In 1980, one of us (SDM) was sent, almost contemporarily, two frozen muscle specimens, one from Dr. Moris J. Danon, then at the University of Chicago, the other from Dr. Shin J. Oh, then (and now) at the University of Alabama in Birmingham. The patients, who were described in the January, 1981 issue of Neurology (1), were remarkably similar both clinically and pathologically. Both were 16-year-old boys, with proximal weakness, cardiomyopathy, and mild mental retardation since childhood. Both had increased serum CK and, sadly, both died at age 17 of cardiac failure. Their muscle biopsies were virtually identical and showed vacuoles reacting intensely both with the periodic acid Schiff (PAS) and with the acid phosphatase reactions, indicating that they were glycogen-laden lysosomes. Ultrastructural studies showed abundant glycogen particles, most of which were contained within lysosomal sacs, either alone or together with cellular debris; in the latter case, the vacuoles had the appearance of autophagic vacuoles. Although heart and brain are typically not affected in the juvenile form of acid maltase deficiency (AMD, glycogen storage disease type II), the pathological features of the muscle biopsy were typical of AMD. The finding of normal or higher-than-normal activities of AM (acid α-glucosidase) both with the artificial fluorogenic substrate and with natural substrates (glycogen and maltose) came as a surprise and prompted us to publish these cases as “Lysosomal glycogen storage disease with normal acid maltase” (1). The one differential feature between the two patients was family history, which was non-contributory in one case, but positive and, in retrospect, very instructive in the other: his mother had died at age 37 of cardiomyopathy, three full siblings were weak and had abnormal EKGs, and one maternal half-sibling had had seizures and mental retardation and died suddenly at age 14.
As more cases were described in the years that followed, it became clear that this was a unique and characteristic entity invariably involving lysosomes but with inconsistent glycogen storage, and clear X-linked inheritance. In 1993, Di Mauro, Servidei, and Tsujino redefined this disorder as “Cardiomyopathy, mental retardation, and autophagic vacuolar myopathy” (2) and stated “… This syndrome clearly involves the lysosomes, but it may not affect glycogen metabolism specifically because the autophagic vacuoles contain various cytoplasmic degradation products besides glycogen, similar to those seen in chloroquine myopathy. The biochemical etiology is unknown”. They also noted that: “The mean age of death in women was 35 years, whereas the mean age of death in men was 16 years. This pattern suggests X-linked dominant transmission, but autosomal dominant inheritance cannot be ruled out” (2).
A feature that distinguished the vacuoles in Danon disease from typical lysosomes was that vacuolar membranes occasionally merged with indentations of the sarcolemma and stained with antibodies to sarcolemmal proteins, such as dystrophin and laminin (3, 4). Based on the shared lysosomal and plasma membrane features of the vacuoles and on the X-linked inheritance of the disease, in 2000, Nishino and coworkers sequenced a candidate gene on chromosome Xq24, LAMP-2, in ten unrelated patients with Danon disease, including one of the two boys described in the original paper. They found pathogenic mutations in all 10 patients and documented lack of LAMP-2 (lysosome-associated membrane protein 2) both by Western blot analysis and by immunohistochemistry (5). Their findings were bolstered by data from LAMP-2 knockout mice, which also showed accumulation of autophagic vacuoles in all tissues, but predominantly in cardiac and skeletal muscle (6). LAMP-2 is a 410 amino acid protein consisting of a small cytoplasmic tail with a lysosomal membrane targeting signal, a transmembrane domain, and a large intraluminal head. The LAMP-2 open reading frame consists of 9 exons: the first 8 exons and part of the ninth encode the luminal domain, and what is left of exon 9 encodes both the transmembrane and the cytoplasmic domains. Human exon 9 exists in two forms, 9a and 9b, which are alternatively spliced, producing two isoforms, LAMP-2a and LAMP-2b. Nishino et al. provided evidence that Danon disease is mostly due to defects of the LAMP-2b isoform, which is predominantly expressed in heart, muscle, and brain, the three “target tissues” in Danon disease (5).
The discovery of LAMP-2 deficiency in Danon disease ushered a new group of lysosomal diseases, those due to defects in lysosomal structural proteins rather than lysosomal enzymes. It also justified why Danon disease should not be included among the glycogenoses, glycogen being but one of many substrates that accumulate within abnormal autophagosomes.
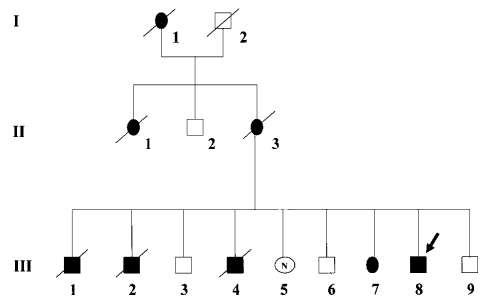
In 2002, Sugie et al. reviewed 38 genetically confirmed cases (20 men and 18 women) and provided a comprehensive description of the typical clinical and pathological features of Danon disease (7). To summarize their clinical findings (Table 1), the age of death was much earlier in men (19 years) than in women (40 years); cardiomyopathy was a defining feature, present in all patients; myopathy was almost invariably seen in men but only in about one third of women; and mental retardation was common in men but uncommon in women. Figure 1 illustrates the X-linked dominant inheritance in a French-Sardinian family (8).
Table 1. Clinical features of 48 patients with Danon disease. Modified from Sugie et al.(7), with permission.
| Feature | Male | Female |
| Subjects (#) | 20 | 18 |
| Age at death | 19 ± 6 (#7) | 40 ± 7 (#6) |
| Cardiac failure | 7/7 (100%) | 6/6 (100%) |
| Myopathy | 18/20 (90%) | 6/18 (33%) |
| Cardiomyopathy | 20/20 (100%) | 18/18 (100%) |
| Mental retardat. | 14/20 (70%) | 1/18 (6%) |
| Affected mother | 14/20 (70%) | 6/18 (33%) |
Figure 1.
Pedigree of a family with Danon disease. Filled symbols indicate affected patients. The pattern of inheritance is compatible with X-linked dominant transmission, with male patients affected earlier and dying younger than hemizygous females. (Reproduced from Echeniz-Laguna et al. (8), with permission).
As already mentioned, hypertrophic cardiomyopathy (HCM) is the clinical hallmark of the disease, often associated with Wolff-Parkinson-White (WPW) syndrome. To assess the frequency of Danon disease in children with HCM, Yang et al. (9) sequenced the LAMP-2 gene in blood DNA from 50 unselected patients and diagnosed Danon disease in two (4%), who also had WPW. They concluded that Danon disease may be under-diagnosed in the pediatric cardiology population. Another study sought to define the importance of glycogenoses (Danon disease was classified as such) as causes of HCM in patients not harboring more common mutations in sarcomere proteins (10). When the analysis was restricted to a cohort (24 patients) with increased left ventricular thickness and electrocardiographic evidence of ventricular preexcitation, four patients harbored mutations in LAMP-2 and seven had mutations in the PRKAG2 gene, encoding subunit γ2 of AMP-activated protein kinase (see the articles by Manfred Kilimann and Paolo Spirito in this issue). The Authors called attention to the fact that LAMP-2 deficiency may present as isolated HCM and should be suspected in young males with preexcitation.
Unusual or unrecognized manifestations of Danon disease include peripheral pigmentary retinopathy, lens changes, and abnormal electroretinogram in four affected women and near-complete loss of pigment in the retinal pigment epithelium in two men (11). The findings of this retrospective study suggest that patients with Danon disease should be subjected routinely to ophthalmological examinations. One patient had HCM, but neither muscle weakness nor mental retardation. He did, however, show exercise intolerance and persistent hyperCKemia, which was considered a clue to the correct diagnosis in patients with isolated HCM (12).
There is no specific therapy for Danon disease and the limitations of palliative medicine are illustrated by the early age of death in both affected boys and hemizygous women. However, cardiac transplantation is an important option and has been used successfully in several cases (7, 8).
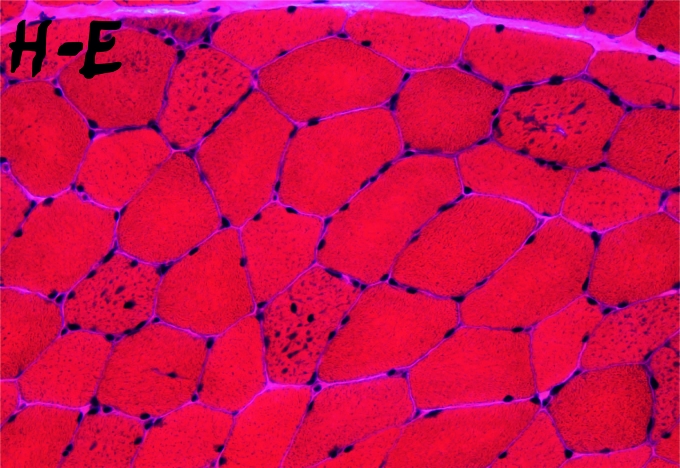
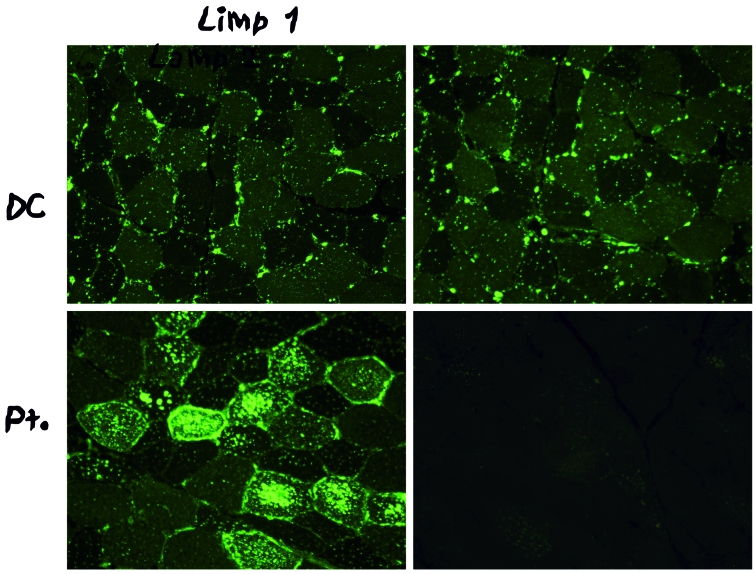
The muscle pathology of Danon disease shows basophilic inclusions by hematoxylin-eosin (Fig. 2A) corresponding to a profusion of vacuoles within type 1 fibers. As mentioned above, immunohistochemistry is diagnostic, as the vacuoles show no reaction whatsoever with antibodies to LAMP-2, contrasting with a hyperintense reaction with antibodies to a different lysosomal membrane protein, lysosomal integral membrane protein 1 (LIMP-1) (Fig. 3). Electron microscopy shows vacuoles filled with a variety of debris, including more or less abundant glycogen particles: for instance, glycogen is not very prominent in the vacuole shown in Figure 4.
Figure 2.
Muscle biopsy from a patient with Danon disease. H & E stain reveals small basophilic inclusions in several fibers.
Figure 3.
Ultrastructure of an autophagic vacuole in the muscle biopsy of a patient with Danon disease. The single membrane-bound vacuole contains various debris but remarkably little glycogen.
Figure 4.
Immunohistochemistry of muscle biopsies from a patient with Danon disease upper panel) and from a control (lower panel). Immunoreaction with antibodies to LIMP-1 (left panel) is more intense in the patient than in the control, whereas immunoreaction with antibodies to LAMP-2 (right panel) is completely lacking in the patient’s muscle.
The precise function of LAMP-2 is not known and the pathogenesis of the “autophagic vacuoles with sarcolemmal features” (AVSF) that are characteristic of Danon disease is likewise unclear (13). In the normal process of autophagy, “isolation membranes” envelop portions of the cytoplasm to form autophagosomes, which fuse with primary lysosomes (containing a battery of acid hydrolases), thus generating autolysosomes, where trapped cytoplasmic components are digested. In the parallel process of endocytosis, portions of the plasma membrane invaginate to form “early endosomes”, which, after fusing with primary lysosomes, become “late endosomes” and finally lysosomes. The formation of AVSF in Danon disease, as envisioned by Sugie et al. (13), hypothesizes as a primary (and earlier) phenomenon the formation of LIMP-1-positive autolysosomes, which are secondarily enveloped by membranes with sarcolemmal features. Alternatively, AVSF may be formed through a dysregulation of exocytosis (or endocytosis), leading to autophagic vacuoles with sarcolemmal features. However, what exactly this “dysregulation” entails remains to be clarified.
Irrespective of its precise pathogenesis, LAMP-2 deficiency illustrates the importance of rare diseases in helping us understand the normal functioning of the cell, or, to quote William Harvey, “Nature is nowhere accustomed more openly to display her secret mysteries than in cases where she shows tracings of her workings apart from the beaten paths; nor is there any better way to advance the proper practice of medicine than to give our minds to the discovery of the usual law of nature, by careful investigation of cases of rarer forms of disease”.
Acknowledgements
Part of this work was supported by grant from the Muscular Dystrophy Association.
References
- 1.Danon MJ, Oh SJ, Di Mauro S, et al. Lysosomal glycogen storage disease with normal acid maltase. Neurology 1981;31:51-7. [DOI] [PubMed] [Google Scholar]
- 2.Di Mauro S, Servidei S, Tsujino S. Disorders of carbohydrate metabolism: Glycogen storage diseases. In: Rosenberg RN, Prusiner SB, Di Mauro S, Barchi RL, eds. The Molecular and Genetic Basis of Neurological Disease. 2nd ed. Boston: Butterworth-Heinemann; 1997. p. 1067-97. [Google Scholar]
- 3.Muntoni F, Catani G, Mateddu A, et al. Familial cardiomyopathy, mental retardation, and myopathy associated with desmin-type intermediate filaments. Neuromusc Disord 1994;4:233-41. [DOI] [PubMed] [Google Scholar]
- 4.Murakami N, Goto Y, Itoh M, et al. Sarcolemmal indentation in cardiomyopathy with mental retardation and vacuolar myopathy. Neuromusc Disord 1995;5:149-55. [DOI] [PubMed] [Google Scholar]
- 5.Nishino I, Fu J, Tanji K, et al. Primary LAMP-2 deficiency causes X-linked vacuolar cardiomyopathy and myopathy (Danon disease). Nature 2000;406:906-10. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka Y, Guhde G, Suter A, et al. Accumulation of autophagic vacuoles and cardiomyopathy in LAMP-2-deficient mice. Nature 2000;406:902-6. [DOI] [PubMed] [Google Scholar]
- 7.Sugie K, Yamamoto A, Murayama K, et al. Clinicopathological features of genetically confirmed Danon disease. Neurology 2002;58:1773-8. [DOI] [PubMed] [Google Scholar]
- 8.Echaniz-Laguna A, Mohr M, Epailly E, et al. Novel LAMP-2 gene mutation and successful treatment with heart transplantation in large family with Danon disease. Muscle Nerve 2006;33:393-7. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, McMahon CJ, Smith LR, et al. Danon disease as an underrecognized cause of hypertrophic cardiomyopathy in children. Circulation 2005;112:1612-7. [DOI] [PubMed] [Google Scholar]
- 10.Arad M, Maron BJ, Gorham JM, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. New Engl J Med 2005;352:362-72. [DOI] [PubMed] [Google Scholar]
- 11.Prall FR, Drack A, Taylor M, et al. Ophthalmic manifestations of Danon disease. Ophthalmology 2006;113:1010-3. [DOI] [PubMed] [Google Scholar]
- 12.Musumeci O, Rodolico C, Nishino I, et al. Asymptomatic hyperCKemia in a case of Danon disease due to a missense mutation in LAMP-2 gene. Neuromusc Disord 2005;15:409-11. [DOI] [PubMed] [Google Scholar]
- 13.Sugie K, Noguchi S, Kozuka Y, et al. Autophagic vacuoles with sarcolemmal features delineate Danon disease and related myopathies. J Neuropath Exp Neurol 2005;64:513-22. [DOI] [PubMed] [Google Scholar]