Abstract
Background.
Executive function (EF) deficits may increase fall risk, even among older adults with no overt cognitive impairment. Indeed, the effects of dual tasking (DT) on gait, a challenge to executive control, are more exaggerated in persons with a history of falls. Prospective evidence is, however, lacking.
Methods.
We prospectively evaluated whether EF predicts falls over a 2-year period among 262 community-living, healthy, and well-functioning older adults, focusing on the 201 who reported no falls during the previous year. At baseline, participants completed a computerized cognitive battery that generated an index of EF and other cognitive domains. Gait was assessed using performance-based tests and by quantifying walking during single- and dual-task conditions.
Results.
The 262 participants (mean age: 76.3 ± 4.3 years, 60.3% women) had intact cognitive function on testing, a low comorbidity index, and good mobility. The EF index predicted future falls. Among those who reported no previous falls, participants in the worst EF quartile were three times more likely to fall during the 2 years of follow-up, and they were more likely to transition from nonfaller to faller sooner. DT gait variability also predicted future falls and multiple falls, whereas other measures of cognitive function, gait, and mobility did not.
Conclusions.
Among healthy older adults, individuals with poorer EF are more prone to falls. Higher-level cognitive functions such as those regulated by the frontal lobes are apparently needed for safe everyday navigation that demands multitasking. Optimal screening, early detection, and treatment of falls should, apparently, also target this cognitive domain.
Keywords: Falls, Executive function, Aging, Cognitive impairment, Gait, Gait variability
FALLS in older adults are a significant source of morbidity and mortality (1,2). Falls have typically been related to multiple risk factors, including muscle strength, motor function, and postural control (2,3). Identification of older adults with a heightened risk of falling is a major medical challenge (1,4). The limited success of multifactorial fall prevention interventions and the inadequacy of early detection screens may stem from the fact that major causes of falls continue to elude us (4–6).
Recently, a decline in cognitive abilities, especially executive function (EF), has been associated with an increased fall risk, even in older adults who have no overt cognitive impairment (7–14). Age-associated reductions in EF abilities are common among older adults (9,15,16). These deficits may impair an older adult’s ability to compensate for age-associated changes in gait and balance by compromising safe negotiation in complex everyday environments (9,12,17–19). Numerous studies have reported that the effects of dual tasking (DT) on gait and balance are much larger in elderly fallers (9,11,20–22). DT walking, the ability to walk while simultaneously performing another task, may be viewed as a feature of and challenge to executive control. Retrospective reports indicate that falls and DT gait abilities are related specifically to EF (7,8,12,18,23), and fall status has been associated with age-related changes in the prefrontal cortex and brain regions related to EF (23–25). However, few studies have prospectively examined the relationship between EF and falls using quantitative measures of cognitive function and gait in healthy older adults.
The relationship between EF and fall risk in healthy community-dwelling older adults has been primarily investigated based on case control, cross-sectional, or retrospective data. In general, previous studies used only nonspecific screening measures of cognitive function or included participants with marked cognitive decline, with various other medical conditions that may have confounded the results, or participants with a history of falls, already a strong predictor of future falls (3,26). The primary purpose of the present study was to prospectively examine whether EF predicts falls in a cohort of healthy older adults, perhaps acting as a prodrome or early marker, while focusing, for the first time, on participants who reported no falls in the year prior to the study. We also sought to gain insight into the putative relationship between EF and falls by examining gait under usual walking and DT conditions. Based on the extant literature, we hypothesized that (i) EF would predict the development of falls (ie, persons with poorer EF would be predisposed to becoming a faller), (ii) DT gait would also predict future falls, and (iii) usual walking abilities and other cognitive domains such as memory would not be associated with future falls.
METHODS
Participants
The overall cohort included 262 community-living healthy older adults whose age ranged from 70 to 90 years and who were participating in a longitudinal study examining the relationships between gait, cognitive function, and fall risk, as detailed previously (18). At baseline, participants could walk independently and were free from disease likely to directly impact gait (eg, vestibular, orthopedic, neurological diseases). Participants were excluded if they had acute illness, history of brain surgery, major depression, or scored less then 25 on the Mini-Mental State Examination. Baseline testing included thorough clinical and neurological evaluations and medical history taking. The Charlson Comorbidity Index quantified disease burden. The study was approved by the local human studies committee, and informed written consent was obtained.
Assessment of Falls
History of falls in the year prior to the study was obtained based on self-report during the baseline evaluation. Subsequently, data on falls were collected prospectively for 2 years using monthly calendars that each participant returned by mail via prepaid and preaddressed envelopes using previously established methods (5,27). Participants were instructed to keep the calendar in a convenient place and to record any falls, defined as unintentionally coming to rest on a lower surface, that occurred immediately or at the end of each day (5,27). About 80% of the diaries were returned on time. If participants failed to return the diary, they were contacted by telephone to obtain the missing information.
Cognitive Function Assessment
To address the primary question of this study, a computerized neuropsychological test battery (Mindstreams; NeuroTrax Corp, Newark, NJ) quantified EF. Other cognitive domains including memory, problem solving (ie, abstraction and analytical abilities that reflect general intelligence), visual spatial perception, and a global cognitive score were also evaluated using this battery (28,29). The EF battery included computerized versions of the Go-No-Go and the Stroop interference tests, both related to response inhibition. The test battery generates composite indices of each cognitive domain (7,11,28–30) on an IQ-like scale, with 100 representing the estimated population mean normalized for age and education. We also evaluated choice reaction time as measured during the first no-interference stage of the computerized Stroop test, verbal fluency, forward and backward digit span, and the Trail Making Tests (TMT) A and B (color version), both time to completion and a normalized time, TMT (B-A)/A, that isolated the executive component of this test.
Assessment of Gait and DT
Gait was quantified under two conditions: “single task,” usual walking at preferred speed, and DT, walking while subtracting serial threes from a predefined three-digit number (without explicit instructions regarding prioritization). Participants walked up and down a 25-m-long 2-m-wide hallway at their self-selected speed for 2 minutes while wearing force-sensitive insoles that enabled quantification of gait speed (mean over the middle 10 m of the walk) and gait variability (11,31–35), as measured by swing time variability, a property independent of gait speed.
Other Potential Mediators
The Berg Balance Scale (BBS) and the Dynamic Gait Index (DGI) evaluated balance and mobility (36) (higher scores indicate better function). The Timed Up and Go (TUG) test (37), a commonly used measure of lower extremity function, assessed functional mobility and fall risk (3). Hand-held dynamometers measured tibialis anterior and grip strength (averaged over three attempts and left and right extremities). The Physical Activity Scale for the Elderly (PASE) (38) quantified activity levels. Depressive symptoms and fear of falling were evaluated using the Geriatric Depression Scale (GDS) (39) and the Activities-specific Balance Confidence (ABC) Scale (40), respectively.
Statistical Analysis
In general, we initially examined the relationship between falls and EF (and other cognitive tests) using Student’s t test or a chi-square test for continuous and dichotomous variables, respectively. Then, we followed these comparisons with multivariate binary logistic regression analyses to identify (i) which EF measures (eg, computerized EF index, the primary measure of EF) were related to fall status, (ii) which other measures might explain any observed associations, and, finally, (iii) which associations persisted in multivariate analyses after adjusting for confounding effects. The EF index was treated as a continuous variable, and, to detect more subtle relationships among the participants with no history of falls, the participants with the upper (better EF index, BEF) and lower (worse EF index, WEF) quartiles were compared. (The results reported are similar to those obtained if participants were divided into five or three groups instead of quartiles.) A similar ranking into quartiles and testing for associations with fall status was performed for the remaining measures of EF (eg, TMT-B, digit span) and the additional cognitive measures. We used binary logistic regression to calculate the predictability of the risk of future falls from EF variables, that is, the odds ratio (OR) and the 95% confidence intervals (CI). To assess whether the relationship between EF and falls persisted after adjusting for baseline measures, any variables that tended to be associated (p < .15) with both the EF index and the fall status after 2 years (yes or no) were entered into multivariate binary logistic regression models using both enter and forward stepwise (Wald) analysis. Both enter and stepwise models were applied to test the robustness of the association and to develop a more parsimonious model. The selection variable of the model included a classification cutoff of 0.5 with entry at 0.05 and exclusion at 0.1. Survival analyses using Kaplan–Meier testing were also performed to assess if EF measures were associated with time to first or time to second fall (ie, multiple faller). p Values reported are based on two-tailed comparisons. The significance level was set at .05. Statistical analyses were performed using SPSS 17.0 for Windows.
RESULTS
Participant Characteristics, EF and Falls Among the Entire Cohort
The participants (n = 262, mean age: 76.3 ± 4.3 years, 60.3% women) were healthy and had few comorbidities, as evidenced by low Charlson scores (0.8 ± 1.1). Neurological examination revealed no abnormalities (eg, normal plantar reflex, sensation, and muscle tone). Performance-based tests of gait were near perfect (eg, BBS: 54.1 ± 2.3, DGI: 22.8 ± 1.5, TUG times: 9.5 ± 1.6 seconds), and the indices of the computerized cognitive battery were at the expected range for cognitively intact older adults (ie, means very near 100.0). The participants were active and mobile (PASE scores: 114 ± 66). Over the 2-year follow-up period, 263 falls were reported (0.50 falls per person-year), with 50% of the participants falling at least once.
In the overall cohort, the EF index was worse (p = .038) among participants who reported new falls during the 2-year follow-up (97.9 ± 10.7) compared with those who reported no new falls (100.6 ± 10.6). Gait variability during DT was worse in the fallers (3.2 ± 1.8%) compared with nonfallers (2.7 ± 1.1%, p = .030). The only other measures showing significant differences in future fallers, compared with nonfallers, were gender (p = .005, 69.0% of the fallers were women), fall history (p < .001, 68.9% of the fallers fell previously), grip strength (fallers: 23.0 ± 7.9 kg, nonfallers: 26.8 ± 8.9 kg; p < .001), GDS scores (fallers: 5.9 ± 5.1, nonfallers: 4.5 ± 3.8; p = .018), and ABC scores (fallers: 90.8 ± 10.1, nonfallers: 93.5 ± 9.4; p = .029). In stepwise binary logistic regression analysis, DT gait variability (p = .026, OR: 1.26, 95% CI: 1.03–1.55), a history of falls (p = .002, OR: 2.17, 95% CI: 1.32–3.58), and grip strength (p = .014, OR: 0.96, 95% CI: 0.93–0.99) were independent predictors of fall status.
Cognitive Function and Falls in the Target Cohort (No Falls Prior to Baseline Testing)
The participants who reported no falls at baseline (n = 201) formed the target cohort. At baseline, they were healthy and active and exhibited good scores on all tests of cognitive function, gait, and mobility (see Supplementary Material Table 1). 42.3% of these participants reported 168 falls (0.42 falls per person-year). The EF index was only marginally related to fall status during the 2 years of follow-up (p = .13). However, when participants were stratified by EF index scores into quartiles, participants with worse EF (WEF) were three times more likely to fall compared with those with better EF (BEF; OR: 3.02, 95% CI: 1.35–6.78; Table 1). Alternatively viewed, those with BEF were less likely to fall, compared with participants from all other quartiles combined (OR: 0.44, 95% CI: 0.22–0.86).
Table 1.
Univariate Logistic Regression Analysis for the Prediction of Fall Status (yes or no) Over the 2-Years of Follow-up for Each Cognitive Measure
| Cognitive Measure | Worse Quartile (M ± SD) | Better Quartile* (M ± SD) | OR (95% confidence interval) | p Value (association with falls) |
| EF index | 86.1 ± 5.6 | 111.8 ± 4.9 | 3.02 (1.35–6.78) | .007 |
| Memory index | 83.9 ± 7.2 | 112.7 ± 2.7 | 1.05 (0.47–2.35) | .901 |
| Visual spatial index | 77.5 ± 4.6 | 120.4 ± 9.2 | 1.86 (0.94–3.65) | .072 |
| Problem solving index | 79.4 ± 9.3 | 118.4 ± 4.1 | 0.89 (0.39–3.65) | .796 |
| Global cognitive index | 88.1 ± 5.2 | 109.7 ± 2.8 | 1.64 (0.74–3.65) | .225 |
| Mini-Mental State Examination | 26.6 ± 0.71 | 30 ± 0.0 | 1.61 (0.67–3.86) | .285 |
| Verbal fluency | 19.4 ± 4.8 | 49.4 ± 5.3 | 1.19 (0.53–2.67) | .679 |
| Backward digit span | 3.5 ± 0.71 | 9.4 ± 1.3 | 1.08 (0.51–2.29) | .835 |
| TMT-A | 123.9 ± 27.9 | 46.1 ± 7.6 | 1.13 (0.52–2.29) | .761 |
| TMT-B | 247.9 ± 49.8 | 90.4 ± 10.9 | 2.01 (0.90–4.47) | .088 |
| TMT-B normalized | 2.01 ± 0.64 | 0.27 ± 0.21 | 1.46 (0.66–3.24) | .355 |
| Reaction time (ms) | 1,440.8 ± 450.3 | 584.7 ± 53.4 | 1.00 (0.44–2.29) | 1.000 |
Note: Significant associations are bolded. *The OR and p value show the association between each cognitive measure (the two extreme quartiles of each measure) and falls. Recall that the index scores are computed on an IQ-like scale. Thus, the mean of 86 on the EF index among those in the worse EF quartile is about 1 SD below the age- and education-adjusted expected population norm of 100. EF = executive function; OR = odds ratio; TMT = Trail Making Tests.
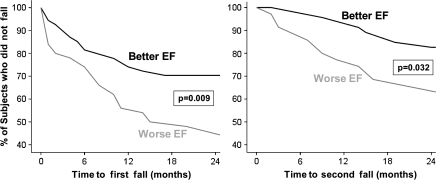
Survival analyses demonstrated that the change in status from nonfaller to faller occurred significantly sooner in the WEF group, compared with the BEF group, both for time to first fall (p = .009) and time to second fall (p = .032; see Figure 1). For example, 35% of participants from the WEF group reported one fall in the first year of follow-up compared with 15% from the BEF group (p < .0001), and 12% of WEF participants had two or more falls compared with none in the BEF group (p < .0001). After 2 years of follow-up, 53% of participants in the WEF reported falling once compared with only 29% in the BEF group (p = .011) and 35% of the lower EF group reported multiple falls compared with only 17% in the BEF group (p = .06).
Figure 1.
Survival curves illustrating the percent of participants who did not fall (the y-axes) as a function of time and executive function group. Among the participants who reported no falls in the year prior to baseline, the target cohort, participants with worse EF (lowest quartile) were more likely to fall sooner (left) and more likely to become multifallers sooner (right) compared to those with better EF (best quartile). Put differently, participants with worse EF were more likely to become fallers and recurrent fallers sooner than those with better EF. EF = executive function.
When scores on the TMT-B were stratified into quartiles, there was also a tendency toward an association with fall status (p = .08), and normalized TMT-B was significantly worse in multiple fallers (1.26 ± 0.78) compared with nonfallers (0.96 ± 0.65, p = .018). In contrast, all other cognitive measures were not significantly associated with falls over the 2-year follow-up period (Table 1). The memory index, for example, was essentially identical in fallers and nonfallers (fallers: 100.1 ± 11.0, nonfallers: 100.1 ± −11.7; p = .96), even if the cohort was stratified into those with better and worse memory index (p = .90).
Other Potential Risk Factors and Multivariate Analyses for Falls in the Target Cohort
High gait variability during the DT condition was significantly associated with future falls (OR: 1.29, 95% CI: 1.04–1.59; p = .02). At baseline, small but significant differences between the BEF and the WEF groups were observed for gait speed and performance-based measures of mobility. However, none of these measures were associated with de novo falls (p > .22). Grip strength and tibialis anterior muscle strength were also significantly different between the EF groups at baseline, but only grip strength was significantly associated with future fall status (OR = 0.95, 95% CI: 0.92–0.99; p = .007).
In general, EF measures and DT gait variability were predictive of both falls status and multiple falls status in univariate and multivariate analyses (see Table 2 and Supplementary Material Table 2). Investigation of multiple falls status (ie, two or more falls) demonstrated that the EF index (OR = 2.81, 95% CI: 1.01–7.83), normalized TMT-B (OR = 1.82, 95% CI: 1.09–3.02), grip strength (OR = 0.94, 95% CI: 0.89–0.99), and gait variability during DT (OR = 1.47, 95% CI: 1.13–1.92) were significant predictors of multiple falls status in univariate analyses.
Table 2.
Multivariate Predictors of Fall Status (yes or no) Over the 2-Year Follow-up Period*
| Enter Method |
Forward Stepwise (Wald) Method |
|||
| OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Model 0 | ||||
| EF index | 2.55 (1.08–6.00) | .032 | 3.02 (1.35–6.78) | .007 |
| Age | 1.05 (0.95–1.16) | .334 | .383 | |
| Gender | 0.52 (0.22–1.21) | .129 | .142 | |
| Model 1 | ||||
| EF index | 2.54 (1.07–6.02) | .034 | 2.45 (1.05–5.74) | .038 |
| Grip strength | 0.95 (0.89–0.97) | .037 | 0.95 (0.89–0.99) | .043 |
| Visual spatial | 1.01 (0.98–1.04) | .578 | .577 | |
| Model 2 | ||||
| EF index | 2.86 (1.11–7.41) | .030 | 2.78 (1.18–6.55) | .019 |
| Grip strength | 0.94 (0.88–0.99) | .034 | .061 | |
| Usual walking speed | 3.54 (0.39–32.08) | .260 | .817 | |
| Model 3 | ||||
| EF index | 3.35 (1.31–8.58) | .012 | 3.68 (1.51–8.93) | .004 |
| Grip strength | 0.94 (0.89–1.00) | .063 | .058 | |
| Usual walking | 0.94 (0.72–1.21) | .624 | .643 | |
| Gait variability | ||||
| Model 4 | ||||
| EF index | 1.70 (0.66–4.38) | .269 | 0.93 (0.87–0.99) | .137 |
| Grip strength | 0.93 (0.87–0.99) | .038 | 1.46 (1.04–2.03) | .024 |
| Dual tasking gait variability | 1.39 (0.99–1.96) | .055 | .027 | |
Notes: OR and CI obtained using enter and stepwise methods analysis are shown. CI = confidence interval; EF = executive function; OR = odds ratio.
See the footnote to Supplementary Material Table 2.
DISCUSSION
As hypothesized, EF measures at baseline were associated with falls that occurred during the 2-year prospective follow-up in the overall cohort and, most significantly, in the target cohort, that is, the subset of participants who reported no falls in the year prior to the study. Among these participants, better EF may have shielded them from falling or poor EF may have prevented compensation for age-associated changes in gait and balance, increasing the risk of falls. Like the neuropsychological index of EF, DT walking ability, an everyday task requiring executive control, was also related to future falls, both in the overall and target cohorts, whereas usual walking measures were not.
The association between EF, DT gait, and falls can, a priori, be interpreted in several ways. Perhaps, the EF deficits are sensitive but nonspecific signs of relatively accelerated aging or broad changes in cognitive function. Brain imaging studies of older adults have shown that white matter changes are associated with gait impairment and falls, perhaps due to decreased connectivity between different brain areas (41). Thus, EF could be considered a general biomarker of overall brain reserve.
The present findings suggest, however, that the relationship between EF and falls is rather specific. Memory, general measures of cognitive function, and other cognitive domains were not related to falls, consistent with retrospective reports that memory was not associated with fall status (7,8,11). Several studies reported that the associations between falls and white matter changes in older adults were primarily the result of deep frontal and periventricular changes (24,25), regions that could readily explain the unique associations between EF and falls observed in the present study. Although EF likely involves a complex network of frontal–cortical and subcortical circuitries (42), EF is generally associated with the frontal cortex, including the dorsolateral prefrontal cortex, which in turn has been related to DT and to gait variability (42,43), supporting the idea that fall risk depends, in part, on EF.
In this present study, both the EF index and the TMT-B were related to falls. The EF index is based on tests of response inhibition, and the TMT-B primarily challenges flexibility, set shifting, and task switching, undertakings also related to response inhibition. Of note, poor performance on the TMT-B was previously associated with decreased gait speed in older adults, especially during complex walking tasks (eg, walking over an obstacle course, DT walking) (12,17,44). Conversely, digit span and verbal fluency, reflecting other aspects of EF, were not associated with falls, whereas DT gait variability was. Indeed, the multivariate analyses suggest that the EF index and DT gait variability were interchangeable with respect to their relationship to future falls (recall Table 2 and Supplementary Material Table 2). In other words, DT gait abilities can be viewed as a functional measure of EF. Perhaps, older adults whose gait has become less resilient and automatic rely on intact EF to successfully allocate cognitive resources among competing activities, including those critical to gait, while inhibiting diversions, in order to minimize fall risk. This is consistent with the idea that intact EF enables the use of real-time control to continuously adapt to perturbations and postural challenges that arise while multitasking and navigating in complex environments (9,11,12,18,20). Several recent pilot studies have demonstrated that pharmacological agents, typically prescribed to enhance cognitive function, also apparently improve gait, DT walking, and other fall risk measures (45–48). Thus, the intriguing possibility that reduced EF is not simply an early risk marker, but it may also contribute to fall risk, perhaps via its role in multitasking, a specific form of EF, is supported by (i) these preliminary pharmacological studies, (ii) the previous reports that documented reduced EF and increased sensitivity to DT in elderly fallers (7–13,21,22), and (iii) the present findings that the EF index, TMT-B, and DT gait were all predictive of future falls.
Strengths and Limitations
Self-report of falls is problematic due to the subjective nature of the data collection. Although the rate of compliance in the present study was high, some falls may have been missed. Nonetheless, currently, this is the most widely used method for longitudinal assessment of falls. It might be objected that by design, the target cohort may not represent aging in general but rather reflects more successful aging. This choice, however, could be considered as a major strength because it enabled examination of the subtle role of EF in the absence of significant motor and cognitive changes and separated from the confounding consequences of previous falls. Other strengths of the present investigation include the relatively large number of participants, the similarity between fall-related characteristics of the overall cohort and previous population studies (1,34), the use of a standardized computerized neuropsychological battery of several cognitive domains, along with a traditional test of EF (eg, TMT-B), and the quantitative assessment of gait during DT. Of special note is the finding that DT gait variability and several measures of EF were predictive of future falls in all participants, in general, and, more importantly, in the target cohort, that is, those participants who had no falls in the year prior to the study.
Future Studies and Clinical Implications
Many of the “accidental falls” previously attributed to no specific cause (1) may actually be related to EF deficits and reduced DT abilities, but future work is needed to address this question more directly. Recent studies have demonstrated that aerobic exercise, resistance training, and cognitive training can improve EF in community-living older adults (49,50). The present findings raise the intriguing possibility that these intervention modalities can also be used to reduce fall risk via their effect on EF. Future investigations designed to reduce fall risk by improving EF will be helpful both for further elucidation of the mechanisms that connect EF to falls and for developing optimal interventions. In the meantime, the present findings indicate that deficits in EF and DT performance are prodromes of fall risk, predating clinically significant impairment in motor or cognitive function. An EF index score 1 SD below the age-expected norm appears to be a warning sign for an increased fall risk. To address the need for better screening options (1,4), evaluation and early detection of fall risk should apparently expand its focus from the lower extremity, gait, and balance to include cortical influences, perhaps, by incorporating tests of EF to identify a risk factor that might otherwise escape detection during a routine clinical examination in healthy older adults.
FUNDING
This work was supported by the National Institutes of Health (AG-14100).
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomed.gerontologyjournals.org/.
Supplementary Material
Acknowledgments
The authors are indebted to the participants and staff of the “Holchim Rachok” project for their invaluable contribution and to biostatistician Dr. Elliot Sprecher.
References
- 1.Rubenstein LZ. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35(suppl 2):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 2.Kannus P, Sievanen H, Palvanen M, Jarvinen T, Parkkari J. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–1893. doi: 10.1016/S0140-6736(05)67604-0. [DOI] [PubMed] [Google Scholar]
- 3.AGS Guidelines. Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49:664–672. [PubMed] [Google Scholar]
- 4.Gates S, Smith LA, Fisher JD, Lamb SE. Systematic review of accuracy of screening instruments for predicting fall risk among independently living older adults. J Rehabil Res Dev. 2008;45:1105–1116. [PubMed] [Google Scholar]
- 5.Leveille SG, Jones RN, Kiely DK, et al. Chronic musculoskeletal pain and the occurrence of falls in an older population. JAMA. 2009;302:2214–2221. doi: 10.1001/jama.2009.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD007146.pub2. CD007146. [DOI] [PubMed] [Google Scholar]
- 7.Hausdorff JM, Doniger GM, Springer S, et al. A common cognitive profile in elderly fallers and in patients with Parkinson’s disease: the prominence of impaired executive function and attention. Exp Aging Res. 2006;32:411–429. doi: 10.1080/03610730600875817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtzer R, Friedman R, Lipton RB, et al. The relationship between specific cognitive functions and falls in aging. Neuropsychology. 2007;21:540–548. doi: 10.1037/0894-4105.21.5.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23:329–342. doi: 10.1002/mds.21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson HH, Rosano C, Simonsick EM, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2007;62:844–850. doi: 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- 11.Springer S, Giladi N, Peretz C, et al. Dual-tasking effects on gait variability: the role of aging, falls, and executive function. Mov Disord. 2006;21:950–957. doi: 10.1002/mds.20848. [DOI] [PubMed] [Google Scholar]
- 12.van Iersel MB, Kessels RP, Bloem BR, Verbeek AL, Olde Rikkert MG. Executive functions are associated with gait and balance in community-living elderly people. J Gerontol A Biol Sci Med Sci. 2008;63:1344–1349. doi: 10.1093/gerona/63.12.1344. [DOI] [PubMed] [Google Scholar]
- 13.Persad CC, Jones JL, Ashton-Miller JA, Alexander NB, Giordani B. Executive function and gait in older adults with cognitive impairment. J Gerontol A Biol Sci Med Sci. 2008;63:1350–1355. doi: 10.1093/gerona/63.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander NB, Hausdorff JM. Linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2008;63:1325–1328. doi: 10.1093/gerona/63.12.1325. [DOI] [PubMed] [Google Scholar]
- 15.Prakash RS, Erickson KI, Colcombe SJ, et al. Age-related differences in the involvement of the prefrontal cortex in attentional control. Brain Cogn. 2009;71:328–335. doi: 10.1016/j.bandc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Royall DR, Palmer R, Chiodo LK, Polk MJ. Declining executive control in normal aging predicts change in functional status: the Freedom House Study. J Am Geriatr Soc. 2004;52:346–352. doi: 10.1111/j.1532-5415.2004.52104.x. [DOI] [PubMed] [Google Scholar]
- 17.Coppin AK, Shumway-Cook A, Saczynski JS, et al. Association of executive function and performance of dual-task physical tests among older adults: analyses from the InChianti study. Age Ageing. 2006;35:619–624. doi: 10.1093/ageing/afl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hausdorff JM, Schweiger A, Herman T, Yogev-Seligmann G, Giladi N. Dual-task decrements in gait: contributing factors among healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1335–1343. doi: 10.1093/gerona/63.12.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 20.Woollacott M, Shumway-Cook A. Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture. 2002;16:1–14. doi: 10.1016/s0966-6362(01)00156-4. [DOI] [PubMed] [Google Scholar]
- 21.Verghese J, Buschke H, Viola L, et al. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc. 2002;50:1572–1576. doi: 10.1046/j.1532-5415.2002.50415.x. [DOI] [PubMed] [Google Scholar]
- 22.Bootsma-van der Wiel A, Gussekloo J, de Craen AJ, et al. Walking and talking as predictors of falls in the general population: the Leiden 85-Plus Study. J Am Geriatr Soc. 2003;51:1466–1471. doi: 10.1046/j.1532-5415.2003.51468.x. [DOI] [PubMed] [Google Scholar]
- 23.Anstey KJ, Wood J, Kerr G, Caldwell H, Lord SR. Different cognitive profiles for single compared with recurrent fallers without dementia. Neuropsychology. 2009;23:500–508. doi: 10.1037/a0015389. [DOI] [PubMed] [Google Scholar]
- 24.Blahak C, Baezner H, Pantoni L, et al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. J Neurol Neurosurg Psychiatry. 2009;80:608–613. doi: 10.1136/jnnp.2008.154633. [DOI] [PubMed] [Google Scholar]
- 25.Soumare A, Elbaz A, Zhu Y, et al. White matter lesions volume and motor performances in the elderly. Ann Neurol. 2009;65:706–715. doi: 10.1002/ana.21674. [DOI] [PubMed] [Google Scholar]
- 26.Friedman SM, Munoz B, West SK, Rubin GS, Fried LP. Falls and fear of falling: which comes first? A longitudinal prediction model suggests strategies for primary and secondary prevention. J Am Geriatr Soc. 2002;50:1329–1335. doi: 10.1046/j.1532-5415.2002.50352.x. [DOI] [PubMed] [Google Scholar]
- 27.Mackenzie L, Byles J, D’Este C. Validation of self-reported fall events in intervention studies. Clin Rehabil. 2006;20:331–339. doi: 10.1191/0269215506cr947oa. [DOI] [PubMed] [Google Scholar]
- 28.Dwolatzky T, Whitehead V, Doniger GM, et al. Validity of a novel computerized cognitive battery for mild cognitive impairment. BMC Geriatr. 2003;3:4. doi: 10.1186/1471-2318-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schweiger A, Doniger GM, Dwolatzky T, Jaffe D, Simon ES. Reliability of a novel computerized neuropsychological battery for mild cognitive impairment. Acta Neuropsychologica. 2003;1:407–413. [Google Scholar]
- 30.Giladi N, Mordechovich M, Gruendlinger L, et al. “Brain Screen”: a self-referral, screening program for strokes, falls and dementia risk factors. J Neurol. 2006;253:307–315. doi: 10.1007/s00415-005-0986-6. [DOI] [PubMed] [Google Scholar]
- 31.Hausdorff JM, Edelberg HK, Mitchell SL, Goldberger AL, Wei JY. Increased gait unsteadiness in community-dwelling elderly fallers. Arch Phys Med Rehabil. 1997;78:278–283. doi: 10.1016/s0003-9993(97)90034-4. [DOI] [PubMed] [Google Scholar]
- 32.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 33.Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil. 2001;82:1050–1056. doi: 10.1053/apmr.2001.24893. [DOI] [PubMed] [Google Scholar]
- 34.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative gait markers and incident fall risk in older adults. J Gerontol A Biol Sci Med Sci. 2009;64:896–901. doi: 10.1093/gerona/glp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hausdorff JM, Nelson ME, Kaliton D, et al. Etiology and modification of gait instability in older adults: a randomized controlled trial of exercise. J Appl Physiol. 2001;90:2117–2129. doi: 10.1152/jappl.2001.90.6.2117. [DOI] [PubMed] [Google Scholar]
- 36.Berg KO, Wood-Dauphinee SL, Williams JI, Maki B. Measuring balance in the elderly: validation of an instrument. Can J Public Health. 1992;83(suppl 2):S7–S11. [PubMed] [Google Scholar]
- 37.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 38.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 39.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 40.Powell LE, Myers AM. The Activities-specific Balance Confidence (ABC) Scale. J Gerontol A Biol Sci Med Sci. 1995;50A:M28–M34. doi: 10.1093/gerona/50a.1.m28. [DOI] [PubMed] [Google Scholar]
- 41.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008;70:935–942. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 42.Leh SE, Petrides M, Strafella AP. The neural circuitry of executive functions in healthy subjects and Parkinson’s disease. Neuropsychopharmacology. 2010;35:70–85. doi: 10.1038/npp.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53:410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 45.Montero-Odasso M, Wells J, Borrie M. Can cognitive enhancers reduce the risk of falls in people with dementia? An open-label study with controls. J Am Geriatr Soc. 2009;57:359–360. doi: 10.1111/j.1532-5415.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben-Itzhak R, Giladi N, Gruendlinger L, Hausdorff JM. Can methylphenidate reduce fall risk in community-living older adults? A double-blind, single-dose cross-over study. J Am Geriatr Soc. 2008;56:695–700. doi: 10.1111/j.1532-5415.2007.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Assal F, Allali G, Kressig RW, Herrmann FR, Beauchet O. Galantamine improves gait performance in patients with Alzheimer’s disease. J Am Geriatr Soc. 2008;56:946–947. doi: 10.1111/j.1532-5415.2008.01657.x. [DOI] [PubMed] [Google Scholar]
- 48.Auriel E, Hausdorff JM, Herman T, Simon ES, Giladi N. Effects of methylphenidate on cognitive function and gait in patients with Parkinson’s disease: a pilot study. Clin Neuropharmacol. 2006;29:15–17. doi: 10.1097/00002826-200601000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 50.Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.