Summary
Distal myopathy with rimmed vacuoles (DMRV) or hereditary inclusion body myopathy (hIBM) is an adult onset slowly progressive myopathy secondary to mutations in the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) gene that encodes a bifunctional enzyme which catalyzes the rate-limiting step in sialic acid biosynthesis. Many hypotheses have been proposed to explain why patients develop weakness and atrophy, but are most views are obscure and thus are still considered controversial, partly because of the lack of an appropriate model with which these theories could be clarified. In this review, we briefly summarize the progress in DMRV research, and highlight efforts of researchers in generating the animal model for this myopathy.
Keywords: Amyloid, autophagy, muscular dystrophy
Introduction
Distal myopathy with rimmed vacuoles (DMRV), as its name imply, is a myopathy involving distal muscles, although other muscles are likewise involved. It affects young adults and commonly presents as foot drop due to the early involvement of tibialis anterior muscles. It was reported as Nonaka myopathy among the Japanese population (1) and also known as hereditary inclusion body myopathy (hIBM), which was described as “ quadriceps-sparing rimmed vacuolar myopathy” among Middle Eastern patients. DMRV and hIBM are now known to afflict populations of diverse ethnicities around the world.
Clinical Features
Clinically, distal muscles, especially the tibialis anterior muscles, are preferentially involved during the early part of the disease, but other muscles including those in proximal regions can be affected. In fact, the gastrocnemius muscle can be severely affected, albeit in rare cases (2). The quadriceps muscles are relatively spared during the initial course of the disease; hence previously DMRV was reported as quadriceps-sparing myopathy. Upper limb involvement is usually limited to the scapular and proximal muscles, but distal arm and hand muscles are later affected; this is in contrast to the early involvement of finger flexor muscles in sporadic inclusion body myositis.
The course of the disease is gradually progressive, whereby patients usually become wheelchair-bound around 12 years after the onset of myopathy (1, 2), but there were anecdotes that the age of being wheelchair bound could become variable (2). More interesting is the identification of seemingly asymptomatic patients who are genetically diagnosed as DMRV in the Japanese population (3) and others from the Middle Eastern population (Dr. Argov, personal communication). DMRV is known to affect skeletal muscles, but cardiac involvement actually is not rare and can be seen in 18% of patients (Dr. Tanaka, personal communication), although the severity varies from mild to life-threatening arrhythmias. In fact, there was one patient genetically diagnosed with the diseases who succumbed to ventricular arrhythmia. This indicates that even in patients with no apparent cardiac abnormality, careful examination of the cardiac function is necessary.
Serum creatine kinase (CK) is mildly to moderately elevated in most cases, but there are several cases wherein CK levels are markedly increased.
Pathomechanistic Clues seen in Pathology
The pathological characteristics of DMRV are the presence of rimmed vacuoles in muscle fibers, but are not specific to this myopathy, and the presence of scattered small angular and atrophic fibers. These rimmed vacuoles are actually empty spaces created by the aggregation of autophagic vacuoles (2). The presumption of autophagic process in these areas is due to high acid phosphatase activity and reactivity with lysosomal markers, including LAMPs 1 and 2, and cathepsins. Autophagic process is confirmed by presence of aggregation of vacoules, various cellular debris, and multilammelar bodies by electron microscopy (1). Other ultrastructural findings include proliferation of the Golgi apparatus, mitochondrial degeneration, and myofibrillar loss. Tubulofilamentous inclusions measuring 15-20 nm in diameter were also seen in the nucleus and cytoplasm (1).
The rimmed vacuoles have been shown to be immunoreactive to amyloid (4), phosphorylated tau (1, 2), although the role of these proteins in this disorder has not been clarified. The activation of ubiquitin proteasome system and lysosomal system may be a secondary response to the presence of accumulated proteins, like amyloid. The presence of muscle fiber atrophy, fiber degeneration and apoptosis (5) which can seemingly explain why patients develop weakness may be downstream findings in this disease. Recently, activated capase-3 and caspase-9 were shown to be strongly enhanced in hIBM myoblasts after apoptosis induction, and pAkt remained upregulated in hIBM cells compared to control, implying impaired apoptotic signaling in hIBM (6). Further, the authors theorize that this phenomenon could contribute to the muscle mass loss seen in patients. However, satellite cell abnormality has not been reported in DMRV.
The pathologic findings found in DMRV seem diverse. Several propositions and hypotheses have been made in an attempt to reconcile these complex findings, the histologic findings are still unexplained. Although these findings in pathology offer clues to what is going on in muscles of patients afflicted with this condition, the precise pathomechanism of this disease remains enigmatic.
Genetic etiology
DMRV and hIBM are known to be associated with mutations in the GNE gene which encodes UDP-GlcNAc-2-epimerase/ManNAc kinase in chromosome 9p12-13 (1, 2, 7–9). Most of the mutations were missense, including the most common p.V572L among Japanese patients (3) and the p.M712T among Persian-Jewish families (9). Only a few null mutations were found, but no patient had null mutations in both alleles, consistent with the fact that embryonic lethality results from knocking out the GNE gene in mice (10).
After report of mutations among the Japanese and Middle Eastern patients, several groups found out similar mutations among patients of varied nationalities, indicating the worldwide occurrence of DMRV (11–15).
Molecular Pathogenesis: GNE hypoactivity and hyposialylation
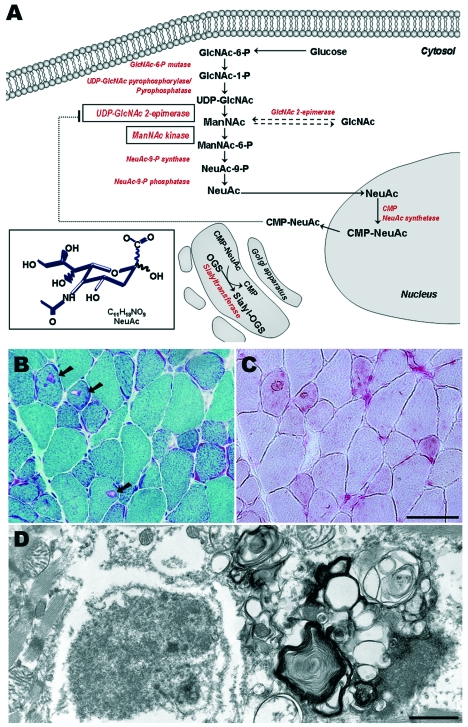
The GNE gene is the key enzyme in sialic acid biosynthesis (Fig. 1A). The UDP-GlcNAc-2-epimerase domain catalyzes the epimerization of UDP-GlcNAc to ManNAc with simultaneous release of UDP, while the ManNAc kinase domain phosphorolyzes ManNAc to ManNAc-6-P. The succeeding steps involve the condensation of ManNAc-6-P and phospoenolpyruvate to NeuAc and its activation into CMP-NeuAc, which is used for the synthesis of sialyl oligosaccharides. CMP-sialic acid regulates GNE activity through negative feedback inhibition by binding to its allosteric site. Sialic acids are a group of charged sugars found at the terminal end of glycoconjugates having roles in molecular interaction and regulatory functions in various biological processes; however, the understanding of its role in muscle has remained remote.
Figure 1.
Mutations in UDP-N-acetylglucosamine 2-epimerase /N-acetylmannosamine kinase (GNE) gene causes DMRV/hIBM. The pathway of sialic acid synthesis is shown, A. Glucose is converted to UDP-GlcNAc, which is later epimerized to ManNAc by UDP-GlcNAc 2-epimerase. ManNAc undergoes phosphorylation, catalyzed by ManNAc kinase. Subsequent steps involves the conversion of ManNAc to NeuAC, and activation of into CMP-NeuAc in the nucleus. CMP-NeuAc is utilized in the Golgi apparatus for synthesis of Sialyl-oligosaccharides (OGS). GNE is regulated by the amount of cytosolic CMP-NeuAc by negative feedback inhibition. Sections from Gne(-/-)hGNED176VTg, B-D. Modified Gomori-trichrome (B) shows the characteristic fibers with rimmed vacuoles (RV), shown in arrows. These fibers with RV are strongly highlighted by acid phosphatase (C), indicating activation of lysosomal system. On electron microscopy (D), the RVs are actually aggregation of autophagic vacuoles, composed of myeloid bodies and autophagosomes. Various intracellular deposits are likewise seen. Bar in B and C represents 50μ m; in D, 1μ m.
In DMRV, therefore, the next matter-of-course was to determine the enzymatic activity of GNE in patients. Nishino et al. measured the epimerase activity in patients’ leukocytes using tritium-labeled UDP-GlcNAc (3). The epimerase activity was markedly decreased in terms of the mean value, albeit the fact that the standard deviation was too large. Thus, Noguchi et al. analyzed recombinant GNE with several DRMV mutations among Japanese patients and expressed these in COS cells (16). All epimerase mutants had remarkable reduction in epimerase activity while kinase activity was generally maintained. In contrast, kinase mutants had modest reduction in epimerase activities, while kinase activities were markedly reduced. Hinderlich et al. also measured enzymatic activities in M712T mutation expressed in insect cells using baculovirus expression system, and found 30% reduction only in kinase activities (17) and they speculated that the minor effect of this mutation on enzyme activities might be due to the probable location of M712 outside the core of the kinase domain. When they measured enzyme activities using a cell-based system in patient-derived lymphoblastoid cell lines, however, they discovered a 35% reduction only in the epimerase activity in patient cells, and pointed out that the cell-based assays may greatly hamper the precise determination of ManNAc kinase activity because of the low expression of GNE and high expression of sugar kinases other than GNE in lymphoblastoid cell lines. Penner et al. further demonstrated the phenomenon of GNE hypoactivity by analyzing 10 GNE mutants expressed in insect cells (18), showing 20-80% reduction of epimerase/kinase activities in different mutations; they further implied that mutations may also influence the function of the domain not harboring them. From these studies, it can be seen that the enzymatic activity is variably reduced, but do not seem to correlate to the clinical phenotype in patients.
While it may be natural to assume that sialic acid production should be decreased in patients who have mutations in the GNE gene, this notion is not without controversy, as results from previous reports do not provide unanimous conclusion. By using lectin staining, Noguchi et al. (16) clearly demonstrated that the levels of sialic acid in fibroblasts from patients were reduced to 60-75% of control cells. They also showed that the hyposialylation in DMRV cells can be recovered by the addition of ManNAc, the precursor for sialic acid synthesis, or sialic acid itself. Interestingly, the sialylation of alpha-dystroglycan, a major glycoprotein in the sarcolemma, was not consistent among patients, suggesting that hyposialylation of alpha-dystroglycan may be a downstream phenomenon. In contrast, Hinderlich et al. demonstrated that in lymphoblastoid cell lines expressing M712T, the membrane-bound sialic acid levels in patients did not differ from control (17). Salama et al. compared the sialylation status of cultured muscle cells from patients harboring M712T mutation with other patients having mutations in the epimerase domain (19). They have found that all patients have lower membrane-bound sialic acid levels but with overlapping values with control cells; M712T cells have lower sialic acid levels but no statistical significance was seen. Other groups analyzed muscle glycoproteins and revealed that sialic acid levels are reduced (19–21). Nevertheless, it is still unclear why this disease should involve primarily the skeletal muscle, while GNE is ubiquitously expressed, and sialic acid is involved in various physiologic processes in various organs.
Functions of GNE beyond sialic acid synthesis
Although GNE has been believed to be present in cytosol, it was surprising that it was also localized within the Golgi apparatus, as it colocalizes with golgin-97, a Golgi-specific protein (22). One possible explanation offered by the authors is the fact that sialylation of glycoconjugates occur in the Golgi complex. In addition, GNE was also detected in the nucleus, although this is controversial at present. These results suggested that GNE, apart from its role in sialic acid synthesis, might influence gene expression modulation when targeted to the nucleus. This observation also led to the hypothesis that GNE can act as a nucleocytoplasmic shuttling protein (22), but proving this would necessitate in vivo labeling of GNE to determine its precise subcellular targeting.
Nonetheless, in a more recent paper, the subcellular distribution of GNE in skeletal muscles and primary myoblasts/myotubes from DMRV patients remain unaltered (23), suggesting that other pathomechanistic factors await further elucidation to explain how GNE mutations contribute to the phenotype of DMRV.
Because hyposialylation in DMRV is not fully agreed upon, other authors consider a disparate between a low GNE enzymatic levels and variable reduction of sialic acid as reported by several groups. Thus they believed that there should be a role of GNE in addition to the already-established role of GNE in sialic acid synthesis. Wang et al. demonstrated that GNE controlled sialyltransferase expression, ganglioside production (GM3 and GD3), and the subsequent modulation of proliferation and apoptosis, independent of sialic acid production (24). However, although the influence of GNE on sialyltranferase expression is far from being understood and may pose a challenge for further studies, this is not actually totally out of the context in the role of GNE in sialic acid synthesis.
Animal models
The progress in DMRV research was limited by the absence of a model with which analysis could be performed more extensively. Various culture cell models have been developed but understandably, these do not necessarily represent the phenotype in patients. Thus there has been a clamor for an appropriate animal model, the production of which was greatly limited by the fact that knocking out the Gne gene in mice led to embryonic lethality (10).
Two independent groups have attempted to produce models for this disease. A knock-in mouse that harbored the M712T mutation in the kinase domain, the most common mutation among Middle Eastern patients, was reported by the NIH group. These mice, however, did not show the phenotype seen among DMRV patients, but a lethal phenotype consisting of early glomerular disease with proteinuria, and podocytopathy, segmental splitting of the glomerular basement membrane, and effacement of podocyte foot processes resulting in death within 72 hours after birth (25). Podocalyxin, a major glycoprotein in the glomerular basement membrane, was hyposialylated, in addition to the decreased Gne expression and activity, implying that reduction of sialic acid may be responsible for the lethal phenotype in these mice. Galeano et al. then proceeded with administration of ManNAc in pregnant heterozygous mice and with this treatment 43% of homozygous mutants survived beyond postnatal day 3, exhibiting improved renal histology, increased sialylation of podocalyxin, and increased Gne expression and activity. Galeano et al. then concluded that M712T Gne-knock-in mice provide a novel animal model of hyposialylation-related podocytopathy and segmental splitting of the glomerular basement membrane, and demonstrated the significance of sialic acid synthesis in kidney development and function.
Our group took a different approach in generating an animal model for this disease. As attempts to generate a Gne knock-out mouse did not produce a homozygote mouse, we generated a transgenic mice which expressed D176V in the epimerase domain of Gne, one of the common mutations among Japanese patients, and crossed this with Gne knock-out line (26). The Gne(-/-)hGNED176VTg appeared normal at birth, but what was remarkable was the decreased levels of sialic acid evident in the serum and all organs, regardless of age, underscoring the role of hyposialylation in the pathogenesis of DMRV. The mice gradually developed muscle phenotype starting from 30 weeks of age, seen as poor motor performance and gradually increasing serum CK levels, albeit minor changes in muscle pathology which included variation in fiber type and the presence of scattered small angular fibers. Around the age of 40 weeks, significant changes were seen in muscle pathology, comprising of intracytoplasmic rimmed vacuoles (Fig. 1B) which were highlighted by acid phosphatase staining (Fig. 1C), and were immunoreactive to lysosomal markers, amyloid, phosphorylated tau, and neurofilaments. It was also demonstrated that these mice exhibited autophagy confirmed both by immunohistochemical analysis and electron microscopy studies (Fig. 1D) (27), further adding to the similarity of the Gne(-/-)hGNED176VTg with human DMRV patients. In some of these mice, rimmed vacuoles were also seen in the cardiac myofibers, indicating that the skeletal muscle is not the only organ involved. It will thus be important to look into other organs as well, especially that hyposialylation is not confined to the serum and skeletal muscles. Interestingly, intracellular amyloid deposition were seen by age 34-38 weeks of age, implying the role of amyloid misprocessing as an upstream event to the formation of rimmed vacuoles. Overall, Gne(-/-)hGNED176VTg mouse resembled the clinical, biochemical, and pathological phenotype of DMRV, and thus is aptly regarded as the first animal model of DMRV or hIBM.
The results obtained from this DMRV mouse model have underscored the key role of hyposialylation in the pathomechanism of this myopathy. This phenomenon pre-dated all findings that refer to the clinical phenotype in human patients, namely histological changes and clinical weakness. The generation of this DMRV model certainly paves the way for a more detailed study of the pathogenesis of the disease, and, more importantly, the evaluation of relevant therapeutic drugs for future clinical trials.
Acknowledgments
This study is supported partly by: the “ Research on Psychiatric and Neurological Diseases and Mental Health” from the Japanese Health Sciences Foundation; the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO); the “ Research Grant (17A-10, 19A-7) for Nervous and Mental Disorders” from the Ministry of Health Labour and Welfare; the Kato Memorial Trust for Nambyo Research; and the Neuromuscular Disease Foundation.
References
- 1.Nonaka I, Noguchi S, Nishino I. Distal myopathy with rimmed vacuoles and hereditary inclusion body myopathy. Curr Neurol Neurosci Rep 2005;5:61-5. [DOI] [PubMed] [Google Scholar]
- 2.Nishino I, Malicdan MC, Murayama K, et al. Molecular pathomechanism of distal myopathy with rimmed vacuoles. Acta Myol 2005;24:80-3. [PubMed] [Google Scholar]
- 3.Nishino I, Noguchi S, Murayama K, et al. Distal myopathy with rimmed vacuoles is allelic to hereditary inclusion body myopathy. Neurology 2002;59:1689-93. [DOI] [PubMed] [Google Scholar]
- 4.Askanas V, Engel WK. Hereditary inclusion myopathies, in The molecular and genetic basis of neurologic and psychiatric disease. Woburn, MA: Butterworth-Heinemann 2003. [Google Scholar]
- 5.Yan C, Ikezoe K, Nonaka I. Apoptotic muscle fiber degeneration in distal myopathy with rimmed vacuoles. Acta Neuropathol 2001;101:9-16. [DOI] [PubMed] [Google Scholar]
- 6.Amsili S, Schlomai Z, Levitzki R, et al. Characterization of hereditary inclusion body myopathy myoblasts: possible primary impairment of apoptotic events. Cell Death Differ 2007 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 7.Tomimitsu H, Shimizu J, Ishikawa K, et al. Distal myopathy with rimmed vacuoles (DMRV): new GNE mutations and splice variant. Neurology 2004;62:1607-10. [DOI] [PubMed] [Google Scholar]
- 8.Argov Z, Eisenberg I, Grabov-Nardini G, et al. Hereditary inclusion body myopathy: the Middle Eastern genetic cluster. Neurology 2003;60:1519-23. [DOI] [PubMed] [Google Scholar]
- 9.Eisenberg I, Grabov-Nardini G, Hochner H, et al. Mutations spectrum of GNE in hereditary inclusion body myopathy sparing the quadriceps. Hum Mutat 2003;21:99. [DOI] [PubMed] [Google Scholar]
- 10.Schwarzkopf M, Knobeloch KP, Rohde E, et al. Sialylation is essential for early development in mice. Proc Natl Acad Sci USA 2002;99:5267-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broccolini A, Ricci E, Cassandrini D, et al. Novel GNE mutations in Italian families with autosomal recessive hereditary inclusion-body myopathy. Hum Mutat 2004;23:632. [DOI] [PubMed] [Google Scholar]
- 12.Liewluck T, Pho-Iam T, Limwongse C, et al. Mutation analysis of the GNE gene in distal myopathy with rimmed vacuoles (DMRV) patients in Thailand. Muscle Nerve 2006;34:775-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim BJ, Ki CS, Kim JW, et al. Mutation analysis of the GNE gene in Korean patients with distal myopathy with rimmed vacuoles. J Hum Genet 2006;51:137-40. [DOI] [PubMed] [Google Scholar]
- 14.Ro LS, Lee-Chen GJ, Wu YR, et al. Phenotypic variability in a Chinese family with rimmed vacuolar distal myopathy. J Neurol Neurosurg Psychiatry 2005;76752-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu CC, Kuo HC, Yeh TH, et al. Heterozygous mutations affecting the epimerase domain of the GNE gene causing distal myopathy with rimmed vacuoles in a Taiwanese family. Clin Neurol Neurosurg 2007;109:250-6. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi S, Keira Y, Murayama K, et al. Reduction of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase activity and sialylation in distal myopathy with rimmed vacuoles. J Biol Chem 2004;279:11402-7. [DOI] [PubMed] [Google Scholar]
- 17.Hinderlich S, Salama I, Eisenberg I, et al. The homozygous M712T mutation of UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase results in reduced enzyme activities but not in altered overall cellular sialylation in hereditary inclusion body myopathy. FEBS Lett 2004;566:105-9. [DOI] [PubMed] [Google Scholar]
- 18.Penner J, Mantey LR, Elgavish S, et al. Influence of UDP-GlcNAc 2-Epimerase/ManNAc kinase mutant proteins on hereditary inclusion body myopathy. Biochemistry 2006;45:2968-77. [DOI] [PubMed] [Google Scholar]
- 19.Salama I, Hinderlich S, Shlomai Z, et al. No overall hyposialylation in hereditary inclusion body myopathy myoblasts carrying the homozygous M712T GNE mutation. Biochem Biophys Res Commun 2005;328:221-6. [DOI] [PubMed] [Google Scholar]
- 20.Saito F, Tomimitsu H, Arai K, et al. A Japanese patient with distal myopathy with rimmed vacuoles: missense mutations in the epimerase domain of the UDP-N-acetylglucosamine 2-epimerase/N-acetylmannosamine kinase (GNE) gene accompanied by hyposialylation of skeletal muscle glycoproteins. Neuromuscul Disord 2004;14:158-61. [DOI] [PubMed] [Google Scholar]
- 21.Ricci E, Broccolini A, Gidaro T, et al. NCAM is hyposialylated in hereditary inclusion body myopathy due to GNE mutations. Neurology 2006;66:755-8. [DOI] [PubMed] [Google Scholar]
- 22.Krause S, Hinderlich S, Amsili S, et al. Localization of UDP-GlcNAc 2-epimerase/ManAc kinase (GNE) in the Golgi complex and the nucleus of mammalian cells. Exp Cell Res 2005;304:365-79. [DOI] [PubMed] [Google Scholar]
- 23.Krause S, Aleo A, Hinderlich S, et al. GNE protein expression subcellular distribution are unaltered in HIBM. Neurology 2007;69:655-9. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Sun Z, Li AV, et al. Roles for GNE outside of sialic acid biosynthesis: modulation of sialyltransferase and BiP expression, GM3 and GD3 biosynthesis, proliferation and apoptosis, and ERK1/2 phosphorylation. J Biol Chem 2006;281:27016-28. [DOI] [PubMed] [Google Scholar]
- 25.Galeano B, Klootwijk R, Manoli I, et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest 2007;117:1585-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malicdan MC, Noguchi S, Nonaka I, et al. A Gne knockout mouse expressing human GNE D176V mutation develops features similar to distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Hum Mol Genet 2007 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 27.Malicdan, MC, Noguchi S, Nishino I. Autophagy in a mouse model of distal myopathy with rimmed vacuoles or hereditary inclusion body myopathy. Autophagy 2007;3:396-8. [DOI] [PubMed] [Google Scholar]