Abstract
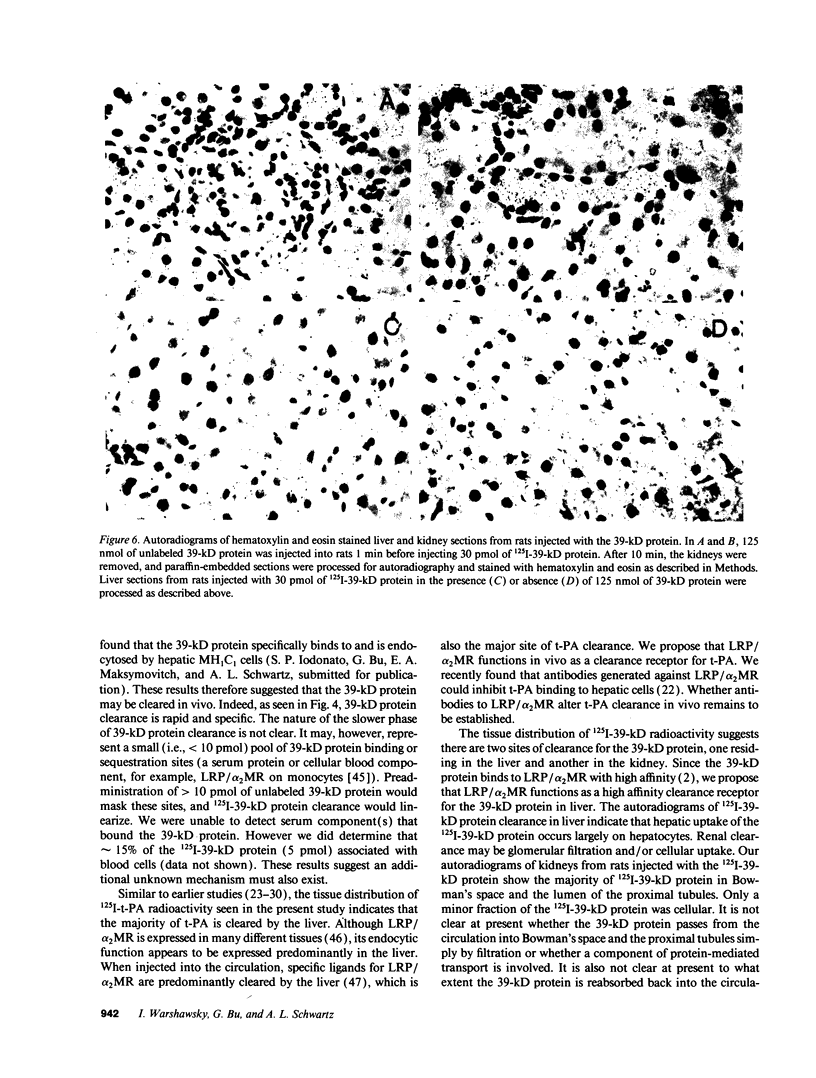
Tissue-type plasminogen activator (t-PA) is a plasma serine protease that catalyzes the initial and rate-limiting step in the fibrinolytic cascade. t-PA is widely used as a thrombolytic agent in the treatment of acute myocardial infarction. However, its use has been impaired by its rapid hepatic clearance from the circulation following intravenous administration. Studies with both rat hepatoma MH1C1 cells (G. Bu, S. Williams, D. K. Strickland, and A. L. Schwartz, 1992. Proc. Natl. Acad. Sci. USA. 89:7427-7431) and human hepatoma HepG2 cells (G. Bu, E. A. Maksymovitch, and A. L. Schwartz. 1993. J. Biol. Chem. 28:13002-13009) have shown that binding of t-PA to its clearance receptor, the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor, is inhibited by a 39-kD protein that copurifies with this receptor. Herein we investigated whether administration of purified recombinant 39-kD protein would alter t-PA clearance in vivo. We found that intravenous administration of purified 39-kD protein to rats prolonged the plasma half-life of 125I-t-PA from 1 min to approximately 5-6 min. The plasma half-life of t-PA enzymatic activity was similarly prolonged following intravenous administration of purified 39-kD protein. In addition we found that the 39-kD protein itself was rapidly cleared from the circulation in vivo. Clearance of 125I-39-kD protein was a biphasic process with half-lives of 30 s and 9 min and the liver was the primary organ of clearance. Preadministration of excess unlabeled 39-kD protein slowed 125I-39-kD protein clearance in rats in a dose-dependent manner, suggesting that specific clearance receptors were responsible for this process. Administration of increasing doses of unlabeled 39-kD protein along with labeled 39-kD protein resulted in a decrease in the amount of labeled 39-kD protein associating with the liver and a concomitant increase in the amount of labeled 39-kD protein associating with the kidneys, indicating two clearance mechanisms exist for the 39-kD protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angles-Cano E. A spectrophotometric solid-phase fibrin-tissue plasminogen activator activity assay (SOFIA-tPA) for high-fibrin-affinity tissue plasminogen activators. Anal Biochem. 1986 Mar;153(2):201–210. doi: 10.1016/0003-2697(86)90082-5. [DOI] [PubMed] [Google Scholar]
- Ashcom J. D., Tiller S. E., Dickerson K., Cravens J. L., Argraves W. S., Strickland D. K. The human alpha 2-macroglobulin receptor: identification of a 420-kD cell surface glycoprotein specific for the activated conformation of alpha 2-macroglobulin. J Cell Biol. 1990 Apr;110(4):1041–1048. doi: 10.1083/jcb.110.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwell G., Harford J. Carbohydrate-specific receptors of the liver. Annu Rev Biochem. 1982;51:531–554. doi: 10.1146/annurev.bi.51.070182.002531. [DOI] [PubMed] [Google Scholar]
- Beebe D. P., Aronson D. L. Turnover of human tissue plasminogen activator (tPA) in rabbits. Thromb Res. 1986 Sep 15;43(6):663–674. doi: 10.1016/0049-3848(86)90103-9. [DOI] [PubMed] [Google Scholar]
- Beisiegel U., Weber W., Bengtsson-Olivecrona G. Lipoprotein lipase enhances the binding of chylomicrons to low density lipoprotein receptor-related protein. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8342–8346. doi: 10.1073/pnas.88.19.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisiegel U., Weber W., Ihrke G., Herz J., Stanley K. K. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989 Sep 14;341(6238):162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- Bu G., Maksymovitch E. A., Schwartz A. L. Receptor-mediated endocytosis of tissue-type plasminogen activator by low density lipoprotein receptor-related protein on human hepatoma HepG2 cells. J Biol Chem. 1993 Jun 15;268(17):13002–13009. [PubMed] [Google Scholar]
- Bu G., Morton P. A., Schwartz A. L. Identification and partial characterization by chemical cross-linking of a binding protein for tissue-type plasminogen activator (t-PA) on rat hepatoma cells. A plasminogen activator inhibitor type 1-independent t-PA receptor. J Biol Chem. 1992 Aug 5;267(22):15595–15602. [PubMed] [Google Scholar]
- Bu G., Williams S., Strickland D. K., Schwartz A. L. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor is an hepatic receptor for tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7427–7431. doi: 10.1073/pnas.89.16.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeis J. J., van den Hoogen C. M., Jense D. Hepatic clearance of tissue-type plasminogen activator in rats. Thromb Haemost. 1985 Oct 30;54(3):661–664. [PubMed] [Google Scholar]
- Fuchs H. E., Berger H., Jr, Pizzo S. V. Catabolism of human tissue plasminogen activator in mice. Blood. 1985 Mar;65(3):539–544. [PubMed] [Google Scholar]
- Furukawa T., Ozawa M., Huang R. P., Muramatsu T. A heparin binding protein whose expression increases during differentiation of embryonal carcinoma cells to parietal endoderm cells: cDNA cloning and sequence analysis. J Biochem. 1990 Aug;108(2):297–302. doi: 10.1093/oxfordjournals.jbchem.a123197. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Herz J., Goldstein J. L., Strickland D. K., Ho Y. K., Brown M. S. 39-kDa protein modulates binding of ligands to low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. J Biol Chem. 1991 Nov 5;266(31):21232–21238. [PubMed] [Google Scholar]
- Herz J., Hamann U., Rogne S., Myklebost O., Gausepohl H., Stanley K. K. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988 Dec 20;7(13):4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Kowal R. C., Goldstein J. L., Brown M. S. Proteolytic processing of the 600 kd low density lipoprotein receptor-related protein (LRP) occurs in a trans-Golgi compartment. EMBO J. 1990 Jun;9(6):1769–1776. doi: 10.1002/j.1460-2075.1990.tb08301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz J., Kowal R. C., Ho Y. K., Brown M. S., Goldstein J. L. Low density lipoprotein receptor-related protein mediates endocytosis of monoclonal antibodies in cultured cells and rabbit liver. J Biol Chem. 1990 Dec 5;265(34):21355–21362. [PubMed] [Google Scholar]
- Jensen P. H., Moestrup S. K., Gliemann J. Purification of the human placental alpha 2-macroglobulin receptor. FEBS Lett. 1989 Sep 25;255(2):275–280. doi: 10.1016/0014-5793(89)81105-6. [DOI] [PubMed] [Google Scholar]
- Kanalas J. J., Makker S. P. Identification of the rat Heymann nephritis autoantigen (GP330) as a receptor site for plasminogen. J Biol Chem. 1991 Jun 15;266(17):10825–10829. [PubMed] [Google Scholar]
- Kerjaschki D., Farquhar M. G. Immunocytochemical localization of the Heymann nephritis antigen (GP330) in glomerular epithelial cells of normal Lewis rats. J Exp Med. 1983 Feb 1;157(2):667–686. doi: 10.1084/jem.157.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjaschki D., Miettinen A., Farquhar M. G. Initial events in the formation of immune deposits in passive Heymann nephritis. gp330-anti-gp330 immune complexes form in epithelial coated pits and rapidly become attached to the glomerular basement membrane. J Exp Med. 1987 Jul 1;166(1):109–128. doi: 10.1084/jem.166.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korninger C., Stassen J. M., Collen D. Turnover of human extrinsic (tissue-type) plasminogen activator in rabbits. Thromb Haemost. 1981 Oct;46(3):658–661. [PubMed] [Google Scholar]
- Kounnas M. Z., Argraves W. S., Strickland D. K. The 39-kDa receptor-associated protein interacts with two members of the low density lipoprotein receptor family, alpha 2-macroglobulin receptor and glycoprotein 330. J Biol Chem. 1992 Oct 15;267(29):21162–21166. [PubMed] [Google Scholar]
- Kounnas M. Z., Morris R. E., Thompson M. R., FitzGerald D. J., Strickland D. K., Saelinger C. B. The alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein binds and internalizes Pseudomonas exotoxin A. J Biol Chem. 1992 Jun 25;267(18):12420–12423. [PubMed] [Google Scholar]
- Kowal R. C., Herz J., Goldstein J. L., Esser V., Brown M. S. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal R. C., Herz J., Weisgraber K. H., Mahley R. W., Brown M. S., Goldstein J. L. Opposing effects of apolipoproteins E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J Biol Chem. 1990 Jun 25;265(18):10771–10779. [PubMed] [Google Scholar]
- Krause J., Seydel W., Heinzel G., Tanswell P. Different receptors mediate the hepatic catabolism of tissue-type plasminogen activator and urokinase. Biochem J. 1990 May 1;267(3):647–652. doi: 10.1042/bj2670647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper J., Otter M., Rijken D. C., van Berkel T. J. Characterization of the interaction in vivo of tissue-type plasminogen activator with liver cells. J Biol Chem. 1988 Dec 5;263(34):18220–18224. [PubMed] [Google Scholar]
- Lehrman M. A., Hill R. L. The binding of fucose-containing glycoproteins by hepatic lectins. Purification of a fucose-binding lectin from rat liver. J Biol Chem. 1986 Jun 5;261(16):7419–7425. [PubMed] [Google Scholar]
- Lennartz M. R., Cole F. S., Shepherd V. L., Wileman T. E., Stahl P. D. Isolation and characterization of a mannose-specific endocytosis receptor from human placenta. J Biol Chem. 1987 Jul 25;262(21):9942–9944. [PubMed] [Google Scholar]
- Lijnen H. R., Collen D. Strategies for the improvement of thrombolytic agents. Thromb Haemost. 1991 Jul 12;66(1):88–110. [PubMed] [Google Scholar]
- Lucore C. L., Fujii S., Sobel B. E. Dependence of fibrinolytic activity on the concentration of free rather than total tissue-type plasminogen activator in plasma after pharmacologic administration. Circulation. 1989 Jun;79(6):1204–1213. doi: 10.1161/01.cir.79.6.1204. [DOI] [PubMed] [Google Scholar]
- Lund H., Takahashi K., Hamilton R. L., Havel R. J. Lipoprotein binding and endosomal itinerary of the low density lipoprotein receptor-related protein in rat liver. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9318–9322. doi: 10.1073/pnas.86.23.9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moestrup S. K., Gliemann J. Analysis of ligand recognition by the purified alpha 2-macroglobulin receptor (low density lipoprotein receptor-related protein). Evidence that high affinity of alpha 2-macroglobulin-proteinase complex is achieved by binding to adjacent receptors. J Biol Chem. 1991 Jul 25;266(21):14011–14017. [PubMed] [Google Scholar]
- Moestrup S. K., Kaltoft K., Petersen C. M., Pedersen S., Gliemann J., Christensen E. I. Immunocytochemical identification of the human alpha 2-macroglobulin receptor in monocytes and fibroblasts: monoclonal antibodies define the receptor as a monocyte differentiation antigen. Exp Cell Res. 1990 Oct;190(2):195–203. doi: 10.1016/0014-4827(90)90185-d. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Nykjaer A., Petersen C. M., Møller B., Jensen P. H., Moestrup S. K., Holtet T. L., Etzerodt M., Thøgersen H. C., Munch M., Andreasen P. A. Purified alpha 2-macroglobulin receptor/LDL receptor-related protein binds urokinase.plasminogen activator inhibitor type-1 complex. Evidence that the alpha 2-macroglobulin receptor mediates cellular degradation of urokinase receptor-bound complexes. J Biol Chem. 1992 Jul 25;267(21):14543–14546. [PubMed] [Google Scholar]
- Orlando R. A., Kerjaschki D., Kurihara H., Biemesderfer D., Farquhar M. G. gp330 associates with a 44-kDa protein in the rat kidney to form the Heymann nephritis antigenic complex. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6698–6702. doi: 10.1073/pnas.89.15.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth K., Madison E. L., Gething M. J., Sambrook J. F., Herz J. Complexes of tissue-type plasminogen activator and its serpin inhibitor plasminogen-activator inhibitor type 1 are internalized by means of the low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7422–7426. doi: 10.1073/pnas.89.16.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychowdhury R., Niles J. L., McCluskey R. T., Smith J. A. Autoimmune target in Heymann nephritis is a glycoprotein with homology to the LDL receptor. Science. 1989 Jun 9;244(4909):1163–1165. doi: 10.1126/science.2786251. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Emeis J. J. Clearance of the heavy and light polypeptide chains of human tissue-type plasminogen activator in rats. Biochem J. 1986 Sep 15;238(3):643–646. doi: 10.1042/bj2380643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schwartz A. L. Receptor-mediated endocytosis. J Clin Invest. 1986 Mar;77(3):657–662. doi: 10.1172/JCI112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland D. K., Ashcom J. D., Williams S., Battey F., Behre E., McTigue K., Battey J. F., Argraves W. S. Primary structure of alpha 2-macroglobulin receptor-associated protein. Human homologue of a Heymann nephritis antigen. J Biol Chem. 1991 Jul 15;266(20):13364–13369. [PubMed] [Google Scholar]
- Verstraete M., Bounameaux H., de Cock F., Van de Werf F., Collen D. Pharmacokinetics and systemic fibrinogenolytic effects of recombinant human tissue-type plasminogen activator (rt-PA) in humans. J Pharmacol Exp Ther. 1985 Nov;235(2):506–512. [PubMed] [Google Scholar]
- Williams S. E., Ashcom J. D., Argraves W. S., Strickland D. K. A novel mechanism for controlling the activity of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein. Multiple regulatory sites for 39-kDa receptor-associated protein. J Biol Chem. 1992 May 5;267(13):9035–9040. [PubMed] [Google Scholar]