Introduction
McArdle disease (MCA) is the muscle glycogenosis due to defect of myophosphorylase. The pathological hallmark of the disease is the accumulation in the skeletal muscle of normal glycogen, and the absence of histochemical staining for glycogen phosphorylase in muscle. The pathology reflects the biochemical functional block in access to muscle glycogen, which while causing the local storage, is the physiopathological basis of the clinical signs associated with the disease. Patients with MCA show exercise intolerance which is maximal for the efforts which depend upon the rapid mobilization of muscle glycogen. Acute anaerobic efforts, when sustained after the first minute, depend heavily upon glycolytic metabolism, which in skeletal muscle utilises blood born glucose and glucose-1-P obtained from glycogen breakdown, which is blocked in MCA patients (1). Indeed, one of the most typical sign of MCA is the second-wind phenomenon, by which the patient, who experienced exhaustion after few minutes of acute effort slightly above the anaerobic threshold, is able to resume the effort with a much improved capacity and resistance (2). There are two rational approaches to circumvent this metabolic limitation, either the provision of a sufficient and continuous blood glucose flux, or a more efficient utilization of the available fuels.
The first approach is efficiently achieved by timely oral administration of sugar (2), which was shown to significantly improve perceived exhaustion and sustainable workload. This approach however cannot cover for all the unforecasted efforts, and has obvious limitation in terms of sustainable amount of sugar ingested. The second approach aims to a more chronic improvement of fuel use by muscle cells during various degrees of efforts in any circumstance.
The efficiency of muscle adaptation to training has been shown to be associated with polymorphic variants of the gene for angiotensin converting enzyme (ACE). In particular the insertion/deletion (I/D) polymorphism is associated with muscle performance in exercise and training (3, 4). Persons with I alleles, which is associated with lower ACE activity (5), show better results after aerobic training and higher muscle performance especially in tasks entailing resistance. The I/D ACE polymorphism was also associated with severity in a large group of MCA patients (6), and ACE activity modulation is easily achieved by drugs with excellent record of efficacy and tolerability. ACE activity modulation thus appears as a suitable target for chronic therapeutic intervention. The use of the ACE inhibitor Ramipril may mimic the condition associated with I alleles, and may improve functioning in MCA patients.
Materials and methods
8 subjects with biochemically and molecularly proven MCA were recruited. Inclusion criteria were age 18-60, absence of major additional medical condition (hypertension, diabetes, cardiopathy, kidney or lung diseases), absence of pregnancy and presence of adequate birth control procedures during the duration of the study, absence of other chronic therapy, adhesion to the study. The study was double-blinded and placebo controlled. The subjects sustained a cycle ergometer exercise test during which maximal workload, maximal heart rate, maximal oxygen uptake (VO2max) were recorded, and were randomly allocated to placebo or active treatment (2.5 mg Ramipril daily).
After 12 weeks of treatment the patients repeated the exercise test. All subjects observed a one month wash-out period, and were then crossed to the opposite treatment. After 12 more weeks of treatment, all patients completed another exercise test. After each period of treatment the patients also completed the WHO-DAS II: a questionnaire meant to quantify the disability associated with their health condition (7).
Exercise test was an incremental cycloergometer (Seca Cardiotest, Hamburg, Germany) effort conducted until exhaustion. HR, Ventilation, VO2, VCO2 were continuously recorded with a portable telemetric system (Cosmed K4, Rome, Italy).
The t test for paired samples was used to assess inter-treatment changes in parametric variables, and the Wilcoxon test for repeated measures for changes in non-parametric variables. Significance was set at p < 0.05.
Results
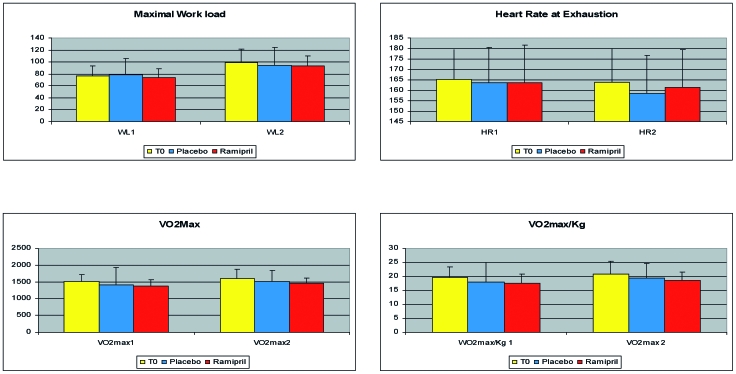
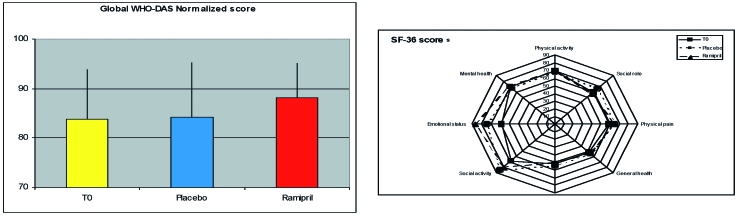
All patients completed the protocol, the treatment was well tolerated and no undesired effect was recorded. All patients exhibited the typical reduction, compared to expected values, of maximal workload and VO2max, with much higher HR/WL ratio. There were no differences in any of the parameters recorded during the exercise testing after any of the treatments (Fig. 1). A significant reduction of global disability score was consistently observed after treatment with Ramipril but not after placebo (Fig. 2). This was true both considering the global score (28.11 ± 16.7 after treatment with placebo, 34.02 ± 17.4 after treatment with Ramipril; p < 0.02), and the normalized score, i.e. the score projected toward the reference control population (84.2 ± 11.4 after treatment with placebo, 88.1 ± 7.2 after treatment with Ramipril, p < 0.05). At least 2 patients upon completion of the two arms of treatment were able to correctly guess the placebo from the active drug.
Figure 1.
Results of exercise testing at baseline, after placebo and after treatment with Ramipril.
Figure 2.
Results of Disability interview and Quality of life questionnaire.
Discussion
Our study explored the possibility that the pharmacological manipulation of ACE activity in MCA patients, by mimicking the condition associated with the ACE I allele, would alleviate the impairments of physiological parameters registered during and after the exercise test and reduce the chronic disability experienced by persons with MCA. While we could not demonstrate any effect of the proposed treatment on the physiological parameters of the cycloergometer testing, we observed a significant albeit small effect on objective disability as registered using the WHO-DAS II. The different results registered in the objective physiological parameters and the disability assessment may be explained considering the profound qualitative difference in the two sets of parameters. The exercise test explores the response to an acute standardised effort in a very short time (exhaustion is usually reached within less than 10 min of incremental effort), while the DAS records the patients’ experience of life in the previous 30 days. It is undeniable that the solidity of the parametric values obtained from the exercise test are much more palatable to the objective statistical perspective that a double blind randomised trial entails, but it is equally true that the significance for the patient quality of life is more adequately reflected by a longer term survey of activity limitation and restriction of participation in life situation. Therefore, while acknowledging that the lack of effect in the primary outcome measures does not allow the generalized and uncontrolled proposal of Ramipril use in MCA, the observation of the changes in the disability score suggests that more studies on a larger population are warranted, and that a careful evaluation of the appropriate outcome measure to be used is an important pre-requisite.
The study has several limitations, mainly linked to the small size of the tested population, and to the relatively small dose of drug employed and the short time of treatment. A recently published trial tested and demonstrated the ability of Ramipril to modify the risk of developing diabetes in subjects with impaired glucose tolerance test (8). The patients enrolled were over 3000, the time of treatment was 3 years, the mean dose employed was 10-15 mg. Given the excellent safety profile demonstrated by Ramipril, it may be worth considering a larger dose in future follow-up trials. The small but significant effect observed in a validated measure of disability should not be overlooked, and deserves a verification in a larger group of patients. In such a trial it may be interesting to compare the specificity and efficiency of QoL and disability measures over more objective exercise test parameters. This is particularly relevant in a disease like MCA where the main limitation is difficult to assess a-priori, and where the size of impairments observed in exercise testing not always correlate with disease severity. It will also be interesting to explore if the effects of an aerobic training, which was recently shown to improve functioning in MCA patients (9) are magnified by concurrent Ramipril treatment.
In conclusion our pilot study while not proving the effectiveness of daily 2.5 mg Ramipril treatment on physiological exercise parameters in MCA indicates its possible effect on reducing disability. A larger trial may be needed to definitely establish Ramipril place in treatment for MCA patients.
Acknowledgments
The financial support of Telethon Italy (Grant GUP03501 to A.M.) is acknowledged.
We thank all patients for their collaboration, and Sanofi Aventis for the generous gift of the drug and the placebo.
References
- 1.Di Mauro S, Tsujino S. Non-lysosomal glycogenoses. In: Engel AG, Franzini-Armstrong C, eds. Myology. 2nd ed. New York: McGraw-Hill, 1994. p. 1554-76. [Google Scholar]
- 2.Vissing J, Haller RG. The effect of oral sucrose on exercise tolerance in patients with McArdle’s disease. N Engl J Med 2003;349:2503-9. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery HE, Clarkson P, Barnard M, et al. Angiotensin-converting enzyme gene insertion/deletion polymorphism and response to physical training. Lancet 1999;353:541-5. [DOI] [PubMed] [Google Scholar]
- 4.Williams AG, Rayson MP, Jubb M, et al. The ACE gene and muscle performance. Nature 2000;403:614. [DOI] [PubMed] [Google Scholar]
- 5.Danser AH, Schalekamp MA, Bax WA, et al. Angiotensin converting enzyme in the human heart: effect of the deletion/insertion polymorphism. Circulation 1995;92:1387-8. [DOI] [PubMed] [Google Scholar]
- 6.Martinuzzi A, Sartori E, Fanin M, et al. Phenotype modulators in myophosphorylase deficiency. Ann Neurol 2003;53:497-502. [DOI] [PubMed] [Google Scholar]
- 7.WHO Mental Health Bulletin. A newsletter on noncommunicable diseases and mental health, WHO Mental Health Bulletin 2000, 6. [Google Scholar]
- 8.The DREAM Trial Investigators. Effect of Ramipril on the incidence of diabetes. N Engl J Med 2006;355:1-12. [DOI] [PubMed] [Google Scholar]
- 9.Haller RG, Wyrick P, Taivassalo T, Vissing J. Aerobic conditioning: an effective therapy in McArdle’s disease. Ann Neurol 2006;59:922-8. [DOI] [PubMed] [Google Scholar]