Summary
Research advances over the last 30 years have shown that key transmembrane proteins at the neuromuscular junction are vulnerable to antibody-mediated autoimmune attack These targets are acetylcholine receptors (AChRs) and muscle specific kinase (MuSK) in myasthenia gravis, voltage-gated calcium channels (VGCCs) in the Lambert-Eaton myasthenic syndrome (LEMS), and voltage-gated potassium channels (VGKCs) in neuromyotonia. In parallel with these immunological advances, mutations identified in genes encoding pre-synaptic, synaptic and post-synaptic proteins that are crucial to neuromuscular transmission have revealed a similar diversity of congenital myasthenic syndromes (CMS). These discoveries have had a major impact on diagnosis and management.
Keywords: Myasthenia gravis, myasthenic syndromes, congenital myasthenia
Introduction
The Mediterranean Society of Myology witnesses at its meetings the many advances that have occurred in the understanding of muscle disease. These advances have been paralleled by the remarkable diversity of disorders of the neuromuscular junction that has emerged over recent years. Crucial to these developments have been the basic science discoveries involving neurophysiology, biophysics, immunology and molecular biology. Crucial too have been the insights provided by the actions of predator neurotoxins such as α-bungarotoxin (BuTx), α-dendrotoxin (Dtx) and ω-conotoxin (CgTx). It is not the place here to describe the well-known and often devastating clinical consequences of the actions of these toxins at the neuromuscular junction. But the availability of these purified toxins, together with advances in molecular genetics, have played a key role in revealing the pathological processes that underlie human disorders of neuromuscular transmission, which are the focus of this brief historical review.
Myasthenia Gravis (MG)
For many years it had been recognized that MG could exist in several forms, namely as a congenital or familial condition, as an acquired disorder affecting individuals of all ages from about one year onwards, and as a transient disorder affecting babies born to MG mothers. This last observation was one of the clinical clues that suggested to Iain Simpson that MG might be an autoimmune disease in which antibodies were directed to the ‘end-plate protein’. This proved to be an accurate prediction, although it took more than a decade before his hypothesis was validated. This was achieved by the discovery that rabbits immunised with electric organ acetylcholine receptors (AChRs), purified using α-BuTx, developed an MG-like disorder, had circulating AChR antibodies and responded to acetylcholinesterase inhibitors (1).
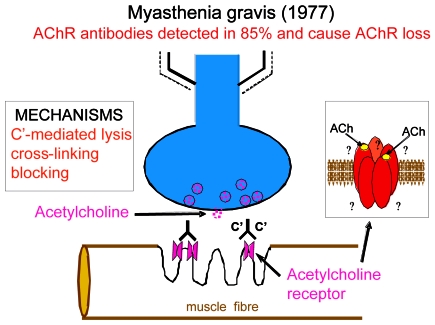
AChR antibodies were then detected in human MG sera (2, 3). Initial uncertainty as to whether the antibodies were protective rather than disease-causing was resolved by the passive transfer of the disorder to mice by injection of MG plasma or immunoglobulins (4) and by the demonstration that plasma exchange (plasmapheresis) that reduces the level of circulating antibodies could induce a striking clinical improvement lasting several weeks (5, 6). Pathological studies showed that AChR loss was caused to varying degrees by complement-mediated lysis, cross-linking and consequent down-regulation, and blocking of the ACh binding site. Figure 1 is a cartoon of the neuromuscular junction based on what was known in 1977. The inset illustrates the five subunit structure of the adult AChR, the ε-subunit being located between the two α-subunits. This subunit replaces the γ subunit at about 33 week’s gestation in man.
Figure 1.
Myasthenia gravis (1977).
It soon became clear that AChR antibodies could only be detected in about 85% of patients with generalised MG. Nevertheless, clinical evidence strongly suggested the presence of pathological antibodies in these ‘seronegative’ patients. Plasma exchange improved the patient’s strength, neonatal MG could affect babies born to mothers seronegative for AChR antibodies and seronegative plasmas or IgG injected into mice could induce an MG-like disorder of neuromuscular transmission (7, 8). Studies in the last few years have implicated antibodies to Muscle Specific Kinase (MuSK) in many of these patients
Antibodies to Muscle Specific Kinase (MuSK)
MuSK is a postsynaptic transmembrane protein at the neuromuscular junction with an extracellular Ig-like domain. It plays a key role during muscle development. Agrin, released by in-growing motor nerve terminals, leads via an intermediary protein to the activation of MuSK and subsequently to phosphorylation of rapsyn, thereby triggering AChR clustering and the formation of a neuromuscular junction. Hoch et al. (9) using an ELISA assay and rat MuSK as antigen detected MuSK antibodies in many patients with MG who were seronegative for AChR antibodies, confirmed by Scuderi et al. (10) using an alternative experimental approach. MuSK antibodies were not detected in healthy controls, in other neurological disorders or in MG patients with restricted ocular MG or whose serum harboured AChR antibodies. Further studies showed that MuSK antibodies could be detected in about 40% of MG patients (‘Musk MG’) who were seronegative for AChR antibodies (11).
The extracellular domain of MuSK can be ‘seen’ by circulating antibodies. Passive immunisation of mice with IgG (7) that was subsequently found to be MuSK positive, and active immunisation of rabbits with rat MuSK (12) can both induce a myasthenic disorder, suggesting that MuSK antibodies may be the effector mechanism in those harbouring them. Babies born to mothers with Musk MG can exhibit transient myasthenia with a similar distribution of muscle weakness.
Clinically, MuSK MG patients show some characteristic features that help to distinguish them from AChR MG. Bulbar weakness and sometimes respiratory weakness are often dominant, and tongue wasting may be present (11, 13– 15). Onset can be at any age from about one year onwards. Females are much more often affected than males (4:1). Thymoma does not seem to associate with MuSK MG and studies of the thymus show that the changes do not differ significantly from healthy thymus, in striking contrast to the changes of hyperplasia seen in early onset MG (16, 17). The response to anticholinesterase medication (e.g. pyridostigmine) is often weak and sometimes absent. Electromyography shows typical changes of MG.
Immunopathogenesis update
Table 1 is an update of the immunopathogenesis of generalised MG. The prevalence figures are approximations. Recent evidence shows that the prevalence of late onset MG is progressively increasing in contrast to early onset disease where the prevalence appears stable (18, 19).
Table 1. Immunopathogeneis of generalised MG.
| Antibody | AChR | ‘Seronegative’ | Musk | ||
| Prevalence ~% | 10 | 30 | 45 | 8 | 7 |
| Thymus | Thymoma | Hyperplasia | Involuted | Mild hyperplasia | Normal |
| Onset age ~yrs | Any | < 45 | > 45 | Any | > 1 |
| Gender (M:F) | 1:1 | 1:3 | 2:1 | ? 1:2 | 1:4 |
Many of those cases shown in the Table as ‘seronegative’ for both AChR and MuSK antibodies may in fact have low affinity AChR antibodies. Consistent with that is the observation that the thymus in these patients can show mild thymic hyperplasia (16). Recognizing these different subgroups is important because they influence the response to treatment.
Neonatal MG
Neonatal MG affects about 1 in 8 of babies born to MG mothers. There may be fetal akinesia, and evidence of weakness at birth that responds to anticholinesterase medication. It is caused by the placental transfer of maternal AChR antibodies and is typically transient, recovering completely within 3 months.
In rare cases, however, neonatal MG can associate with Arthrogryposis Multiplex Congenita, oesophageal atresia, hydramnios and fetal death (20, 21). This appears to occur when the maternal AChR antibodies target the fetal form of AChR (α2, β, γ, δ). The fetal form persists until about the 33rd week of gestation when the γ subunit is replaced by an ε-subunit (see Fig. 1 inset). In exceptional cases, the mother herself may exhibit no manifestations of MG, presumably because her antibodies are mainly or exclusively targeting fetal AChR, thus sparing her own ‘adult’ AChRs.
Neuromyotonia, limbic encephalopathy, thymoma and MG
It has been known for many years that thymoma can associate with other autoimmune diseases besides MG (for example, red cell aplasia). Neuromyotonia (NMT) or Isaacs’ syndrome has also been observed to associate with thymoma or MG. NMT is characterized by hyperexcitability of motor nerves, causing myokymia and fasciculations, and characteristic EMG changes of doublet or multiplet motor unit (‘myokymic’) discharges, spontaneous neuromyotonic burst discharges and after discharges (22). The spontaneous discharges continue during sleep and general anaesthesia. Patients may also experience sensory symptoms that appear to arise from a similar hyperexcitability of sensory nerves. The autoimmune associations with neuromyotonia suggested that it too might have an autoimmune origin (22).
Further studies showed that the clinical and electromyographic abnormalities improved following plasma exchange (23), implicating a serum antibody. Neuronal voltage-gated potassium channels (VGKCs) were identified as a likely target since their down-regulation would prolong depolarisation, increasing the quantal release of transmitter and causing repetitive firing. Injection of NMT IgG into mice reproduced the electrophysiological abnormalities, and rat dorsal root ganglion cells exposed to NMT IgG in culture showed repetitive firing following a step depolarisation, in contrast to controls (24). An assay based on the radio-labelled snake toxin 125I-α-dendrotoxin detected VGKC antibodies in about 40% of patients with NMT (25). Moreover, immunostaining of peripheral nerves shows that NMT IgG labels VGKCs at juxta-paranodes, co-extensive with experinmentally raised rabbit antisera to VGKCs (26). Interestingly, these antibodies can be detected in some patients with the cramp-fasciculation syndrome, indicating that NMT and CFS lie on the same spectrum (25). Thus antibody-mediated autoimmunitry needs to be added to the known causes of peripheral nerve hyperexcitability (Table 2).
Table 2. Principal causes of peripheral nerve hyperexcitability.
|
An unexpected development has been the recognition that VGKC antibodies are implicated in limbic encephalopathy, and also in Morvan’s syndrome that had long been an unexplained disorder (27, 28). Buckley et al. (29) described a patient with thymoma and long-standing AChR antibody positive MG who developed limbic encephalopathy late in her illness. At this point, for the first time, VGKC antibodies became detectable, declining in parallel with recovery of her encephalitis in response to immunosuppressive therapy. The likely involvement of VGKC antibodies in limbic encephalitis and Morvan’s syndrome is now increasingly recognized and has been reviewed elsewhere (30) although the issue of whether the antibodies are the effector mechanism in these conditions is unresolved.
Lambert-Eaton Myasthenic Syndrome (LEMS)
The myasthenic disorder that can associate with lung cancer was first characterised electromyographically by Lambert and colleagues (31). With Elmqvist (32), Lambert later showed that LEMS was a presynaptic disorder in which the quantal release of transmitter was strikingly reduced. In man, 30 or more quanta are released by each nerve impulse, but in LEMS the number may be fewer than 10. Clinically these patients have proximal weakness that first affects their gait, augmentation of strength during the first few seconds of a maximal effort, and post-tetanic potentiation. Importantly, they may also have autonomic disturbances: dry mouth constipation and erectile failure in males.
The commonest underlying tumour is the smoking-associated small cell lung cancer (SCLC). LEMS can precede the appearance of the underlying SCLC by at least 2 years and occasionally for as long as 5 years (33). It soon became clear that not all patients with LEMS were harbouring a neoplasm. Many patients followed for 5 years or more and who were non-smokers failed to develop a tumour. Moreover these ‘non-paraneoplastic’ patients had a markedly increased association with other autoimmune diseases, notably thyroid disease and vitiligo. This non-paraneoplastic form of LEMS can affect children and presents with the features of a myopathy including a pronounced lumbar lordosis. Enquiry may reveal autonomic symptoms that provide a clue to the real nature of the disorder.
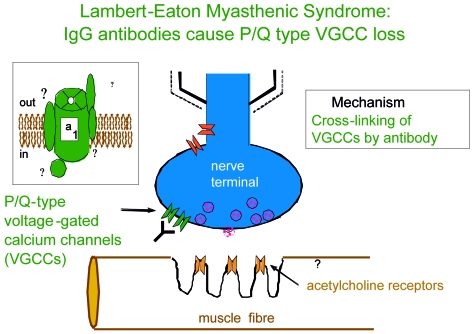
The association with other autoimmune disorders suggested a possible autoimmune pathogenesis, confirmed by the improvement that followed plasmapheresis (34) and the successful passive transfer of the pathophysiological (35) and morphological changes (36) of LEMS to mice. Antibodies to P/Q-type voltage-gated calcium channels (VGCCs) were detected in over 90% of patients (37), whether or nor they had an associated cancer, in a radioimmunoassay using the iodinated cone snail toxin (125I-ω-conotoxin) that is specific for this channel sub-type (Fig. 2). Moreover these antibodies block post-ganglionic cholinergic and adrenergic VGCCs, providing an explanation for the observed autonomic changes (38).
Figure 2.
Lambert-Eaton Myasthenic Syndrome: IgG antibodies cause P/Q type VGCC loss.
VGCCs that are known to be expressed by SCLC cells appear to be the provoking factor in paraneoplastic LEMS because LEMS IgG significantly reduces K+ stimulated Ca++ influx into cultured SCLC cells (39). Interestingly, non-paraneoplastic LEMS IgG acts similarly but the triggering factor for the disorder in these patients in unknown.
Congenital Myasthenic Syndromes (CMS)
Although Congenital Myasthenic Syndromes are the rarest of the myasthenic disorders affecting man (estimated at up to 3 per million), they have nevertheless shown the greatest emerging diversity. They arise from mutations affecting crucial presynaptic, synaptic or post-synaptic proteins at the neuromuscular junction on which synaptic formation and function depend. The majority are recessively inherited. They have been the subject of recent reviews (40, 41).
Although many of these disorders can present as fetal akinesia or in the perinatal period with hypotonia, feeding or breathing difficulties, ptosis, ophthalmoplegia, and sometimes arthrogryposis, some only become first evident during adolescence or even adult life, for example the Slow Channel syndrome (42), thus making the diagnosis especially challenging. Others have a limb-girdle pattern that can be mistaken for a myopathy.
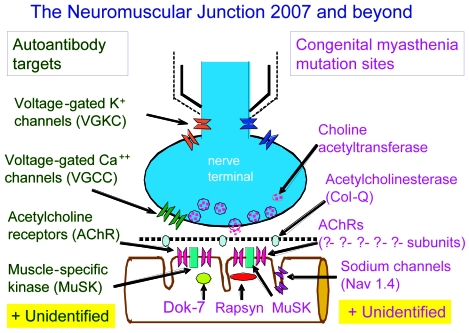
Figure 3 shows the proteins that are currently known to be mutated in CMS. However, as the figure makes clear, some gene targets remain to be identified.
Figure 3.
The Neuromuscular Junction 2007 and beyond.
The commonest site for mutations in CMS is the ε-subunit of the AChR, giving rise to a congenital AChR deficiency syndrome in most instances. Deletions or single nucleotide substitutions typically result in complete loss of function of the subunit. However, in man the fetal γ-subunit has the capacity to substitute for the ε-subunit, though resulting in less efficient neuromuscular transmission. Mutations in rapsyn (43), which plays a key role in AChR clustering during development, are another relatively frequent cause of CMS and can occur as an early-onset or late-onset phenotype (Table 3) (44).
Table 3. Distinct phenotypes associated with Rapsyn mutations.
| Early Onset (85%) |
|
|
Late Onset (15%)
|
| Both groups |
|
An interesting recent discovery has been the demonstration of mutations in Dok-7 (45), a post-synaptic protein (Fig. 3) that, like MuSK, is crucial for AChR clustering (46). These result in a CMS with a limb-girdle pattern of weakness. The clinical features, summarised in Table 4, have recently been described (47–49).
Table 4. Clinical features of Dok-7 neuromuscular junction synaptopathy.
|
The principal differential diagnosis for Dok-7 mutations is Limb-girdle CMS with tubular aggregates in which Dok-7 mutations were not found (48). A distinguishing clinical feature in this disorder is the strongly positive response to acetylcholinesterase inhibitors (Edrophonium, pyridostigmine) in contrast to the transient or absent response in Dok-7 synaptopathy.
Mutations are not always identified in patients thought to have CMS on clinical grounds. Thus although at least 10 genes have now been identified as sites of mutations that can cause CMS, there are others yet to be identified.
References
- 1.Patrick J, Lindstrom J. Autoimmune response to acetylcholine receptor. Science 1973;180:871-2. [DOI] [PubMed] [Google Scholar]
- 2.Lindstrom JM, Seybold ME, Lennon VA, et al. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology 1976;26:1054-9. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A, Newsom DJ. Anti-acetylcholine receptor antibodies. J Neurol Neurosurg Psychiatry 1980;43:590-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyka KV, Drachman DB, Griffin DE, et al. Myasthenia gravis. Study of humoral immune mechanisms by passive transfer to mice. N Engl J Med 1977;296:125-31. [DOI] [PubMed] [Google Scholar]
- 5.Pinching AJ, Peters DK, Newsom-Davis J. Remission of myasthenia gravis following plasma-exchange. Lancet 1976;2:1373-6. [DOI] [PubMed] [Google Scholar]
- 6.Newsom-Davis J, Pinching AJ, Vincent A, Wilson SG. Function of circulating antibody to acetylcholine receptor in myasthenia gravis: investigation by plasma exchange. Neurology 1978;28:266-72. [DOI] [PubMed] [Google Scholar]
- 7.Burges J, Vincent A, Molenaar PC, et al. Passive transfer of seronegative myasthenia gravis to mice. Muscle Nerve 1994;17:1393-400. [DOI] [PubMed] [Google Scholar]
- 8.Mossman S, Vincent A, Newsom-Davis J. Myasthenia gravis without acetylcholine-receptor antibody: a distinct disease entity. Lancet 1986;1:116-9. [DOI] [PubMed] [Google Scholar]
- 9.Hoch W, McConville J, Helms S, et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001;7:365-8. [DOI] [PubMed] [Google Scholar]
- 10.Scuderi F, Marino M, Colonna L, et al. Anti-p110 autoantibodies identify a subtype of “seronegative” myasthenia gravis with prominent oculobulbar involvement. Lab Invest 2002;82:1139-46. [DOI] [PubMed] [Google Scholar]
- 11.Vincent A, Bowen J, Newsom-Davis J, McConville J. Seronegative generalised myasthenia gravis: clinical features, antibodies, and their targets. Lancet Neurol 2003;2:99-106. [DOI] [PubMed] [Google Scholar]
- 12.Shigemoto K, Kubo S, Maruyama N, et al. Induction of myasthenia by immunization against muscle-specific kinase. J Clin Invest 2006;116:1016-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanders DB, El-Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology 2003;60:1978-80. [DOI] [PubMed] [Google Scholar]
- 14.Evoli A, Tonali PA, Padua L, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003;126:2304-11. [DOI] [PubMed] [Google Scholar]
- 15.Farrugia ME, Robson MD, Clover L, et al. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain 2006;129:1481-92. [DOI] [PubMed] [Google Scholar]
- 16.Leite MI, Strobel P, Jones M, et al. Fewer thymic changes in MuSK antibody-positive than in MuSK antibody-negative MG. Ann Neurol 2005;57:444-8. [DOI] [PubMed] [Google Scholar]
- 17.Lauriola L, Ranelletti F, Maggiano N, et al. Thymus changes in anti-MuSK-positive and -negative myasthenia gravis. Neurology 2005;64:536-8. [DOI] [PubMed] [Google Scholar]
- 18.Somnier FE. Increasing incidence of late-onset anti-AChR antibody-seropositive myasthenia gravis. Neurology 2005;65:928-30. [DOI] [PubMed] [Google Scholar]
- 19.Vincent A, Clover L, Buckley C, Grimley EJ, Rothwell PM. Evidence of underdiagnosis of myasthenia gravis in older people. J Neurol Neurosurg Psychiatry 2003;74:1105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent A, Newland C, Brueton L, et al. Arthrogryposis multiplex congenita with maternal autoantibodies specific for a fetal antigen. Lancet 1995;346:24-5. [DOI] [PubMed] [Google Scholar]
- 21.Riemersma S, Vincent A, Beeson D, et al. Association of arthrogryposis multiplex congenita with maternal antibodies inhibiting fetal acetylcholine receptor function. J Clin Invest 1996;98:2358-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Newsom-Davis J, Mills KR. Immunological associations of acquired neuromyotonia (Isaacs’ syndrome). Report of five cases and literature review. Brain 1993;116:453-69. [DOI] [PubMed] [Google Scholar]
- 23.Sinha S, Newsom-Davis J, Mills K, et al. Autoimmune aetiology for acquired neuromyotonia (Isaacs’ syndrome). Lancet 1991;338:75-7. [DOI] [PubMed] [Google Scholar]
- 24.Shillito P, Molenaar PC, Vincent A, et al. Acquired neuromyotonia: evidence for autoantibodies directed against K+ channels of peripheral nerves. Ann Neurol 1995;38:714-22. [DOI] [PubMed] [Google Scholar]
- 25.Hart IK, Maddison P, Newsom-Davis J, Vincent A, Mills KR. Phenotypic variants of autoimmune peripheral nerve hyperexcitability. Brain 2002;125:1887-95. [DOI] [PubMed] [Google Scholar]
- 26.Kleopa KA, Elman LB, Lang B, et al. Neuromyotonia and limbic encephalitis sera target mature Shaker-type K+ channels: subunit specificity correlates with clinical manifestations. Brain 2006;129:1570-84. [DOI] [PubMed] [Google Scholar]
- 27.Serratrice G, Azulay JP. What is left of Morvan’s fibrillary chorea? Rev Neurol (Paris) 1994;150:257-65. [PubMed] [Google Scholar]
- 28.Liguori R, Vincent A, Clover L, et al. Morvan’s syndrome: peripheral and central nervous system and cardiac involvement with antibodies to voltage-gated potassium channels. Brain 2001;124:2417-26. [DOI] [PubMed] [Google Scholar]
- 29.Buckley C, Oger J, Clover L, et al. Potassium channel antibodies in two patients with reversible limbic encephalitis. Ann Neurol 2001;50:73-8. [DOI] [PubMed] [Google Scholar]
- 30.Serratrice G, Azulay JP, Serratrice J, Attarian S. From Morvan’s disease to potassium channelopathies. Bull Acad Natl Med 2004;188:233-44. [PubMed] [Google Scholar]
- 31.Eaton LM, Lambert EH. Electromyography and electric stimulation of nerves in diseases of motor unit; observations on myasthenic syndrome associated with malignant tumours. J Am Med Assoc 1957;163:1117-24. [DOI] [PubMed] [Google Scholar]
- 32.Lambert Eh, Elmqvist D. Quantal components of end-plate potentials in the myasthenic syndrome. Ann N Y Acad Sci 1971;183:183-99. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill JH, Murray NM, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome. A review of 50 cases. Brain 1988;111:577-96. [DOI] [PubMed] [Google Scholar]
- 34.Newsom-Davis J, Murray N, Wray D, et al. Lambert-Eaton myasthenic syndrome: electrophysiological evidence for a humoral factor. Muscle Nerve 1982;5:S17-S20. [PubMed] [Google Scholar]
- 35.Lang B, Newsom-Davis J, Wray D, Vincent A, Murray N. Autoimmune aetiology for myasthenic (Eaton-Lambert) syndrome. Lancet 1981;2:224-6. [DOI] [PubMed] [Google Scholar]
- 36.Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci USA 1983;80:7636-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motomura M, Johnston I, Lang B, Vincent A, Newsom-Davis J. An improved diagnostic assay for Lambert-Eaton myasthenic syndrome. J Neurol Neurosurg Psychiatry 1995;58:85-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waterman SA, Lang B, Newsom-Davis J. Effect of Lambert-Eaton myasthenic syndrome antibodies on autonomic neurons in the mouse. Ann Neurol 1997;42:147-56. [DOI] [PubMed] [Google Scholar]
- 39.Roberts A, Perera S, Lang B, Vincent A, Newsom-Davis J. Paraneoplastic myasthenic syndrome IgG inhibits 45Ca2+ flux in a human small cell carcinoma line. Nature 1985;317:737-9. [DOI] [PubMed] [Google Scholar]
- 40.Engel AG, Sine SM. Current understanding of congenital myasthenic syndromes. Curr Opin Pharmacol 2005;5:308-21. [DOI] [PubMed] [Google Scholar]
- 41.Beeson D, Hantai D, Lochmuller H, Engel AG. 126th International Workshop: congenital myasthenic syndromes, 24-26 September 2004, Naarden, the Netherlands. Neuromuscul Disord 2005;15:498-512. [DOI] [PubMed] [Google Scholar]
- 42.Engel AG, Lambert EH, Mulder DM, et al. A newly recognized congenital myasthenic syndrome attributed to a prolonged open time of the acetylcholine-induced ion channel. Ann Neurol 1982;11:553-69. [DOI] [PubMed] [Google Scholar]
- 43.Ohno K, Engel AG, Shen XM, et al. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am J Hum Genet 2002;70:875-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke G, Cossins J, Maxwell S, et al. Rapsyn mutations in hereditary myasthenia: distinct early- and late-onset phenotypes. Neurology 2003;61:826-8. [DOI] [PubMed] [Google Scholar]
- 45.Beeson D, Higuchi O, Palace J, et al. Dok-7 mutations underlie a neuromuscular junction synaptopathy. Science 2006;313:1975-8. [DOI] [PubMed] [Google Scholar]
- 46.Okada K, Inoue A, Okada M, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 2006;312:1802-5. [DOI] [PubMed] [Google Scholar]
- 47.Slater CR, Fawcett PR, Walls TJ, et al. Pre- and post-synaptic abnormalities associated with impaired neuromuscular transmission in a group of patients with ‘limb-girdle myasthenia’. Brain 2006;129:2061-76. [DOI] [PubMed] [Google Scholar]
- 48.Palace J, Lashley D, Newsom-Davis J, et al. Clinical features of the DOK7 neuromuscular junction synaptopathy. Brain 2007. [DOI] [PubMed] [Google Scholar]
- 49.Muller JS, Herczegfalvi A, Vilchez JJ, et al. Phenotypical spectrum of DOK7 mutations in congenital myasthenic syndromes. Brain 2007. [DOI] [PubMed] [Google Scholar]