morphological changes in the corpus callosum in chronic alcoholism. Folia Neuropathologica. 1995;33:25–29. [PubMed] [Google Scholar]
- Thompson PM, Moussai J, Zohoori S, Goldkorn A, Khan AA, Mega MS, Small GW, Cummings JL, Toga AW. Cortical variability and asymmetry in normal aging and Alzheimer's disease. Cerebral Cortex. 1998;8:492–509. doi: 10.1093/cercor/8.6.492. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, Scapin M, Di Paola M, Le Nezet P, Fadda L, Musicco M, Caltagirone C, Collins DL. Gross anatomy of the corpus callosum in Alzheimer's disease: regions of degeneration and their neuropsychological correlates. Dementia and Geriatric Cognitive Disorders. 2007;23:96–103. doi: 10.1159/000097371. [DOI] [PubMed] [Google Scholar]
- Ukmar M, Makuc E, Onor ML, Garbin G, Trevisiol M, Cova MA. Evaluation of white matter damage in patients with Alzheimer's disease and in patients with mild cognitive impairment by using diffusion tensor imaging. La Radiologia Medica. 2008;113:915–922. doi: 10.1007/s11547-008-0286-1. [DOI] [PubMed] [Google Scholar]
- Wang L, Goldstein FC, Veledar E, Levey AI, Lah JJ, Meltzer CC, Holder CA, Mao H. Alterations in cortical thickness and white matter integrity in mild cognitive impairment measured by whole-brain cortical thickness mapping and diffusion tensor imaging. American Journal of Neuroradiology. 2009;30:893–899. doi: 10.3174/ajnr.A1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PJ, Saykin AJ, Flashman LA, Wishart HA, Rabin LA, Santulli RB, McHugh TL, MacDonald JW, Mamourian AC. Regionally specific atrophy of the corpus callosum in AD, MCI and cognitive complaints. Neurobiology of Aging. 2006;27:1613–1617. doi: 10.1016/j.neurobiolaging.2005.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins RC, Gorman A, Rolsten C, Samorajski T, Ballinger WE, Jr, Freund G. Effects of aging and alcohol on the biochemical composition of histologically normal human brain. Metabolic Brain Disease. 1988;3:67–80. doi: 10.1007/BF01001354. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Haroutunian V, Ho L, Purohit D, Pasinetti GM. Microglia activation in the brain as inflammatory biomarker of Alzheimer's disease neuropathology and clinical dementia. Disease Markers. 2006;22:95–102. doi: 10.1155/2006/276239. [DOI] [PMC free article] [PubMed] [Google Scholar]