Summary
Myasthenia gravis (MG) is caused by the failure of neuromuscular transmission mediated by autoantibodies. That is, the binding of autoantibodies to postsynaptic membranes in neuromuscular junctions (NMJ) results in weakening of the ocular, bulbar and limb muscles and produces the characteristic syndrome of MG. This relatively rare disease serves as a model not only for study of the pathogenesis and treatment of all autoimmune disorders but also for understanding the basic mechanisms of neuromuscular transmission at the NMJ. About 80 to 85% of patients with MG have autoantibodies against acetylcholine receptors (AChR). Although a number of studies have shown the possible existence of other autoantibodies in the remaining ~20% of MG patients, the responsible autoantigens have remained elusive. However, antibodies against muscle-specific kinase (MuSK) have been found in 30% of MG patients without AChR antibodies. MuSK, a tyrosine kinase receptor, is required for the development of NMJ’s postsynaptic membranes. Still, the pathogenicity of MuSK antibodies as a cause of muscle weakness in patients with MG remains a matter of dispute, because the experimental autoimmune MG caused by MuSK antibodies in animals was absent. Here we describe recent progress toward understanding the pathogenic role of MuSK antibodies in the decline of muscle strength that typifies MG.
Keywords: myasthenia gravis, experimental autoimmune MG, muscle-specific kinase
Myathenia gravis caused by antibodies to AChR
Myasthenia gravis (MG) is a rare neuromuscular disease, but a well-recognized disorder because of such characteristic clinical features as ptosis with fluctuating general fatigue and muscle weakness that worsens with repeated activity (1, 2) but tends to improve with rest. Ptosis and diplogia occur early in the majority of these patients. With passing time, when the weakness of bulbar and respiratory muscle worsens, the disease becomes life-threatening so that intubation with mechanical ventilation is required. About 80% of patients with MG have autoantibodies against acetylcholine receptors (AChR) (1, 2). Patrick and Lindstrom provided the first piece of evidence indicating the pathogenicity of AChR antibodies by experimentally induced MG in 1973 (3). While a number of studies showed the pathogenic roles of AChR antibodies in causing structural and functional damage of the neuromuscular junction (NMJ) (4–8), autoantigens of the remaining MG patients (~20%) were undefined (5). Although these patients do not have AChR antibodies, they respond to immunotherapy (1, 2), and their serum antibodies can transfer a defect in neuromuscular transmission to mice (2), indicating that the muscle weakness is also induced by autoantibodies against neuromuscular junctions (NMJ).
MuSK antibodies in MG patients
For the last three decades, causative autoantibodies other than those to AChR have been sought in MG patients but have eluded identification in spite of extensive research efforts (1, 2). In 2001, Hoch et al. found autoantibodies against muscle-specific kinase (MuSK) in a proportion of patients with generalized MG (5). MuSK is essential during the development of NMJ, when it organizes fetal AChR clustering at the postsynaptic membrane. Subsequently, in mature NMJ, MuSK is expressed predominantly at the postsynaptic membrane. Studies by Vincent and others showed that the frequency of MuSK antibodies in “seronegative MG patients”, i.e., those who lack autoantibodies to AChR, varied from 4 to 50% (4–8). Ohta et al. detected MuSK antibodies in about 30% of seronegative MG patients but not in any MG patients with AChR antibodies (seropositive MG) or other autoimmune diseases (9–11). The clinical features of MG with MuSK antibodies are distinctive. These individuals often suffer from a severe bulbar dysfunction that is difficult to resolve with immunosuppressive and immunomodulatory treatments, and muscular atrophy of facial and tongue muscles is common (1, 2). The response to AChE inhibitors is generally unsatisfactory with risk of worsening symptoms, especially when starting treatment in patients with bulbar symptoms or an impending respiratory crisis. Thymectomy does not alleviate the symptoms of MuSK-positive MG. In short-term therapy, patients with MuSK MG respond as well to plasma exchange and intravenous immunoglobulin as those with AChR seropositive MG. Even so, those patients whose neck and shoulder muscles are affected often experience respiratory weakness. MG in which weakness is limited to the ocular muscle is not frequent but does occur.
Some workers in this field are now coming to believe that MuSK MG must constitute a distinct subclass of the disease (4, 6, 7). The reason is that many patients with MuSK antibodies develop severe muscle weakness and eventual atrophy, which is rare in AChR seropositive MG, and the former respond differently to therapy than persons in the latter group. After the identification of MuSK antibodies in an MG patient, laboratory testing is now required to confirm the diagnosis of MG, to seek AChR antibodies and to formulate the clinical treatment.
MuSK functions in NMJ
MuSK plays multiple roles in clustering AChR during development of the postsynaptic membranes of NMJ (12, 13). Contact of the motor-nerve growth cone with the muscle induces a narrow, distinct endplate zone in the mid-muscle that is marked by a high density of AChR clustering. In this step, agrin released from motoneurons activates MuSK and redistributes AChR clusters to synaptic sites (12). However, the direct physical interaction between MuSK and agrin has so far not been demonstrated despite many attempts to do so (13). Thus, the mechanism(s) of MuSK activation and the following events remain obscure, although a co-receptor of MuSK, a co-ligand of agrin or alternative post-translational modification of either agrin or MuSK have been postulated (13). Intriguingly, MuSK is also required for organizing a primary synaptic scaffold to establish the post-synaptic membrane (12). Prior to muscle innervation, AChR clusters form at the central regions of muscle fibers, creating an endplate zone that is somewhat broader than that in innervated muscle. Thus, MuSK is required for pre-patterning of AChR clustering in the absence of motor innervation. However, establishing a scenario for MuSK’s participation in the process is somewhat complicated. For example, an element other than agrin may activate MuSK and trigger the postsynaptic specialization at NMJ. Simultaneously or alternatively, MuSK could act as a primary scaffold molecule without activation. The listed pleiotropic roles of MuSK in AChR clustering at developmental NMJ could also be required for the maintenance of mature NMJ (14–16). Studies performed in vivo have shown that synaptic AChRs intermingle among themselves completely over a period of four days and that many extra-synaptic AChRs are incorporated into the synapse at the mature NMJ, although the synaptic membrane in adult muscle appears macroscopically to be stable (17). Therefore, the mechanisms at play during AChR clustering in developing NMJ are also required in mature NMJ where postsynaptic complexes including those with AChR and MuSK are dynamically turning over for the maintenance of muscle function.
Do MuSK antibodies cause myasthenia?
Research on the mechanisms of synaptic transmission at the NMJ has uncovered some pathogenic effects of antibodies to AChR that could underlie MG (18). Effective neuromuscular transmission depends on numerous interactions between acetylcholine and its receptor, AChR, and the failure of neuromuscular transmission results in myasthenic weakness and fatigue. To evoke action potential for the contraction of muscle fibers, a large enough number of AChR must be present at postsynaptic membranes. In 1973, Fambrough and colleagues found an abnormal decrease in the number of AChR at postsynaptic membranes of the NMJ of patients with MG (1, 2). Others showed that AChR antibodies affect neuromuscular transmission by three main mechanisms: (a) Complement-mediated lysis of post-synaptic membranes follows the binding and activation of complement at the NMJ; (b) the degradation of AChR molecules accelerates upon cross-linking of those molecules by antibodies (antigenic modulation); (c) AChR antibodies block AChR function. The predominant pathogenicity is caused by the complement-mediated mechanisms, but all three mechanisms tend to reduce the number of available AChR and, thereby, decrease neuromuscular transmission between motor nerve endings and postsynaptic membranes. Therefore, an individual nerve impulse cannot generate enough postsynaptic depolarization to achieve the crucial firing threshold required for opening of sufficient voltage-gated sodium channels to initiate an action potential in the muscle fiber (18).
In contrast to the well-accepted mechanisms by which AChR antibodies function in MG, the pathogenic role of MuSK antibodies has been unclear (19). First, no significant loss of AChR at NMJ was observed in biopsies from biceps brachii muscles of MuSK-positive patients with MG (20). Second, MuSK antibodies are mainly in the IgG4 subclass, which does not activate complement (9), and complement-mediated damage to postsynaptic membranes is considered a major source of pathogenicity in MG patients with AChR antibodies. Third, passive transfer of MuSK serum in MG patients cannot generate the equivalent disease in mice. Fourth, no experimental animal model induced by MuSK had been developed. Although none of these studies seems to support a pathogenic role for MuSK antibodies in human MG, MuSK antibodies from MG patients effectively inhibit MuSK functions in vitro (5).
An experimental animal model of myasthenia (EAMG) induced by MuSK antibodies
The pathogenicity of AChR antibodies was shown experimentally by the induction of muscle weakness and development of paralysis in rabbits immunized with AChR protein purified from the electric eel (3). This AChR protein induced the production of antibodies that cross-reacted with rabbit AChR at the NMJ. The flaccid paralysis that followed and electrophysiological studies of these animals provided a model that resembled the MG of humans (21). Furthermore, this EAMG could be transferred by injecting sera from the paralyzed rabbits into naïve animals, indicating that the antibodies rather than cellular immunity caused the disease. Subsequently, EAMG was also induced in other species by repeated inoculations with purified AChR protein. The pathogenic nature of these antibodies from MG patients was demonstrated by passive transfer of the IgG fraction into mice. In addition to these experimental studies indicating the pathogenicity of AChR antibodies, clinical laboratory analyses determined that the patients had serum antibodies that were specific for AChR. Therefore, the next step was using MuSK antibodies to induce an EAMG model, which was essential for proving their pathogenicity and investigating their mechanisms of eliciting MG.
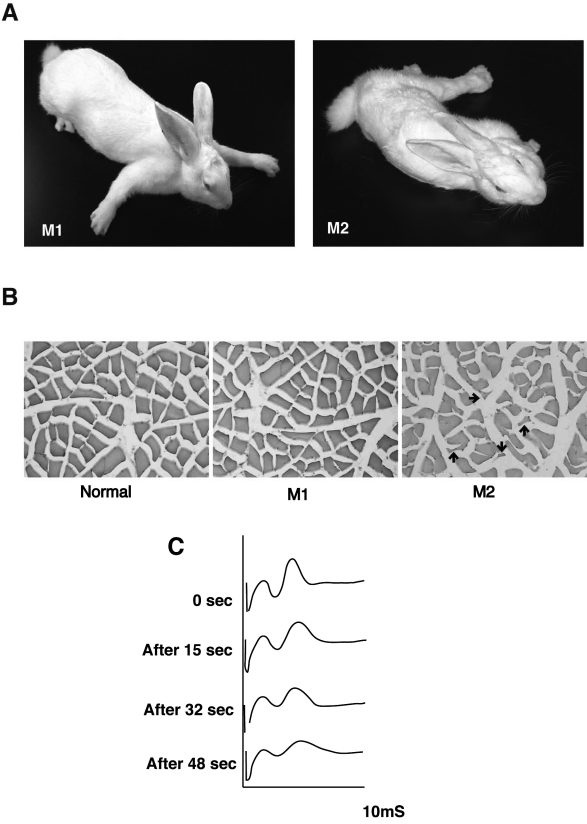
Recently we demonstrated that immunization of rabbits with MuSK ectodomain caused myasthenic weakness and produced electromyographic findings that were compatible with a diagnosis of MG (16), as shown by Patrick and Lindstrom. The extracellular segment of MuSK comprised five distinct domains, i.e., four immunoglobulin-like domains and one cysteine-rich region. The fusion protein expression constructs, which consisted of mouse MuSK ectodomain with the Fc region of human IgG1 or His-tag, were generated and transfected in COS-7 cells. The secreted recombinant MuSK-Fc and MuSK-His proteins were purified by using protein-A Sepharose and histidine affinity columns, respectively. New Zealand White rabbits were then immunized with 100 to 400 mg of purified MuSK recombinant protein. After three to four injections of MuSK protein, all of six rabbits manifested flaccid paralysis (Fig. 1A). Sera from the paretic rabbits contained a high titer of MuSK antibodies that reacted specifically with MuSK molecules on the surfaces of C2C12 myotubes as observed in sera from MG patients who were positive for MuSK antibodies. Histological studies of the muscle tissues from the paretic rabbits, which had manifested severe exhaustion, revealed alterations in muscle fibers ranging from subtle to angular atrophy intermingled with normal muscle tissue (Fig. 1B). The histological changes typical of atrophied muscle fibers can result from MG, reduced mechanical ability or cachexia. In repetitive electromyograms from one of these paretic rabbits, the retroauricular branch of facial nerve was stimulated at 20 Hz, and recordings were taken from adjacent retroauricular muscle (Fig. 1C). The compound muscle action potential (CMAP) showed a decremental pattern, consistent with MG. However, injections of acetylcholine esterase inhibitor did not significantly reverse either the CMAP defect or the paralytic symptoms. Importantly, the induction of EAMG by MuSK antibodies is not confined to rabbit, as we and others can also elicit EAMG in mice by injection of MuSK protein (22).
Figure 1.
Rabbits manifest myasthenia gravis (MG)-like paresis after immunization with MuSK protein. (A) Two rabbits representative of four animals with outcomes manifested myasthenic weakness after immunization with the recombinant MuSK protein. After three injections of MuSK protein, M1 and M2 rabbits manifested flaccid weakness within three and nine weeks respectively. M2 rabbit developed severe exhaustion with muscle weakness. (B) Cross-sections from the soleus muscles of two paretic (M1 and M2) and a normal rabbits (Normal) were stained with H&E. Muscle fibers in M1 paretic rabbit showed only subtle changes in shape and smallness, whereas an atrophy of muscles fibers in M2 paretic rabbit was observed as small angular fibers (indicated by arrows). Scale bar, 50 mm. (C) Electromyograms recorded from M1 paretic rabbit. The retro-auricular branch of the facial nerve was continuously by a constant current stimulator delivered square-wave pulses of 0.1 msec during at 20 Hz, and the compound muscle action potential (CMAP, second peak observed on the oscilloscope screen recorded at the indicated time-points during stimulation) shows a decremental pattern, consistent with MG. Reproduced from Shigemoto et al., 2006 (16), with permission.
How do antibodies to MuSK cause myasthenia?
Next, we focused on demonstrating how MuSK antibodies cause MG. The pathogenicity of MuSK antibodies in MG has been questioned, since MuSK-positive patients with MG do not have a decrease in the number of AChRs nor is complement deposited at the NMJ of their biceps brachii muscles (20). Although the mechanisms of MG caused by AChR antibodies are well delineated, the same pattern does not necessarily apply to MG caused by MuSK antibodies. MuSK antibodies have been identified as predominantly of the IgG4 subclass, which does not activate complement. However, the binding of MuSK antibodies to MuSK molecules could accelerate the latter’s degradation (antigenic modulation) and/or inhibit MuSK functions directly. MuSK is essential for AChR clustering at the developing NMJ, and its deficiency may lead to the complete loss of junctional ultrastructure (12, 13). To reveal the pathogenic role of MuSK antibodies in MG, we still need to know how MuSK acts at mature NMJ and whether MuSK is also required for the maintenance of AChR clustering and the structural stability of mature NMJ.
To elucidate the mechanisms of AChR clustering at NMJ, a number of studies were performed using cultured C2C12 myotubes. Agrin induces clustering of AChR in C2C12 myotubes following autophosphorylation by MuSK. In vitro, this event represents a major cascade of AChR clustering at the NMJ after innervation by motoneurons. Laminin-1 and the Nacetylgalactosamine (GalNAc)-specific lectin Vicia villosa agglutinin (VVA-B4) also induce AChR clustering on C2C12 myotubes, without activation of MuSK. Neither the receptor nor the activation mechanisms of AChR clustering induced by agrin-independent inducers has been identified with certainty. However, these mechanisms may also play important roles in the formation and maintenance of NMJ, the latter via agrin-independent pathways as shown by genetic studies (16).
In a previous study, Hoch et al. observed that the MuSK antibodies of MG patients inhibited agrin-induced AChR clustering in C2C12 myotubes (5). We also found that agrin-induced clustering of AChR was strongly blocked in the presence of MuSK antibodies, whereas absorption of the antibodies with purified MuSK products prevented this blocking effect (16). These results showed that the MuSK antibodies effectively inhibited the formation of agrin-induced AChR clustering. Intriguingly, the monovalent Fab fragments of MuSK antibodies from rabbits with EAMG also inhibited AChR clustering by agrin on C2C12 cells, indicating that complement-mediated mechanisms are not necessarily required for such inhibition (unpublished data). We also noted that MuSK-specific antibodies strongly inhibited AChR clustering induced by all known agrin-independent pathways as well as by agrin itself (16).
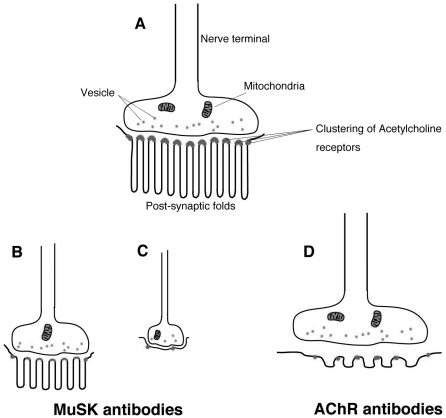
We then determined whether the expression of AChR at NMJ was reduced in soleus muscles of paretic compared to normal rabbits. Using fluorescence microscopy and a digital camera, we examined and recorded the size and optical densities of AChR clusters stained with the rhodamine-conjugated AChR agonist,α-bungarotoxin (α-BTX). The images were measured with NIH image analysis software for comparison with unprocessed digitized NIH images (16). The areas and intensity of AChR fluorescence in muscles of these paretic rabbits were significantly reduced compared with those in normal rabbits. In addition, the structure of NMJ in our paretic rabbits, as well as the size and branching of the motor terminals, were significantly reduced. Electron microscopic observations of NMJ in rabbits with EAMG induced by injection of MuSK protein demonstrated that the normally convoluted synaptic folds (Fig. 2A) underwent a significant simplification of structure (Fig. 2B and C) but no destruction (Fig. 2D). Within these intricately twisted synaptic folds, the high density of voltage-gated sodium channels contained in the membranes’ depths amplify the end-plate current, thus enhancing neuromuscular transmission and muscle contraction (23). Any reduction in the size and branching of the motor terminals contributes to decreases in ACh output. Moreover the simplification of post-synaptic structure increases of the threshold for generating muscle fiber action potential. These structural abnormalities in NMJ, including those in both pre- and post-synaptic structures, thus impair neuromuscular transmission in the EAMG rabbits (16, 22). Intriguingly, similar abnormalities of NMJ structure were also observed in rats with reduced expression of MuSK, as noted by RNA interference (15), in a patient with congenital myasthenic syndromes (CMS) caused by MuSK mutations and also in mice expressing the MuSK missense mutation by electroporation experiments (24). MuSK knock-out mice also displayed presynaptic defects in addition to postsynaptic ones, indicating that MuSK is required for retrograde signals, so far unidentified, to maintain the pre-synaptic structure in mature NMJ.
Figure 2.
Schematic appearances of NMJs observed in normal volunteers and patients. A: Normal NMJ. AChRs are concentrated at the peaks of abundant, well-preserved and intricately twisted junctional folds. B and C: NMJ in EAMG induced by MuSK antibodies, CMS with MuSK or Dok-7 mutations. Small NMJ in both pre- and post-synaptic structures. (B) Attenuation of AChR and reduced twisting of synaptic folds at the post-synaptic membrane without widened synaptic space. (C) Disappearance of post-synaptic folds with preserved synaptic space. D: NMJ in MG patients with AChR antibodies. Complement-mediated lysis of post-synaptic membranes. The myasthenic junction has a reduced number of AChR, simplified synaptic folds and a widened synaptic space with a normal nerve terminal.
Resemblance of clinical features between MuSK MG and CMS with Dok-7 mutations
Recently a MuSK-interacting protein called Dok-7 was discovered (25) and identified as a member of the Dok family of cytoplasmic proteins. Dok-7 is postulated to have three main functional domains: a pleckstrin homology (PH) domain, essential for membrane association; a phosphotyrosine-binding (PTB) domain involved in the Dok-7 induced activation of MuSK; and a large C-terminal domain containing multiple tyrosine residues. Dok-7 knock-out mice showed marked disruption of neuromuscular synaptogenesis that was indistinguishable from the features found in MuSK-deficient mice. Thus, Dok-7 is essential for neuromuscular synaptogenesis through its interaction with MuSK.
Mutations in the Dok-7 protein cause a genetic form of limb-girdle myasthenia (also classed as CMS) (26). Some clinical features in these patients resemble those in the severe type of MG accompanied by MuSK antibodies (27). Proximal muscles are usually more affected than those in distal regions, as evident in MuSK MG patients, and ptosis is often present. Limb-muscle weakness is comparatively less severe. Previous studies showed no reduction of AChR clustering with significant changes in NMJ of MuSK MG patients (20), but further structural analysis of NMJ is required in muscles where severe weakness occurs commonly. The weakness and atrophy are not observed uniformly in muscles of these patients, although both MuSK and Dok-7 are essential for the formation of NMJ during the embryonic stage (25). Of course, one of the major distinctions between acquired MuSK MG and CMS with the Dok-7 mutation is the timing when weakness begins. The CMS patients typically have difficulty in walking after reaching that normal motor milestone during early childhood, whereas the onset of weakness for MG patients, in most instances, occurs in adulthood. Interestingly, AChR clustering and post-synaptic folds are reduced with small motor terminals as observed at NMJ in CMS with Dok-7 mutations. AChR clustering and post-synaptic folds are reduced with small motor terminals as observed at NMJ in CMS with Dok-7 mutations (28). The effect of Dok-7 mutations on post-synaptic structures may also be an alteration of retrograde signaling to the pre-synaptic nerve terminals resulting in a reduced NMJ size in these patients (Figure 2). Dok-7, along with MuSK, is required not only for synaptogenesis but also for the maintenance of NMJ.
Conclusions
We now believe that MuSK antibodies cause MG in humans. Using an experimental model for myasthenia revealed that MuSK antibodies mediate the pathogenesis of this syndrome in rabbits and mice (14, 16, 22). In most cases, the symptoms take more than three months to manifest themselves in animals. Moreover, the symptoms are somewhat difficult to induce experimentally by passive transfer of MuSK antibodies from MG patients into animal hosts. The mechanisms employed by these antibodies include multiple events during which MuSK functions stall in their process of regulating synapse formation and maintenance. MuSK antibodies against compound antigenic determinants in the extracellular domain may engage in their pathogenic activities through antigenic modulation and/or restraint of MuSK functions, and the consequences of these effects range from a partial to entire loss of MuSK function without the involvement of complement-mediated damage. The point that MuSK antibodies in MG patients are mainly of the IgG4 subclass, which does not activate complement, may be relevant here. These diverse possibilities reflect the complexity of clinical features seen in patients ranging from typical MG and throughout its many variants.
MG has long served as model for studying the pathogenesis and treatment of generalized autoimmune disease. In fact, understanding of MG’s pathogenesis has enhanced comprehension of all synaptic functions. Now, the EAMG model with MuSK antibodies will facilitate further progress in resolving the pathogenic basis of MG and CMS at the molecular level and identifying beneficial treatment strategies. Additional areas of relevance are the many physical conditions in which muscles shrink or atrophy, as in patients with cancer or AIDS, termed cahexia, when limbs are immobilized following injury, or even during atrophy from aging, termed sarcopenia. Understanding the molecular basis of NMJ maintenance promises to provide new targets for innovative therapeutics to create healthy, enduring muscles.
Acknowledgments
We thank Ms. P. Minick for excellent editorial assistance. This study was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Science, and Culture, Japan, by a grant from the Health Science Research Grants for Research on Psychiatric and Neurological Diseases and Mental Health from the Ministry of Health, Labor, and Welfare, Japan and by a grant from the Kato Memorial Trust for Nambyo Research. We are also grateful to the stuff of the Integrated Center for Science of Ehime University for assistance with animal care and sequence analysis.
References
- 1.Conti-Fine BM, Milani M, Kaminski HJ. Myasthenia gravis: past, present, and future. J Clin Invest 2006;116:2843-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vincent A, Lang B, Kleopa KA. Autoimmune channelopathies and related neurological disorders. Neuron 2006;52:123-38. [DOI] [PubMed] [Google Scholar]
- 3.Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science 1973;180:871-2. [DOI] [PubMed] [Google Scholar]
- 4.Evoli A, Tonali PA, Padua L, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003;126:2304-11. [DOI] [PubMed] [Google Scholar]
- 5.Hoch W, McConville J, Helms S, et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001;7:365-8. [DOI] [PubMed] [Google Scholar]
- 6.Sanders DB, El-Salem K, Massey JM, et al. Clinical aspects of MuSK antibody positive seronegative MG. Neurology 2003;60:1978-80. [DOI] [PubMed] [Google Scholar]
- 7.Vincent A, Bowen J, Newsom-Davis J, et al. Seronegative generalised myasthenia gravis: clinical features, antibodies, and their targets. Lancet Neurol 2003;2:99-106. [DOI] [PubMed] [Google Scholar]
- 8.Yeh JH, Chen WH, Chiu HC, et al. Low frequency of MuSK antibody in generalized seronegative myasthenia gravis among Chinese. Neurology 2004;62:2131-2. [DOI] [PubMed] [Google Scholar]
- 9.Ohta K, Shigemoto K, Fujinami A, et al. Clinical and experimental features of MuSK antibody positive MG in Japan. Eur J Neurol 2007;14:1029-34. [DOI] [PubMed] [Google Scholar]
- 10.Ohta K, Shigemoto K, Kubo S, et al. MuSK Ab described in seropositive MG sera found to be Ab to alkaline phosphatase. Neurology 2005;65:1988. [DOI] [PubMed] [Google Scholar]
- 11.Ohta K, Shigemoto K, Kubo S, et al. MuSK antibodies in AChR Ab-seropositive MG vs. AChR Ab-seronegative MG. Neurology 2004;62:2132-3. [DOI] [PubMed] [Google Scholar]
- 12.Kummer TT, Misgeld T, Sanes JR. Assembly of the postsynaptic membrane at the neuromuscular junction: paradigm lost. Curr Opin Neurobiol 2006;16:74-82. [DOI] [PubMed] [Google Scholar]
- 13.Strochlic L, Cartaud A, Cartaud J. The synaptic muscle-specific kinase (MuSK) complex: new partners, new functions. Bioessays 2005;27:1129-35. [DOI] [PubMed] [Google Scholar]
- 14.Jha S, Xu K, Maruta T, et al. Myasthenia gravis induced in mice by immunization with the recombinant extracellular domain of rat muscle-specific kinase (MuSK). J Neuroimmunol 2006;175:107-17. [DOI] [PubMed] [Google Scholar]
- 15.Kong XC, Barzaghi P, Ruegg MA. Inhibition of synapse assembly in mammalian muscle in vivo by RNA interference. EMBO Rep 2004;5:183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shigemoto K, Kubo S, Maruyama N, et al. Induction of myasthenia by immunization against muscle-specific kinase. J Clin Invest 2006;116:1016-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akaaboune M, Grady RM, Turney S, et al. Neurotransmitter receptor dynamics studied in vivo by reversible photo-unbinding of fluorescent ligands. Neuron 2002;34:865-76. [DOI] [PubMed] [Google Scholar]
- 18.Ruff RL. Neuromuscular Junction Physiology and Pathophysiology. In: Kaminski, ed. Myasthenia Gravis and Related Disorders. New Jersey: Humana Press Inc. [Google Scholar]
- 19.Lindstrom J. Is “seronegative” MG explained by autoantibodies to MuSK? Neurology 2004;62:1920-1. [DOI] [PubMed] [Google Scholar]
- 20.Shiraishi H, Motomura M, Yoshimura T, et al. Acetylcholine receptors loss and postsynaptic damage in MuSK antibody-positive myasthenia gravis. Ann Neurol 2005;57:289-93. [DOI] [PubMed] [Google Scholar]
- 21.Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol 2002;2:797-804. [DOI] [PubMed] [Google Scholar]
- 22.Shigemoto K, Sachiho K, Chen J, et al. Experimentally induced myasthenia gravis with muscle-specific kinase. Ann N Y Acad Sci 2008 (in press). [DOI] [PubMed] [Google Scholar]
- 23.Wood SJ, Slater CR. Safety factor at the neuromuscular junction. Prog Neurobiol 2001;64:393-429. [DOI] [PubMed] [Google Scholar]
- 24.Chevessier F, Faraut B, Ravel-Chapuis A, et al. MUSK, a new target for mutations causing congenital myasthenic syndrome. Hum Mol Genet 2004;13:3229-40. [DOI] [PubMed] [Google Scholar]
- 25.Okada K, Inoue A, Okada M, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 2006;312:1802-5. [DOI] [PubMed] [Google Scholar]
- 26.Beeson D, Higuchi O, Palace J, et al. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 2006;313:1975-8. [DOI] [PubMed] [Google Scholar]
- 27.Palace J, Lashley D, Newsom-Davis J, et al. Clinical features of the DOK7 neuromuscular junction synaptopathy. Brain 2007. [DOI] [PubMed] [Google Scholar]
- 28.Slater CR, Fawcett PR, Walls TJ, et al. Pre- and post-synaptic abnormalities associated with impaired neuromuscular transmission in a group of patients with “limb-girdle myasthenia”. Brain 2006;129:2061-76. [DOI] [PubMed] [Google Scholar]