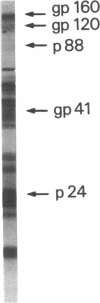
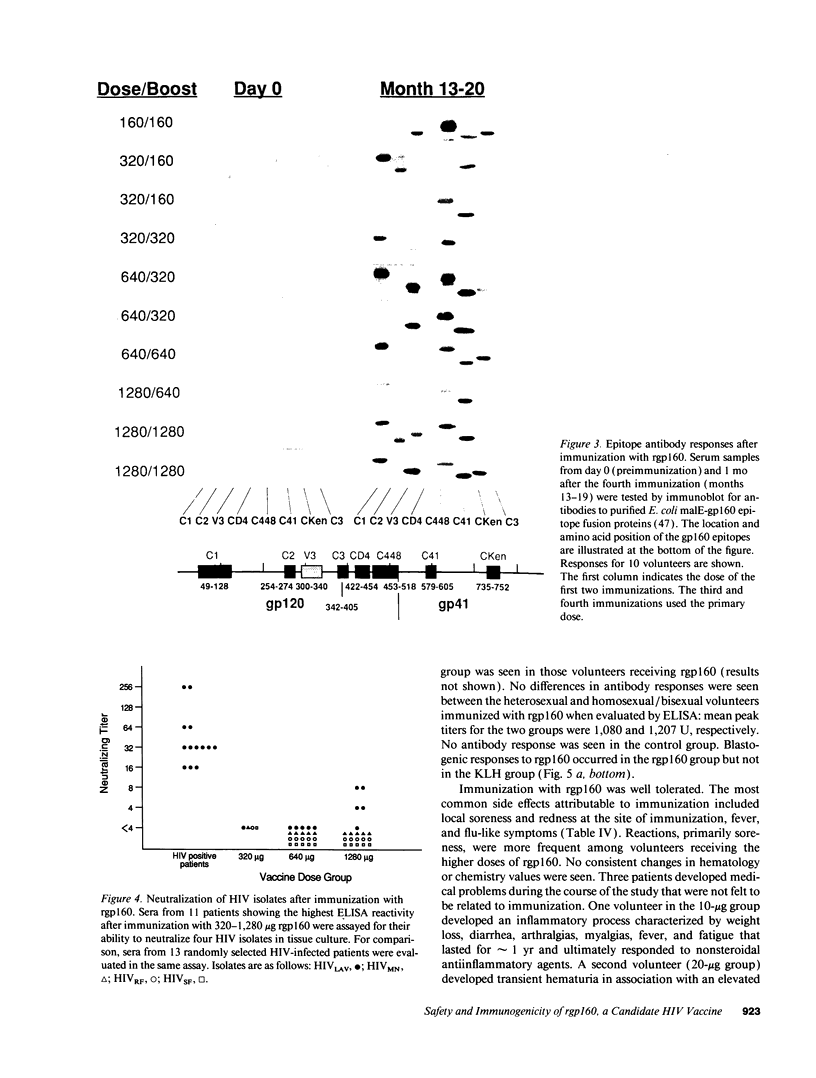
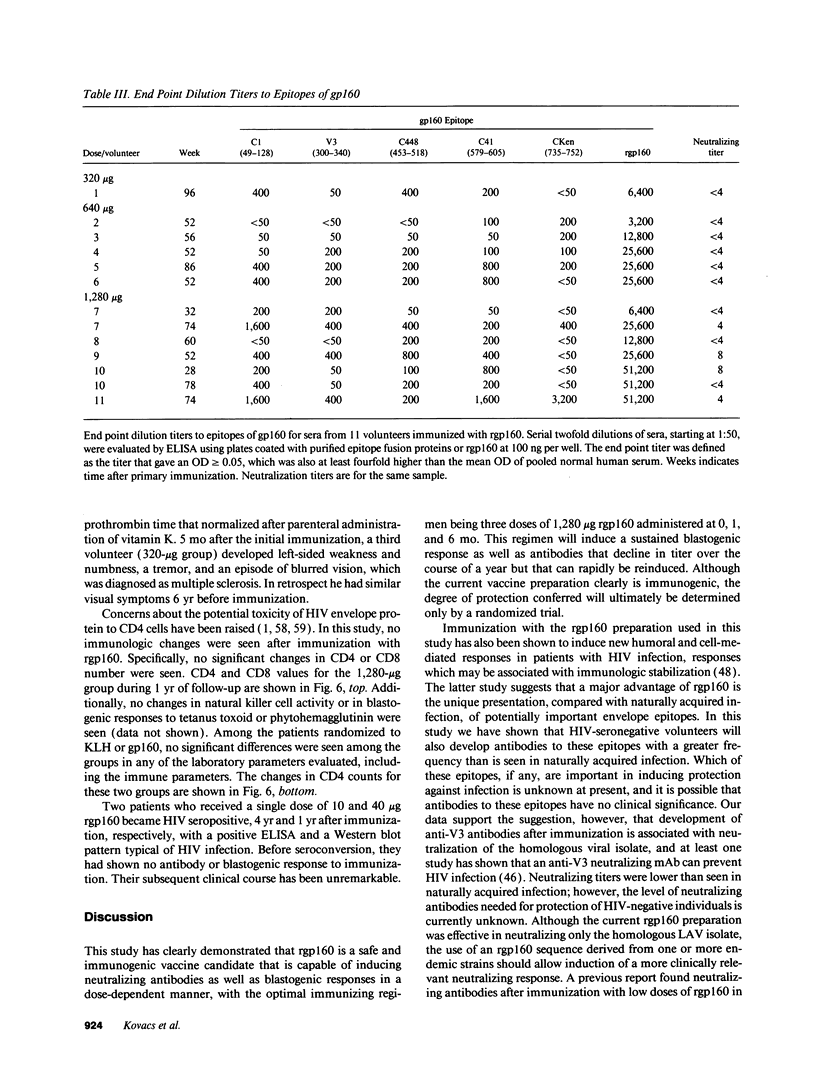
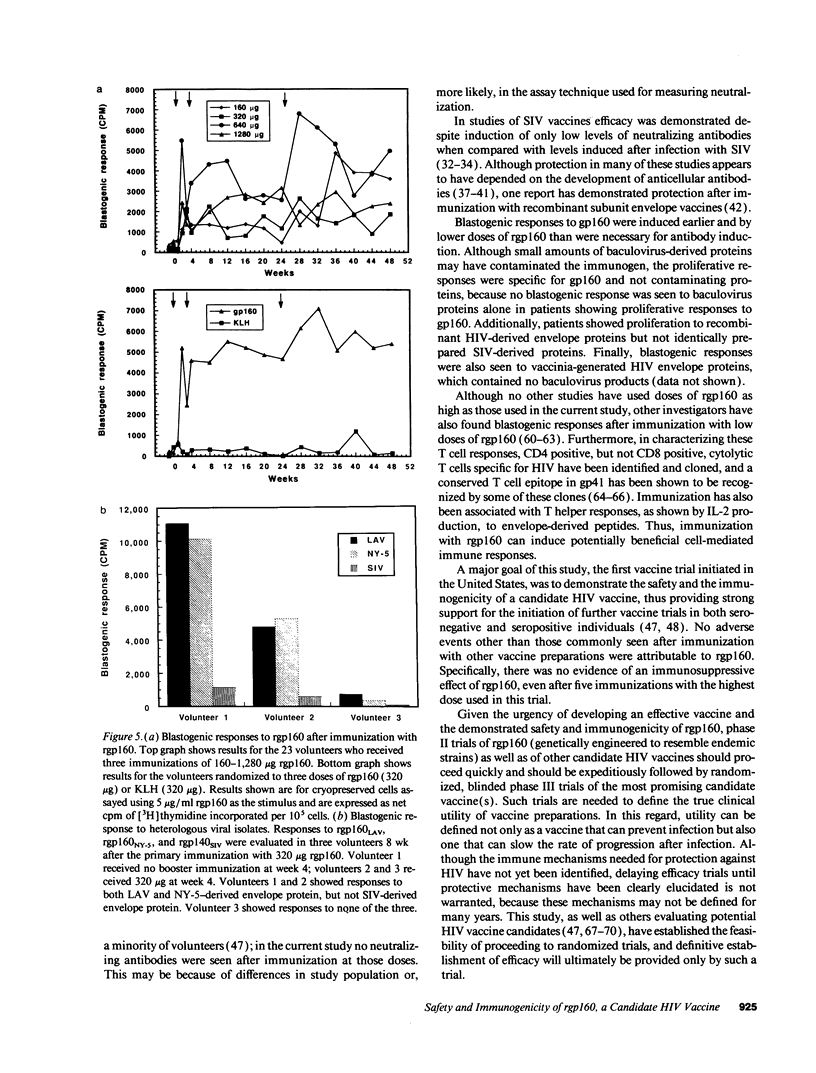
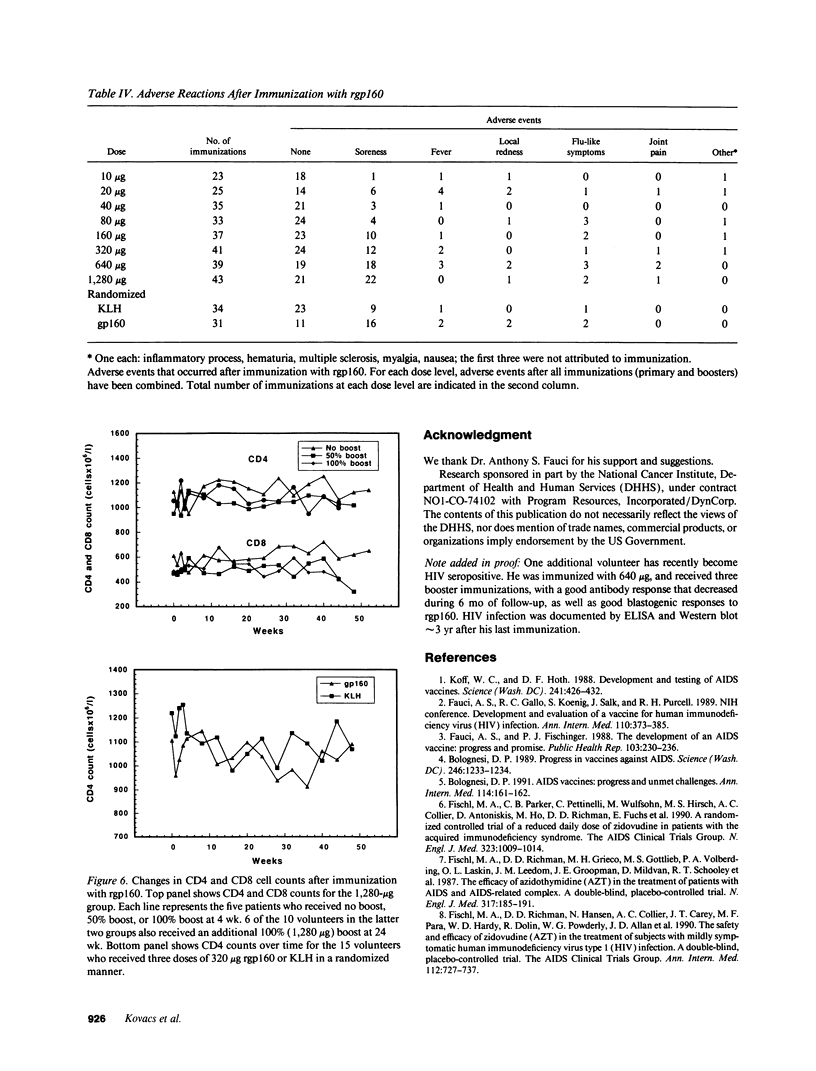
Abstract
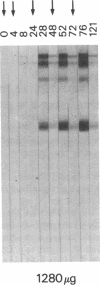
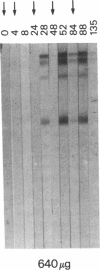
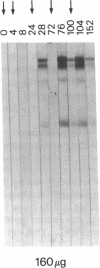
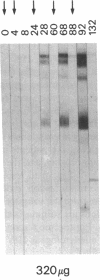
Development of an effective vaccine for prevention of infection with HIV would provide an important mechanism for controlling the AIDS epidemic. In the current study, the first clinical trial of a candidate HIV-1 vaccine initiated in the United States, the safety and immunogenicity of escalating doses (10-1,280 micrograms) of recombinant gp160 (rgp160), were evaluated in 138 HIV-negative volunteers. Maximal antibody responses, as evaluated by ELISA, were seen after immunization with three doses of 1,280 micrograms rgp160. Responses to some specific epitopes of HIV gp160, including the second conserved domain and the CD4 binding site, were seen more frequently than after natural infection. Neutralizing antibodies to the homologous HIV strain, but not heterologous strains, were induced by this regimen. Blastogenic responses to rgp160 were seen in most volunteers receiving at least two doses of > or = 20 micrograms. These envelope-specific T cell responses were also seen against heterologous strains of HIV. No major adverse reactions were seen after immunization. Thus, rgp160 is a safe and immunogenic candidate HIV vaccine; further studies are needed to determine if it will provide any clinical benefit in preventing HIV infection.
Full text
PDF
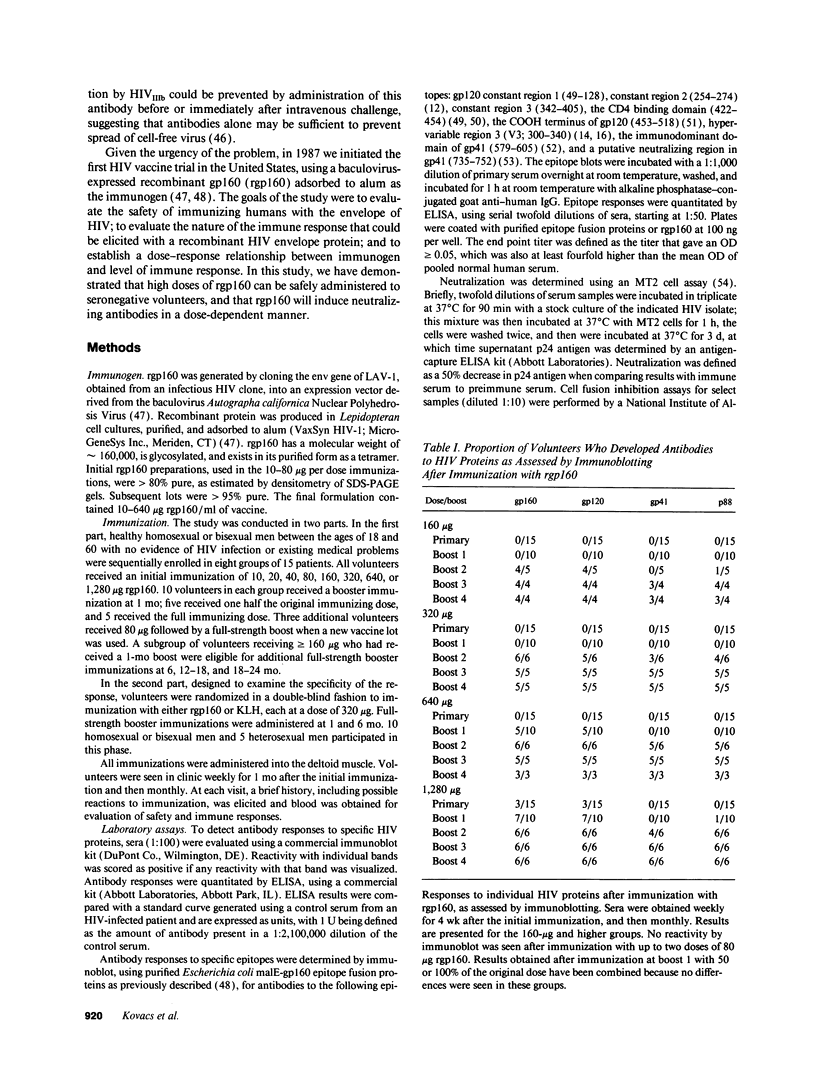
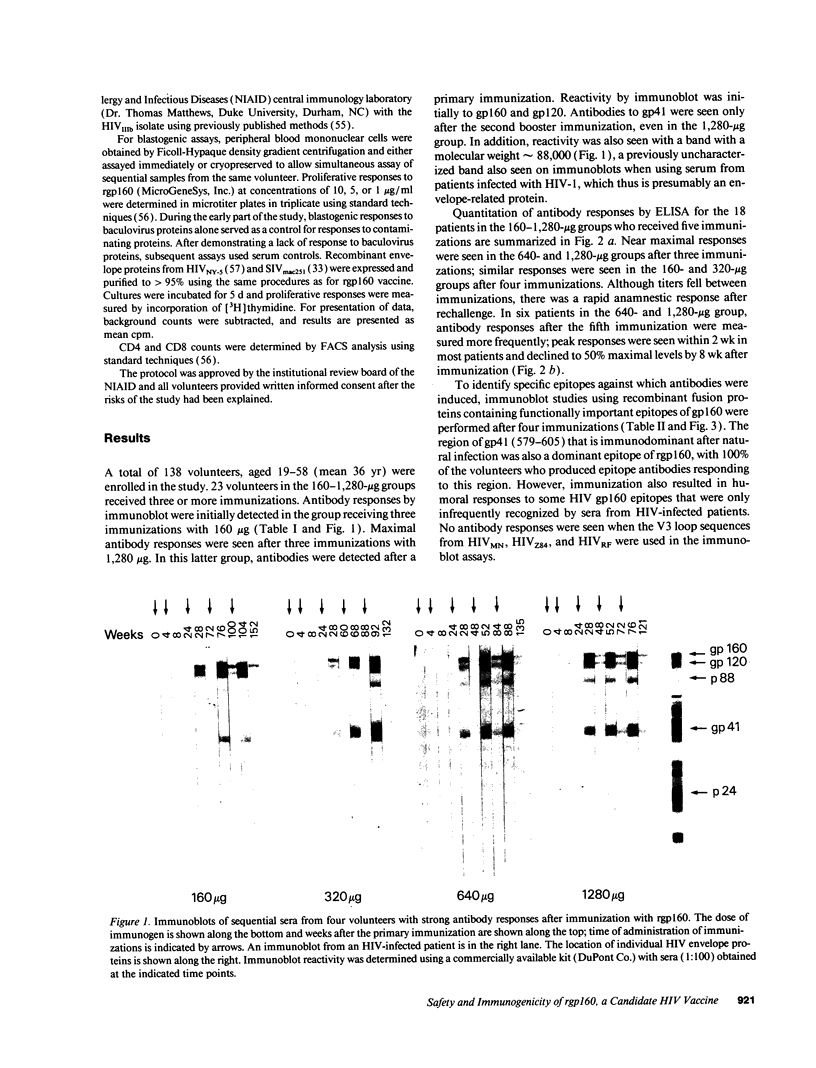
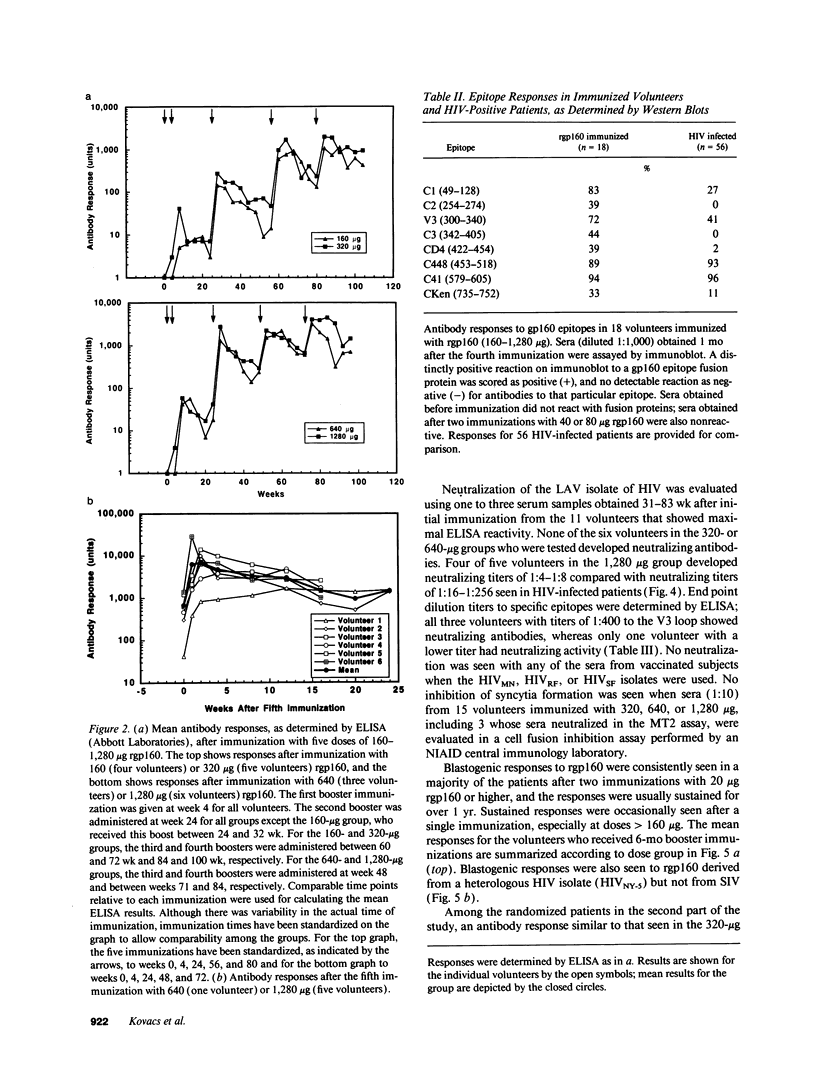


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearne P. M., Matthews T. J., Lyerly H. K., White G. C., Bolognesi D. P., Weinhold K. J. Cellular immune response to viral peptides in patients exposed to HIV. AIDS Res Hum Retroviruses. 1988 Aug;4(4):259–267. doi: 10.1089/aid.1988.4.259. [DOI] [PubMed] [Google Scholar]
- Belo M., Yagello M., Girard M., Greenlee R., Deslandres A., Barré-Sinoussi F., Gluckman J. C. Antibody-dependent cellular cytotoxicity against HIV-1 in sera of immunized chimpanzees. AIDS. 1991 Feb;5(2):169–176. doi: 10.1097/00002030-199102000-00006. [DOI] [PubMed] [Google Scholar]
- Benn S., Rutledge R., Folks T., Gold J., Baker L., McCormick J., Feorino P., Piot P., Quinn T., Martin M. Genomic heterogeneity of AIDS retroviral isolates from North America and Zaire. Science. 1985 Nov 22;230(4728):949–951. doi: 10.1126/science.2997922. [DOI] [PubMed] [Google Scholar]
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P. AIDS vaccines: progress and unmet challenges. Ann Intern Med. 1991 Jan 15;114(2):161–162. doi: 10.7326/0003-4819-114-2-161. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P. Progress in vaccines against AIDS. Science. 1989 Dec 8;246(4935):1233–1234. doi: 10.1126/science.2555922. [DOI] [PubMed] [Google Scholar]
- Bolognesi D. P. Prospects for prevention of and early intervention against HIV. JAMA. 1989 May 26;261(20):3007–3013. [PubMed] [Google Scholar]
- Carlson J. R., McGraw T. P., Keddie E., Yee J. L., Rosenthal A., Langlois A. J., Dickover R., Donovan R., Luciw P. A., Jennings M. B. Vaccine protection of rhesus macaques against simian immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1990 Nov;6(11):1239–1246. doi: 10.1089/aid.1990.6.1239. [DOI] [PubMed] [Google Scholar]
- Clerici M., Tacket C. O., Via C. S., Lucey D. R., Muluk S. C., Zajac R. A., Boswell R. N., Berzofsky J. A., Shearer G. M. Immunization with subunit human immunodeficiency virus vaccine generates stronger T helper cell immunity than natural infection. Eur J Immunol. 1991 Jun;21(6):1345–1349. doi: 10.1002/eji.1830210603. [DOI] [PubMed] [Google Scholar]
- Cooney E. L., Collier A. C., Greenberg P. D., Coombs R. W., Zarling J., Arditti D. E., Hoffman M. C., Hu S. L., Corey L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet. 1991 Mar 9;337(8741):567–572. doi: 10.1016/0140-6736(91)91636-9. [DOI] [PubMed] [Google Scholar]
- Cranage M. P., Ashworth L. A., Greenaway P. J., Murphey-Corb M., Desrosiers R. C. AIDS vaccine developments. Nature. 1992 Feb 20;355(6362):685–686. doi: 10.1038/355685a0. [DOI] [PubMed] [Google Scholar]
- Dalgleish A. G., Chanh T. C., Kennedy R. C., Kanda P., Clapham P. R., Weiss R. A. Neutralization of diverse HIV-1 strains by monoclonal antibodies raised against a gp41 synthetic peptide. Virology. 1988 Jul;165(1):209–215. doi: 10.1016/0042-6822(88)90674-5. [DOI] [PubMed] [Google Scholar]
- Daniel M. D., Kirchhoff F., Czajak S. C., Sehgal P. K., Desrosiers R. C. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992 Dec 18;258(5090):1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Wyand M. S., Kodama T., Ringler D. J., Arthur L. O., Sehgal P. K., Letvin N. L., King N. W., Daniel M. D. Vaccine protection against simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6353–6357. doi: 10.1073/pnas.86.16.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolin R., Graham B. S., Greenberg S. B., Tacket C. O., Belshe R. B., Midthun K., Clements M. L., Gorse G. J., Horgan B. W., Atmar R. L. The safety and immunogenicity of a human immunodeficiency virus type 1 (HIV-1) recombinant gp160 candidate vaccine in humans. NIAID AIDS Vaccine Clinical Trials Network. Ann Intern Med. 1991 Jan 15;114(2):119–127. doi: 10.7326/0003-4819-114-2-119. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Schleif W. A., Nunberg J. H., Conley A. J., Eda Y., Tokiyoshi S., Putney S. D., Matsushita S., Cobb K. E., Jett C. M. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992 Feb 20;355(6362):728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- Evans L. A., Thomson-Honnebier G., Steimer K., Paoletti E., Perkus M. E., Hollander H., Levy J. A. Antibody-dependent cellular cytotoxicity is directed against both the gp120 and gp41 envelope proteins of HIV. AIDS. 1989 May;3(5):273–276. doi: 10.1097/00002030-198905000-00004. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Fischinger P. J. The development of an AIDS vaccine: progress and promise. Public Health Rep. 1988 May-Jun;103(3):230–236. [PMC free article] [PubMed] [Google Scholar]
- Fauci A. S., Gallo R. C., Koenig S., Salk J., Purcell R. H. NIH conference. Development and evaluation of a vaccine for human immunodeficiency virus (HIV) infection. Ann Intern Med. 1989 Mar 1;110(5):373–385. doi: 10.7326/0003-4819-110-5-373. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Parker C. B., Pettinelli C., Wulfsohn M., Hirsch M. S., Collier A. C., Antoniskis D., Ho M., Richman D. D., Fuchs E. A randomized controlled trial of a reduced daily dose of zidovudine in patients with the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1990 Oct 11;323(15):1009–1014. doi: 10.1056/NEJM199010113231501. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Grieco M. H., Gottlieb M. S., Volberding P. A., Laskin O. L., Leedom J. M., Groopman J. E., Mildvan D., Schooley R. T. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987 Jul 23;317(4):185–191. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- Fischl M. A., Richman D. D., Hansen N., Collier A. C., Carey J. T., Para M. F., Hardy W. D., Dolin R., Powderly W. G., Allan J. D. The safety and efficacy of zidovudine (AZT) in the treatment of subjects with mildly symptomatic human immunodeficiency virus type 1 (HIV) infection. A double-blind, placebo-controlled trial. The AIDS Clinical Trials Group. Ann Intern Med. 1990 May 15;112(10):727–737. doi: 10.7326/0003-4819-112-10-727. [DOI] [PubMed] [Google Scholar]
- Fultz P. N., Nara P., Barre-Sinoussi F., Chaput A., Greenberg M. L., Muchmore E., Kieny M. P., Girard M. Vaccine protection of chimpanzees against challenge with HIV-1-infected peripheral blood mononuclear cells. Science. 1992 Jun 19;256(5064):1687–1690. doi: 10.1126/science.256.5064.1687. [DOI] [PubMed] [Google Scholar]
- Girard M., Kieny M. P., Pinter A., Barre-Sinoussi F., Nara P., Kolbe H., Kusumi K., Chaput A., Reinhart T., Muchmore E. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnann J. W., Jr, Schwimmbeck P. L., Nelson J. A., Truax A. B., Oldstone M. B. Diagnosis of AIDS by using a 12-amino acid peptide representing an immunodominant epitope of the human immunodeficiency virus. J Infect Dis. 1987 Aug;156(2):261–267. doi: 10.1093/infdis/156.2.261. [DOI] [PubMed] [Google Scholar]
- Gorse G. J., Belshe R. B., Newman F. K., Frey S. E. Lymphocyte proliferative responses following immunization with human immunodeficiency virus recombinant GP160. The NIAID AIDS Vaccine Clinical Trials Network. Vaccine. 1992;10(6):383–388. doi: 10.1016/0264-410x(92)90068-u. [DOI] [PubMed] [Google Scholar]
- Hammond S. A., Obah E., Stanhope P., Monell C. R., Strand M., Robbins F. M., Bias W. B., Karr R. W., Koenig S., Siliciano R. F. Characterization of a conserved T cell epitope in HIV-1 gp41 recognized by vaccine-induced human cytolytic T cells. J Immunol. 1991 Mar 1;146(5):1470–1477. [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Kaplan J. C., Rackauskas I. E., Gurney M. E. Second conserved domain of gp120 is important for HIV infectivity and antibody neutralization. Science. 1988 Feb 26;239(4843):1021–1023. doi: 10.1126/science.2830667. [DOI] [PubMed] [Google Scholar]
- Hu S. L., Abrams K., Barber G. N., Moran P., Zarling J. M., Langlois A. J., Kuller L., Morton W. R., Benveniste R. E. Protection of macaques against SIV infection by subunit vaccines of SIV envelope glycoprotein gp160. Science. 1992 Jan 24;255(5043):456–459. doi: 10.1126/science.1531159. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer M. C., Bonnez W., Roberts N. J., Jr, Dolin R., Reichman R. C. Human immunodeficiency virus (HIV-1) gp160-specific lymphocyte proliferative responses of mononuclear leukocytes from HIV-1 recombinant gp160 vaccine recipients. J Infect Dis. 1991 Mar;163(3):448–453. doi: 10.1093/infdis/163.3.448. [DOI] [PubMed] [Google Scholar]
- Koenig S., Earl P., Powell D., Pantaleo G., Merli S., Moss B., Fauci A. S. Group-specific, major histocompatibility complex class I-restricted cytotoxic responses to human immunodeficiency virus 1 (HIV-1) envelope proteins by cloned peripheral blood T cells from an HIV-1-infected individual. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8638–8642. doi: 10.1073/pnas.85.22.8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff W. C., Hoth D. F. Development and testing of AIDS vaccines. Science. 1988 Jul 22;241(4864):426–432. doi: 10.1126/science.3293212. [DOI] [PubMed] [Google Scholar]
- Koup R. A., Sullivan J. L., Levine P. H., Brewster F., Mahr A., Mazzara G., McKenzie S., Panicali D. Antigenic specificity of antibody-dependent cell-mediated cytotoxicity directed against human immunodeficiency virus in antibody-positive sera. J Virol. 1989 Feb;63(2):584–590. doi: 10.1128/jvi.63.2.584-590.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Lane H. C., Masur H., Gelmann E. P., Longo D. L., Steis R. G., Chused T., Whalen G., Edgar L. C., Fauci A. S. Correlation between immunologic function and clinical subpopulations of patients with the acquired immune deficiency syndrome. Am J Med. 1985 Mar;78(3):417–422. doi: 10.1016/0002-9343(85)90332-8. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Nakamura G., Smith D. H., Fennie C., Shimasaki C., Patzer E., Berman P., Gregory T., Capon D. J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987 Sep 11;50(6):975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- Le Grand R., Vaslin B., Vogt G., Roques P., Humbert M., Dormont D. AIDS vaccine developments. Nature. 1992 Feb 20;355(6362):684–684. doi: 10.1038/355684a0. [DOI] [PubMed] [Google Scholar]
- Looney D. J., Fisher A. G., Putney S. D., Rusche J. R., Redfield R. R., Burke D. S., Gallo R. C., Wong-Staal F. Type-restricted neutralization of molecular clones of human immunodeficiency virus. Science. 1988 Jul 15;241(4863):357–359. doi: 10.1126/science.3388046. [DOI] [PubMed] [Google Scholar]
- Lyerly H. K., Reed D. L., Matthews T. J., Langlois A. J., Ahearne P. A., Petteway S. R., Jr, Weinhold K. J. Anti-GP 120 antibodies from HIV seropositive individuals mediate broadly reactive anti-HIV ADCC. AIDS Res Hum Retroviruses. 1987;3(4):409–422. doi: 10.1089/aid.1987.3.409. [DOI] [PubMed] [Google Scholar]
- Martínez-A C., Marcos M. A., de la Hera A., Marquez C., Alonso J. M., Toribio M. L., Coutinho A. Immunological consequences of HIV infection: advantage of being low responder casts doubts on vaccine development. Lancet. 1988 Feb 27;1(8583):454–457. doi: 10.1016/s0140-6736(88)91244-5. [DOI] [PubMed] [Google Scholar]
- Matthews T. J., Langlois A. J., Robey W. G., Chang N. T., Gallo R. C., Fischinger P. J., Bolognesi D. P. Restricted neutralization of divergent human T-lymphotropic virus type III isolates by antibodies to the major envelope glycoprotein. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9709–9713. doi: 10.1073/pnas.83.24.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan T. C., Skowron G., Bozzette S. A., Richman D., Uttamchandani R., Fischl M., Schooley R., Hirsch M., Soo W., Pettinelli C. Circulating p24 antigen levels and responses to dideoxycytidine in human immunodeficiency virus (HIV) infections. A phase I and II study. Ann Intern Med. 1989 Feb 1;110(3):189–194. doi: 10.7326/0003-4819-110-3-189. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Martin L. N., Davison-Fairburn B., Montelaro R. C., Miller M., West M., Ohkawa S., Baskin G. B., Zhang J. Y., Putney S. D. A formalin-inactivated whole SIV vaccine confers protection in macaques. Science. 1989 Dec 8;246(4935):1293–1297. doi: 10.1126/science.2555923. [DOI] [PubMed] [Google Scholar]
- Murphey-Corb M., Montelaro R. C., Miller M. A., West M., Martin L. N., Davison-Fairburn B., Ohkawa S., Baskin G. B., Zhang J. Y., Miller G. B. Efficacy of SIV/deltaB670 glycoprotein-enriched and glycoprotein-depleted subunit vaccines in protecting against infection and disease in rhesus monkeys. AIDS. 1991 Jun;5(6):655–662. doi: 10.1097/00002030-199106000-00003. [DOI] [PubMed] [Google Scholar]
- Orentas R. J., Hildreth J. E., Obah E., Polydefkis M., Smith G. E., Clements M. L., Siliciano R. F. Induction of CD4+ human cytolytic T cells specific for HIV-infected cells by a gp160 subunit vaccine. Science. 1990 Jun 8;248(4960):1234–1237. doi: 10.1126/science.2190315. [DOI] [PubMed] [Google Scholar]
- Osterhaus A., de Vries P., Heeney J. AIDS vaccine developments. Nature. 1992 Feb 20;355(6362):684–685. doi: 10.1038/355684b0. [DOI] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Redfield R. R., Birx D. L., Ketter N., Tramont E., Polonis V., Davis C., Brundage J. F., Smith G., Johnson S., Fowler A. A phase I evaluation of the safety and immunogenicity of vaccination with recombinant gp160 in patients with early human immunodeficiency virus infection. Military Medical Consortium for Applied Retroviral Research. N Engl J Med. 1991 Jun 13;324(24):1677–1684. doi: 10.1056/NEJM199106133242401. [DOI] [PubMed] [Google Scholar]
- Sawyer L. A., Katzenstein D. A., Hendry R. M., Boone E. J., Vujcic L. K., Williams C. C., Zeger S. L., Saah A. J., Rinaldo C. R., Jr, Phair J. P. Possible beneficial effects of neutralizing antibodies and antibody-dependent, cell-mediated cytotoxicity in human immunodeficiency virus infection. AIDS Res Hum Retroviruses. 1990 Mar;6(3):341–356. doi: 10.1089/aid.1990.6.341. [DOI] [PubMed] [Google Scholar]
- Shafferman A., Lennox J., Grosfeld H., Sadoff J., Redfield R. R., Burke D. S. Patterns of antibody recognition of selected conserved amino acid sequences from the HIV envelope in sera from different stages of HIV infection. AIDS Res Hum Retroviruses. 1989 Feb;5(1):33–39. doi: 10.1089/aid.1989.5.33. [DOI] [PubMed] [Google Scholar]
- Siliciano R. F., Bollinger R. C., Callahan K. M., Hammond S. A., Liu A. Y., Miskovsky E. P., Rowell J. F., Stanhope P. E. Clonal analysis of T-cell responses to the HIV-1 envelope proteins in AIDS vaccine recipients. AIDS Res Hum Retroviruses. 1992 Aug;8(8):1349–1352. doi: 10.1089/aid.1992.8.1349. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Langlois A. J., McDanal C. B., McDougal J. S., Bolognesi D. P., Matthews T. J. Neutralizing antibodies to an immunodominant envelope sequence do not prevent gp120 binding to CD4. J Virol. 1988 Nov;62(11):4195–4200. doi: 10.1128/jvi.62.11.4195-4200.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stott E. J. Anti-cell antibody in macaques. Nature. 1991 Oct 3;353(6343):393–393. doi: 10.1038/353393a0. [DOI] [PubMed] [Google Scholar]
- Stott E. J., Chan W. L., Mills K. H., Page M., Taffs F., Cranage M., Greenaway P., Kitchin P. Preliminary report: protection of cynomolgus macaques against simian immunodeficiency virus by fixed infected-cell vaccine. Lancet. 1990 Dec 22;336(8730):1538–1541. doi: 10.1016/0140-6736(90)93310-l. [DOI] [PubMed] [Google Scholar]
- Syu W. J., Huang J. H., Essex M., Lee T. H. The N-terminal region of the human immunodeficiency virus envelope glycoprotein gp120 contains potential binding sites for CD4. Proc Natl Acad Sci U S A. 1990 May;87(10):3695–3699. doi: 10.1073/pnas.87.10.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Baqar S., Munoz C., Murphy J. R. Lymphoproliferative responses to mitogens and HIV-1 envelope glycoprotein among volunteers vaccinated with recombinant gp160. AIDS Res Hum Retroviruses. 1990 Apr;6(4):535–542. doi: 10.1089/aid.1990.6.535. [DOI] [PubMed] [Google Scholar]
- Tyler D. S., Lyerly H. K., Weinhold K. J. Anti-HIV-1 ADCC. AIDS Res Hum Retroviruses. 1989 Dec;5(6):557–563. doi: 10.1089/aid.1989.5.557. [DOI] [PubMed] [Google Scholar]
- Tyler D. S., Stanley S. D., Nastala C. A., Austin A. A., Bartlett J. A., Stine K. C., Lyerly H. K., Bolognesi D. P., Weinhold K. J. Alterations in antibody-dependent cellular cytotoxicity during the course of HIV-1 infection. Humoral and cellular defects. J Immunol. 1990 May 1;144(9):3375–3384. [PubMed] [Google Scholar]
- Walker B. D., Chakrabarti S., Moss B., Paradis T. J., Flynn T., Durno A. G., Blumberg R. S., Kaplan J. C., Hirsch M. S., Schooley R. T. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987 Jul 23;328(6128):345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Walker C. M., Moody D. J., Stites D. P., Levy J. A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986 Dec 19;234(4783):1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- Weinhold K. J., Lyerly H. K., Matthews T. J., Tyler D. S., Ahearne P. M., Stine K. C., Langlois A. J., Durack D. T., Bolognesi D. P. Cellular anti-GP120 cytolytic reactivities in HIV-1 seropositive individuals. Lancet. 1988 Apr 23;1(8591):902–905. doi: 10.1016/s0140-6736(88)91713-8. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Clapham P. R., Weber J. N., Dalgleish A. G., Lasky L. A., Berman P. W. Variable and conserved neutralization antigens of human immunodeficiency virus. Nature. 1986 Dec 11;324(6097):572–575. doi: 10.1038/324572a0. [DOI] [PubMed] [Google Scholar]
- Wintsch J., Chaignat C. L., Braun D. G., Jeannet M., Stalder H., Abrignani S., Montagna D., Clavijo F., Moret P., Dayer J. M. Safety and immunogenicity of a genetically engineered human immunodeficiency virus vaccine. J Infect Dis. 1991 Feb;163(2):219–225. doi: 10.1093/infdis/163.2.219. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Mitsuya H., Thomas R. V., Pluda J. M., Hartman N. R., Perno C. F., Marczyk K. S., Allain J. P., Johns D. G., Broder S. In vivo activity against HIV and favorable toxicity profile of 2',3'-dideoxyinosine. Science. 1989 Jul 28;245(4916):412–415. doi: 10.1126/science.2502840. [DOI] [PubMed] [Google Scholar]
- Yarchoan R., Pluda J. M., Thomas R. V., Mitsuya H., Brouwers P., Wyvill K. M., Hartman N., Johns D. G., Broder S. Long-term toxicity/activity profile of 2',3'-dideoxyinosine in AIDS or AIDS-related complex. Lancet. 1990 Sep 1;336(8714):526–529. doi: 10.1016/0140-6736(90)92085-v. [DOI] [PubMed] [Google Scholar]
- Zagury D., Bernard J., Cheynier R., Desportes I., Leonard R., Fouchard M., Reveil B., Ittele D., Lurhuma Z., Mbayo K. A group specific anamnestic immune reaction against HIV-1 induced by a candidate vaccine against AIDS. Nature. 1988 Apr 21;332(6166):728–731. doi: 10.1038/332728a0. [DOI] [PubMed] [Google Scholar]
- Zagury D., Léonard R., Fouchard M., Réveil B., Bernard J., Ittelé D., Cattan A., Zirimwabagabo L., Kalumbu M., Justin W. Immunization against AIDS in humans. Nature. 1987 Mar 19;326(6110):249–250. doi: 10.1038/326249a0. [DOI] [PubMed] [Google Scholar]