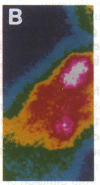
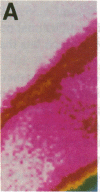
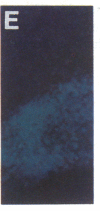
Abstract
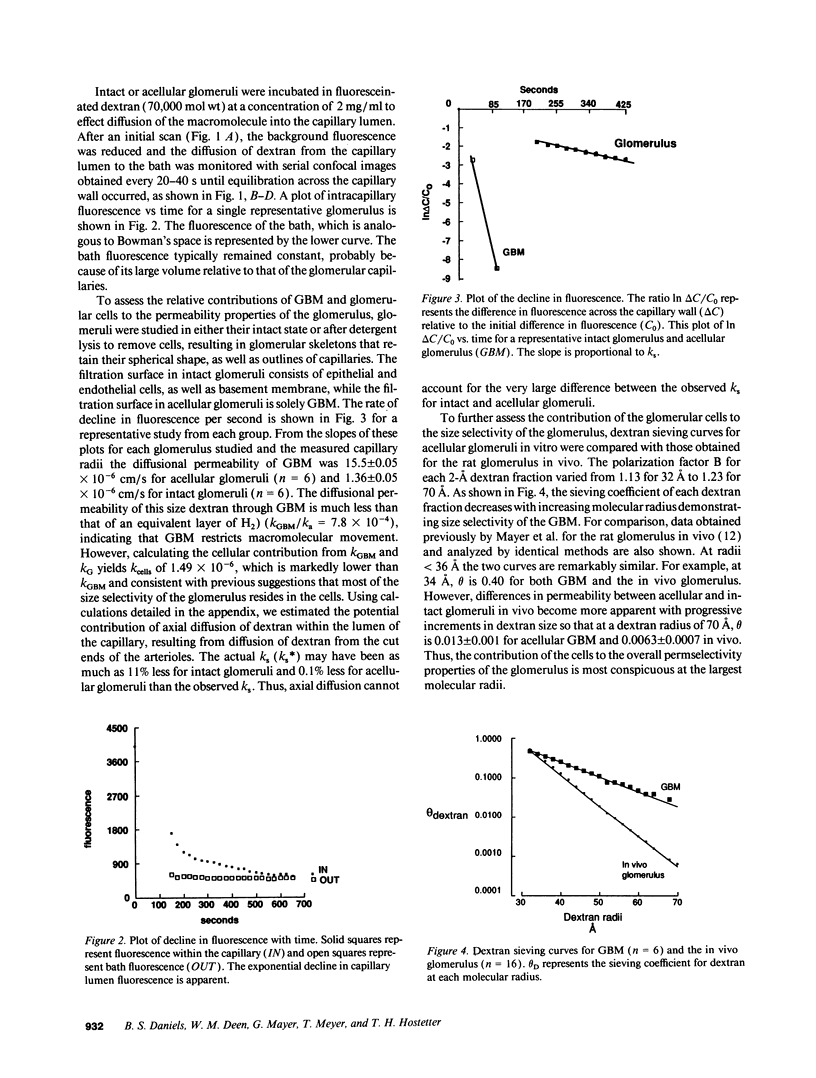
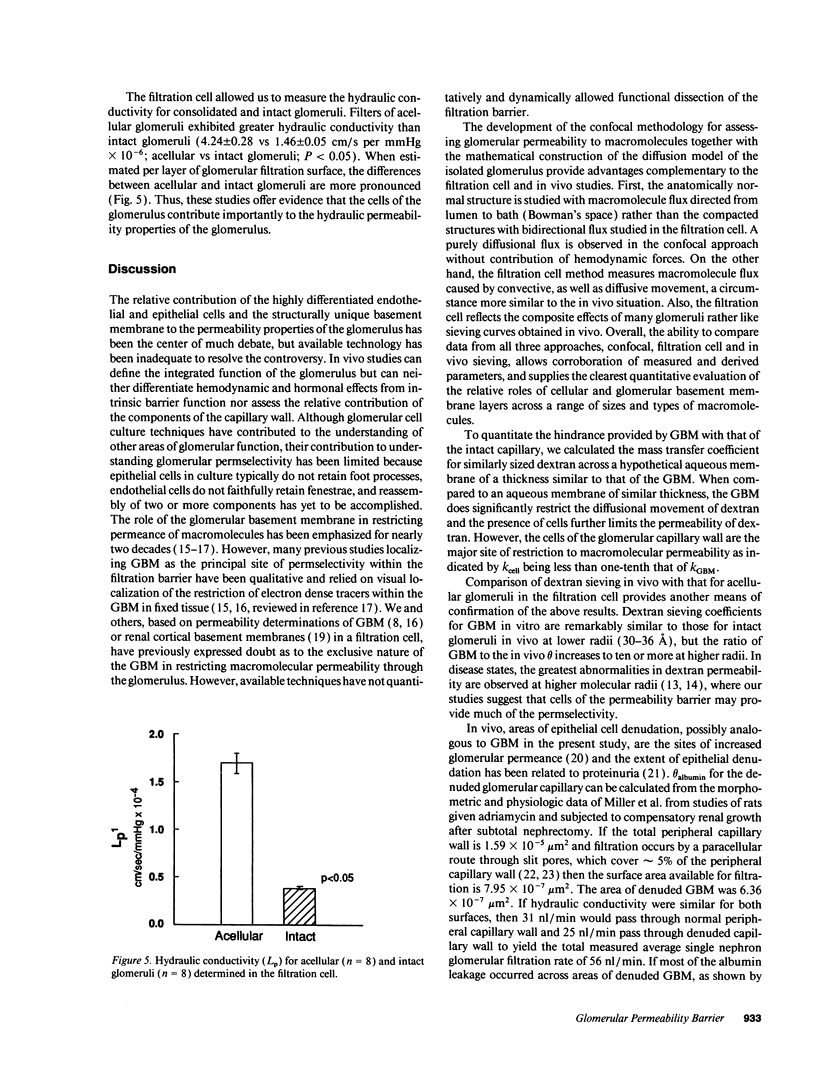
The formation of glomerular ultrafiltrate is dependent on the prevailing hemodynamic forces within the glomerular microcirculation and the intrinsic properties of the filtration barrier. However, direct assessment of the permeability barrier is difficult with most available techniques. We used confocal microscopy to image 1-micron thick optical cross-sections of isolated intact glomeruli and glomeruli denuded of cells and quantitated dextran (70,000 mol wt) diffusion from the capillary lumen. Dextran permeance was 11 times greater for the acellular filtration barrier than the intact peripheral capillary. Consideration of the basement membrane and cells as series resistors demonstrated that cells of the filtration barrier contribute 90% of the total resistance to macromolecular permeance. Using a different approach, dextran sieving coefficients for acellular glomeruli consolidated as a multilayer sheet in a filtration cell were similar to those for intact glomeruli in vivo at radii 30-36 A and approximately 50 times greater at a dextran radius of 60 A. The presence of cells significantly reduced hydraulic permeability determined on consolidated intact or acellular glomeruli in an ultrafiltration cell with 50 mmHg applied pressure. The glomerular basement membrane does restrict macromolecular permeability but cells are important determinants of the overall macromolecular and hydraulic permeability of the glomerulus.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. M., Coffey A. K. Cytoplasmic contractile elements in glomerular cells. Fed Proc. 1983 Nov;42(14):3046–3052. [PubMed] [Google Scholar]
- Bankston P. W., Milici A. J. A survey of the binding of polycationic ferritin in several fenestrated capillary beds: indication of heterogeneity in the luminal glycocalyx of fenestral diaphragms. Microvasc Res. 1983 Jul;26(1):36–48. doi: 10.1016/0026-2862(83)90053-5. [DOI] [PubMed] [Google Scholar]
- Bertolatus J. A., Klinzman D. Macromolecular sieving by glomerular basement membrane in vitro: effect of polycation or biochemical modifications. Microvasc Res. 1991 May;41(3):311–327. doi: 10.1016/0026-2862(91)90031-6. [DOI] [PubMed] [Google Scholar]
- Bray J., Robinson G. B. Influence of charge on filtration across renal basement membrane films in vitro. Kidney Int. 1984 Mar;25(3):527–533. doi: 10.1038/ki.1984.49. [DOI] [PubMed] [Google Scholar]
- Brenner B. M., Bohrer M. P., Baylis Ch, Deen W. M. Determinants of glomerular permselectivity: Insights derived from observations in vivo. Kidney Int. 1977 Oct;12(4):229–237. doi: 10.1038/ki.1977.107. [DOI] [PubMed] [Google Scholar]
- Daniels B. S., Hauser E. B., Deen W. M., Hostetter T. H. Glomerular basement membrane: in vitro studies of water and protein permeability. Am J Physiol. 1992 Jun;262(6 Pt 2):F919–F926. doi: 10.1152/ajprenal.1992.262.6.F919. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Bridges C. R., Brenner B. M. Biophysical basis of glomerular permselectivity. J Membr Biol. 1983;71(1-2):1–10. doi: 10.1007/BF01870670. [DOI] [PubMed] [Google Scholar]
- Deen W. M., Troy J. L., Robertson C. R., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. IV. Determination of the ultrafiltration coefficient. J Clin Invest. 1973 Jun;52(6):1500–1508. doi: 10.1172/JCI107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenckhahn D., Franke R. P. Ultrastructural organization of contractile and cytoskeletal proteins in glomerular podocytes of chicken, rat, and man. Lab Invest. 1988 Nov;59(5):673–682. [PubMed] [Google Scholar]
- Friedman S., Strober S., Field E. H., Silverman E., Myers B. D. Glomerular capillary wall function in human lupus nephritis. Am J Physiol. 1984 May;246(5 Pt 2):F580–F591. doi: 10.1152/ajprenal.1984.246.5.F580. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S. Biophysiology of glomerular filtration and proteinuria. Lab Invest. 1984 Jul;51(1):7–21. [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J. Altered glomerular permeability as a result of focal detachment of the visceral epithelium. Kidney Int. 1982 Apr;21(4):565–574. doi: 10.1038/ki.1982.63. [DOI] [PubMed] [Google Scholar]
- Latta H., Fligiel S. Mesangial fenestrations, sieving, filtration, and flow. Lab Invest. 1985 Jun;52(6):591–598. [PubMed] [Google Scholar]
- Lea P. J., Silverman M., Hegele R., Hollenberg M. J. Tridimensional ultrastructure of glomerular capillary endothelium revealed by high-resolution scanning electron microscopy. Microvasc Res. 1989 Nov;38(3):296–308. doi: 10.1016/0026-2862(89)90007-1. [DOI] [PubMed] [Google Scholar]
- Levick J. R., Smaje L. H. An analysis of the permeability of a fenestra. Microvasc Res. 1987 Mar;33(2):233–256. doi: 10.1016/0026-2862(87)90020-3. [DOI] [PubMed] [Google Scholar]
- Miller P. L., Scholey J. W., Rennke H. G., Meyer T. W. Glomerular hypertrophy aggravates epithelial cell injury in nephrotic rats. J Clin Invest. 1990 Apr;85(4):1119–1126. doi: 10.1172/JCI114543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers B. D., Nelson R. G., Williams G. W., Bennett P. H., Hardy S. A., Berg R. L., Loon N., Knowler W. C., Mitch W. E. Glomerular function in Pima Indians with noninsulin-dependent diabetes mellitus of recent onset. J Clin Invest. 1991 Aug;88(2):524–530. doi: 10.1172/JCI115335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. D., 3rd, Anderson S., Troy J. L., Brenner B. M., Deen W. H. Determination of glomerular size-selectivity in the normal rat with Ficoll. J Am Soc Nephrol. 1992 Aug;3(2):214–228. doi: 10.1681/ASN.V32214. [DOI] [PubMed] [Google Scholar]
- Pinnick R. V., Savin V. J. Filtration by superficial and deep glomeruli of normovolemic and volume-depleted rats. Am J Physiol. 1986 Jan;250(1 Pt 2):F86–F91. doi: 10.1152/ajprenal.1986.250.1.F86. [DOI] [PubMed] [Google Scholar]
- Remuzzi A., Deen W. M. Theoretical effects of network structure on glomerular filtration of macromolecules. Am J Physiol. 1989 Jul;257(1 Pt 2):F152–F158. doi: 10.1152/ajprenal.1989.257.1.F152. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R., Karnovsky M. J. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974 Feb;60(2):423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandling J. D., Myers B. D. Glomerular size-selectivity and microalbuminuria in early diabetic glomerular disease. Kidney Int. 1992 Apr;41(4):840–846. doi: 10.1038/ki.1992.129. [DOI] [PubMed] [Google Scholar]
- Shea S. M., Morrison A. B. A stereological study of the glomerular filter in the rat. Morphometry of the slit diaphragm and basement membrane. J Cell Biol. 1975 Nov;67(2PT1):436–443. doi: 10.1083/jcb.67.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker B. J., Blantz R. C. Effects of glomerular filtration dynamics on the glomerular permeability coefficient. Am J Physiol. 1981 Mar;240(3):F245–F254. doi: 10.1152/ajprenal.1981.240.3.F245. [DOI] [PubMed] [Google Scholar]