Abstract
Changes in progenitor cell biology remain at the forefront of many theories of biologic aging, but there are limited studies evaluating this in humans. Aging has been associated with a progressive depletion of circulating progenitor cells, but age-related bone marrow–resident progenitor cell depletion has not been systematically determined in humans. Patients undergoing total hip replacement were consented, and bone marrow and peripheral progenitor cells were enumerated based on aldehyde dehydrogenase activity and CD34 and CD133 expression. Circulating progenitors demonstrated an age-dependent decline. In contrast, marrow-resident progenitor cell content demonstrated no age association with any progenitor cell subtype. In humans, aging is associated with depletion of circulating, but not marrow-resident, progenitors. This finding has impact on the mechanism(s) responsible for age-related changes in circulating stem cells and important implications for the use of autologous marrow for the treatment of age-related diseases.
Keywords: Bone marrow, Progenitor cell, Endothelial progenitor cells, Aging, Circulating progenitor cell
THE biologic underpinning for aging remains elusive. One of the leading explanations for the markedly poorer outcomes in elderly patients is cumulative cellular damage. Yet, although progressive injury and cell loss are important for the pathogenesis of disease, there is also considerable recent interest how animals and humans adapt to these injuries via cellular repair and/or replacement. Specifically, studies are increasingly demonstrating that most organs in humans and animals have remarkable capacity for healing and rejuvenation. These processes tend to be mediated by progenitor cells; however, as an organism ages, this capacity to repair appears to decrease, leading to an accumulation of cellular damage and ultimate loss of function, “aging” and death.
To date, the effect of aging on progenitor cell biology has been evaluated in a variety of animal models and contexts. The simplest explanation for an age-related loss of progenitor cell–mediated repair would be a decrease in progenitor cell central stores over time. Although this appears present in some animal models (1), in other studies, marrow-resident progenitor cells were found to not change or even increase with age (2–4).
The effect of aging on human marrow-resident progenitors has received less scrutiny; however, the number of circulating progenitors has shown an age-related decline in several studies (5,6). Circulating progenitors may reflect a loss of underlying marrow-resident progenitors but may also reflect poor mobilization of these cells into the circulation or enhanced migration of these cells from the circulation to the areas of ongoing injury. Whether or not bone marrow (BM)–resident progenitor cell stores are affected by aging has important implications for the use of autologous BM sources for cell therapy and is thus a vital question of human progenitor cell biology.
Whether human aging is the result of loss of marrow-resident progenitor cell numbers has received little attention, affected by the difficulty of obtaining the required tissue specimens in a clinical context in which marrow is obtainable without suspected pathology. One opportunity for routine BM sampling is orthopedic joint replacement surgery, in which the femoral head is removed to allow placement of the prosthetic device.
We therefore undertook to determine the relationship between aging and BM-resident progenitor cell content by determining the progenitor cell content of marrow specimens obtained from a group of patients undergoing elective hip replacement surgery, a setting where we could simultaneously determine circulating progenitor cell numbers. We assessed for progenitor cells as defined using well-established cell surface markers, which select for early multipotent progenitors (CD133) or hematopoietic/endothelial lineage cells (CD34). We also performed a similar analysis using a progenitor cell–specific functional assay, which identifies multiple progenitor cell phenotypes (7–16), thereby assessing overall progenitor cell content. To our knowledge, this will be the first comprehensive assessment of marrow-resident and circulating progenitor cell numbers in human patients.
STUDY METHODS
BM and Blood Sampling
Patients undergoing elective hip replacement surgery who provided informed written consent of this Duke Institutional Review Board–approved study were enrolled in the “Bone Marrow in Aging” protocol. Surgery was performed by one of the two participating surgeons (D.E.A. and M.P.B.). During surgery, femoral canal preparation and femoral head harvest were performed as clinically indicated, and contents of the femoral canal and the femoral head were used for marrow analysis.
Analysis of Peripheral Blood Specimens
Blood samples were collected by an experienced phlebotomist using either a 21- or a 23-gauge butterfly needle. Prior to collecting study samples, one 3-mL red top tube was drawn and discarded and blood was drawn into an EDTA-anticoagulated tube. Each study tube was completely filled, gently inverted six to eight times, labeled, double bagged, placed in a metal container at ambient temperature, and transported by a study coordinator.
Peripheral blood mononuclear cells were isolated from 7–10 mL of blood by differential centrifugation. Blood was layered on 35 mL of a 1:1 mixture of Ficoll-Paque Plus (GE Healthcare, Piscataway, NJ) in 50-mL conical tubes and centrifuged at 1,800 rcf for 30 minutes. After removal of the plasma layer, the buffy coat was transferred to a 50-mL conical tube, and recovered cells were washed with phosphate-buffered saline–1% bovine serum albumin (30 mL), recovered after centrifugation at 500 rcf for 10 minutes, resuspended in phosphate-buffered saline–1% bovine serum albumin, and counted, and approximately 2 million cells portioned for analysis.
All cellular analyses were performed within 2 hours of sample acquisition.
Determination of Progenitor Cell Numbers
Progenitor cell numbers were simultaneously assayed using analysis of cell surface expression of CD133, CD34, and vascular endothelial growth factor receptor-2 (VEGFR-2) as well as on the basis of aldehyde dehydrogenase (ALDH) activity.
Identification of Progenitors Based on ALDH Activity
Total human peripheral blood was analyzed for the relative content of cells with low orthogonal light scatter and high ALDH activity content (side scatter low [SSClo] ALDHbr cells) (6). Briefly, 4 × 106 cells were aliquoted for analysis into an Aldecount tube containing 2 mL of Aldecount buffer (Aldagen Inc., Durham, NC). Immediately after addition of the cells, 500 μL was transferred to a tube containing diethylaminobenzaldehyde, a potent inhibitor of ALDH activity (10 μM). After 30 minutes at 37°C, the cells were centrifuged, placed on ice, and flow cytometry was performed.
Identification of Progenitor Cells by Expression of Cell Surface Markers
Isolated mononuclear cells were washed with Iscove’s Modified Dulbecco’s Medium containing 2% fetal calf serum (IMDM+2%), concentrated to 107 cells/ml in 80 μL IMDM+2%. Nonspecific antibody binding was blocked with FcR blocking reagent (Miltenyi Biotec, Auburn, CA; 10 μL) for 10 minutes. Using CD133–allophycocyanin (Miltenyi Biotec), CD34–fluorescein isothiocyanate (Miltenyi Biotec), and VEGFR2–phycoerythrin (R and D Biosystems, Minneapolis, MN) allowed simultaneous staining for all three key cell surface markers. Dead and dying cells were excluded using staining with 7-amino-actinomycin D (1 μg/106 cells; Invitrogen, Carlsbad, CA) added just prior to performance of flow cytometry.
Analysis for Marrow-Resident Progenitors
BM was procured at time of surgery and did not involve an alteration in the planned surgical procedure. Marrow was obtained from the femoral canal and the excised femoral head. The femoral head was reamed of all marrow. The combined marrow was treated with ammonium chloride erythrocyte lysis solution (BD Biosciences, San Jose, CA) to remove any contaminating red blood cells, washed 3× with phosphate-buffered saline, and the resulting mononuclear cell product subjected to analysis.
Marrow-resident progenitor cell content was determined using procedures analogous to those used on peripheral blood.
Flow Cytometry
Flow cytometry was performed by trained technicians blinded to patient identity using an LSR II flow cytometer (BD Biosciences, San Jose, CA) and analyzed using Flow Jo software (Treestar, Costa Mesa, CA). Quality control measures were performed daily using BD Comp Beads (BD Biosciences) incubated with each antibody.
Analysis was performed in a blinded fashion. Dead and dying cells were excluded based on staining with 7-amino-actinomycin D. The numbers of CD34+, CD133+, and VEGFR-2+ cells were identified as subpopulations of a mononuclear cell gate, determined on the basis of light front and side scatter characteristics. The number of cells staining for combinations of these markers was determined by gating on individual markers in sequential fashion. Reported frequencies were expressed as percentages of the mononuclear cell population, as well as percentages of all live cells in the BM.
Analytical Methods
Progenitor cell numbers were plotted as continuous variables versus age, and a Pearson correlation determined to assess the relationship between age and progenitor cell content in both peripheral blood and BM. Marrow-resident progenitor cell content was compared with circulating progenitor cell levels using similar techniques. A p value <.05 was assumed to represent statistical significance.
RESULTS
Patient Population
Between December 2006 and February 2009, we enrolled 107 patients in the “Bone Marrow Composition in Aging” study. BM was successfully obtained for analysis from 81 participants and was analyzed for marrow progenitor cell content based on ALDH activity (n = 80) as well as the expression of cell surface markers CD34, CD133, and VEGFR-2 (n = 80). Reasons for inability to obtain marrow for analysis varied and included inadequate specimen retrieval (n = 12); change in surgical plans (n = 2); redo surgery status (n = 2); or failure to retrieve, properly store, or transport the specimen (n = 2). In addition, we initially planned to enroll patients undergoing both hip or knee replacement; however, after enrollment of several knee replacement patients yielded inadequate specimens (n = 8), we elected to consent only patients undergoing hip replacement. The study population includes the 81 patients in whom BM analysis was performed.
Key baseline characteristics for this study population are shown in Table 1. Patients underwent joint replacement predominantly for osteoarthritis (n = 61, 75%), with other indications including avascular necrosis (n = 12), rheumatoid arthritis (n = 3), and prior trauma (n = 5).
Table 1.
Indications for Joint Replacement
| Indication | N (%) |
| Avascular necrosis | 12 (14.8) |
| Osteoarthritis | 61 (75.2) |
| Rheumatoid arthritis | 3 (3.7) |
| Trauma | 5 (6.2) |
The median age of the population was 62 (interquantile range 52–67, range 18–85) years, with equal gender representation. There was a significant burden of hypertension but limited presence of hyperlipidemia, diabetes, ongoing tobacco abuse, or a family history of premature coronary artery disease. The presence of documented coronary artery disease was rare, with 2.4% of the population having undergone prior percutaneous coronary intervention and 3.5% prior coronary artery bypass grafting. No other patients were known to have coronary disease based on cardiac catheterization. A small proportion of patients underwent stress testing, and these patients lacked demonstrable ischemia. The use of cardiac medication at the time of surgery is listed and is consistent with routine use in a middle-age population for treatment of hypertension and predominantly primary prevention (Table 2). In addition, the use of narcotics, NSAIDs, and cyclo-oxygenase-2 inhibitors in this patient population with chronic joint limitation is listed in Table 2.
Table 2.
Patient Characteristics
| Mean ± SD or % | Median [IQR] | |
| Age | 58.8 ± 14.2 | 62 [52.2, 67.8] |
| Race (Caucasian) | 91.4% | |
| Gender (male) | 47.6% | |
| Hypertension | 58.3% | |
| Hyperlipidemia | 35.7% | |
| Diabetes | 10.7% | |
| Tobacco use | 36.1% | |
| Current Tob | 6.0% | |
| Family history of coronary artery disease | 21.9% | |
| Transient ischemic attack/cerbrovascular accident | 3.6% | |
| Peripheral vascular disease | 4.8% | |
| Q waves on electrocardiogram | 2.8% | |
| Hematocrit | 40.0 ± 4.3% | 40.5 [37, 43] |
| Platelet count | 256.8 ±73.5 | 250.5 [205, 299] |
| White blood count | 7.16 ± 2.04 | 6.9 [5.9, 8.2] |
| Creatinine | 0.95 ± 0.29 | 0.9 [0.8, 1.1] |
| Height | 166.9 ± 24.6 | 172 [162, 180] |
| Weight | 91.6 ± 22.3 | 88.8 [75.5 106] |
| Body mass index | 32.3 ± 7.7 | 30.5 [26.6, 36.4] |
| h/o Percutaneous coronary intervention | 2.4% | |
| h/o Coronary artery bypass grafting | 3.5% | |
| Medication use | ||
| Aspirin | 36.1% | |
| Statin | 33.7% | |
| Beta-blocker | 24.1% | |
| ACE inhibitor | 22.9% | |
| Angiotensin receptor blocker | 18.1% | |
| Plavix | 2.4% | |
| Thiazide diuretic | 23.8% | |
| Estrogen replacement | 8.4% | |
| NSAID | 40.0% | |
| Narcotic | 25.0% | |
| Cyclooxygenase-2 inhibitors | 10.0% |
The gating strategy employed for marrow-resident stem cell identification is displayed in Figure 1. ALDHbr cells were enumerated in one experiment, whereas analysis based on cell surface markers was performed separately.
Figure 1.
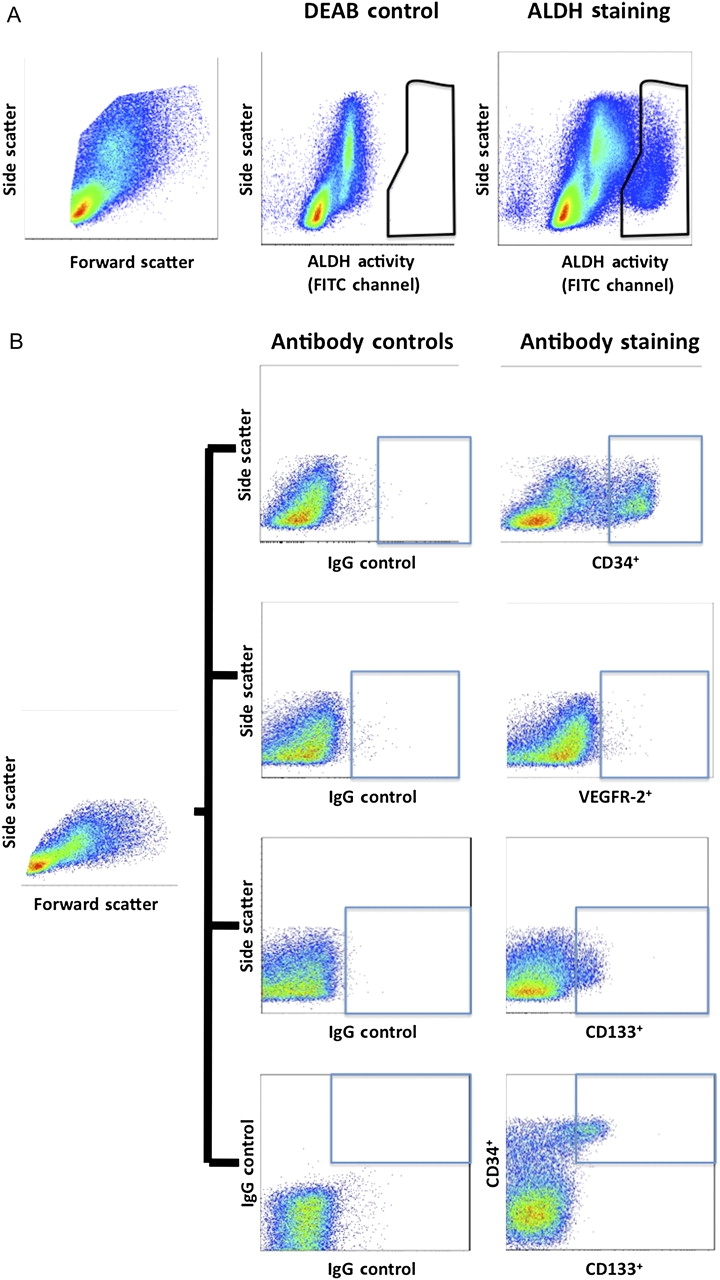
Representative flow cytometry gating strategy. Mononuclear cells were selected based on forward and side scatter characteristics (left panel). Panel (A): Cells were incubated with BODIPY-aminoacetaldehyde in the presence (center panel) or absence (right panel) of diethylaminobenzaldehyde, a specific aldehyde dehydrogenase (ALDH) inhibitor (y axis = side scatter in all panels). Panel (B): Cells were incubated with antibodies to CD34, CD133, and VEGFR-2 or their IgG controls, and the expression of individual markers was determined. For cells expressing combinations of markers (eg, CD133 and CD34), the CD133 gate was dragged into the CD34 gate to obtain dual expressing cells. DEAB = diethylaminobenzaldehyde; FITC = fluorescein isothiocyanate.
Marrow-resident and circulating progenitors were identified on the basis of ALDH activity as well as expression of select cell surface markers. Cells characterized by high levels of ALDH activity (ALDHbr cells) from either marrow (7,8,10–12,14,17,18) or peripheral blood sources (19) comprise hematopoietic progenitors with long-term reconstitution potential; however, ALDHbr cells have also been shown to differentiate into cells with neuronal (9,13,20), mesenchymal (16), and endothelial (16,21) characteristics. We also assessed progenitors based on expression of CD34 (expressed on hematopoietic as well as endothelial progenitors), CD133 (a marker of early progenitor cell phenotypes of multiple lineages), and VEGFR-2 (expressed on mature and immature endothelial cells). Each of these markers is commonly used to define hematopoietic progenitor cell content but has also been used to define endothelial progenitors (22–28).
The number of circulating progenitor cells is consistent with those reported in most previous studies, with a mean and median number of ALDHbr cells of 0.069 and 0.052% of mononuclear cells, respectively, and with similar numbers of CD133+ cells and CD34+ cells represented at approximately double that frequency (Table 3).
Table 3.
Mean and Median Numbers of Circulating Progenitor Cells
| Cell Type | % of MNCs (M ± SD) | % of MNCs, Median [interquantile range] |
| ALDHbr | 0.068 ± 0.056 | 0.052 [0.024–0.087] |
| CD133+ | 0.061 ± 0.043 | 0.053 [0.030–0.079] |
| CD34+ | 0.127 ± 0.092 | 0.090 [0.061–0.173] |
| VEGFR-2+ | 0.337 ± 0.225 | 0.274 [0.202–0.424] |
| CD34+–CD133+ | 0.024 ± 0.018 | 0.019 [0.011–0.038] |
Note: ALDH = aldehyde dehydrogenase; MNCs = mononuclear cells; VEGFR-2 = vascular endothelial growth factor receptor-2.
Progenitor cell content in the BM was normally distributed, with mean and median numbers that were closely related (Table 4). We calculated marrow-resident progenitors as a percentage of both the mononuclear cell population and the total BM cell population. In general, approximately 50% of cells were mononuclear, and the percentage of marrow-resident cells was approximately 2× higher among the mononuclear cell population.
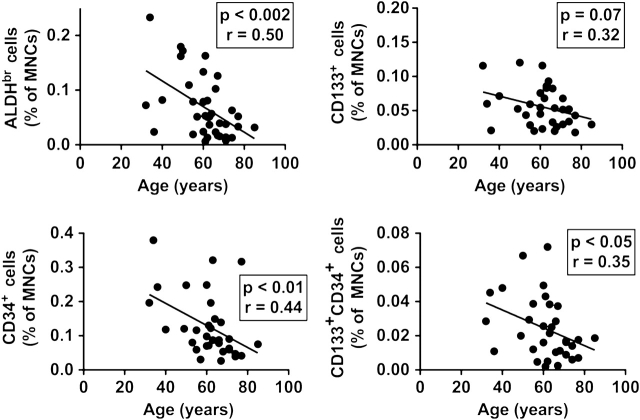
We observe a strong inverse relationship between age and the numbers of circulating ALDHbr, CD133+, CD34+, and CD133+–CD34+ progenitor cells (Figure 2). We did not observe an age-related loss of cells expressing VEGFR-2.
Figure 2.
Relationship between age and circulating progenitor cell content. Circulating progenitor cells were enumerated in peripheral blood samples drawn prior to surgery. The percentage of mononuclear cells expressing a progenitor cell phenotype (high levels of aldehyde dehydrogenase activity [upper left panel], CD133 expression [upper right panel], CD34 expression [lower left panel], CD133 and CD34 cell surface expression [lower right panel]) is plotted vs patient age, and the Pearson correlation coefficient and p value for correlation shown.
Table 4.
Mean and Median Bone Marrow (BM) Progenitor Cell Content
| Cell Type | % of MNCs in BM (M ± SD) | % of MNCs in BM, Median [IQR] | % of All Cells in BM (M ± SD) | % of All Cells in BM, Median [IQR] |
| ALDHbr | 3.32 ± 1.93 | 3.24 [1.87–4.57] | 1.14 ± 0.57 | 1.12 [0.85–1.51] |
| CD133+ | 2.02 ± 1.31 | 1.73 [1.04–2.85] | 0.76 ± 0.42 | 0.69 [0.45–0.93] |
| CD34+ | 6.33 ± 3.04 | 6.18 [4.33–8.58] | 2.15 ± 1.01 | 2.08 [1.53–2.75] |
| VEGFR-2+ | 1.11 ± 0.73 | 0.96 [0.45–1.63] | 0.66 ± 0.53 | 0.58 [0.25–0.92] |
| CD34+–CD133+ | 1.63 ± 1.15 | 1.39 [0.77–2.46] | 0.58 ± 0.72 | 0.49 [0.26–0.74] |
Note: ALDH = aldehyde dehydrogenase; IQR = interquantile range; MNCs = mononuclear cells; VEGFR-2 = vascular endothelial growth factor receptor-2.
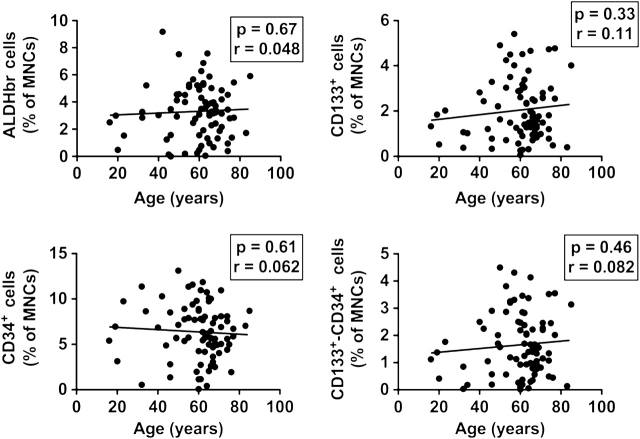
We assessed the numbers of marrow-resident progenitors using identical techniques and markers as were used to define circulating progenitors, with the exception that we did not observe a consistent population of marrow-resident VEGFR-2+ cells. We found no relationship between marrow-resident progenitor cell content and patient age, either among progenitor cells expressed as a proportion of all marrow cells or marrow mononuclear cells (Figure 3). This observation was consistent whether marrow-resident progenitors were defined on the basis of ALDH activity, single cell surface markers expression, or the use of a combination of cell surface markers.
Figure 3.
Relationship between age and bone marrow (BM)–resident progenitor cell content. BM-resident progenitor cells were enumerated in marrow samples obtained during surgery. The percentage of marrow mononuclear cells expressing a progenitor cell phenotype (high levels of aldehyde dehydrogenase (ALDH) activity [upper left panel], CD133 expression [upper right panel], CD34 expression [lower left panel], CD133 and CD34 cell surface expression [lower right panel]) is plotted vs patient age, and the Pearson correlation coefficient and p value for correlation shown.
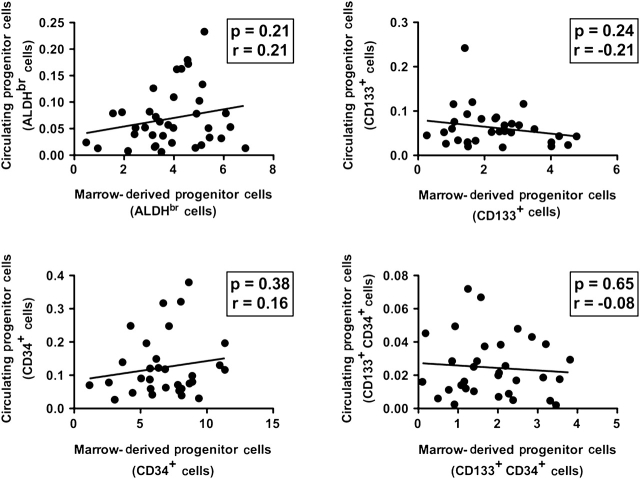
We also explored the relationship between circulating and marrow-resident progenitors. We found no relationship between any marrow-resident progenitor cell type and the numbers of circulating progenitors as identified by the same enumeration technique (Figure 4).
Figure 4.
Relationship between bone marrow–resident and circulating progenitor cells. The percentage of circulating mononuclear cells expressing a progenitor cell phenotype (high levels of aldehyde dehydrogenase (ALDH) activity [upper left panel], CD133 expression [upper right panel], CD34 expression [lower left panel], CD133 and CD34 cell surface expression [lower right panel]) is plotted vs marrow content of the same phenotype in samples drawn before and during surgery. The Pearson correlation coefficient and p value for correlation shown.
DISCUSSION
Age is often the most critical factor prognosticating outcome in a variety of clinical conditions, but the biologic underpinnings to the relationship remain unknown. A leading theory of aging is that chronic injury leads to eventual depletion of organismal reparative capacity. Repair has been best characterized in organs with rapid turnover such as the skin, intestine, and the hematopoietic system and is thought to emanate from a small population of long-lived resident stem cells with significant proliferative capacity.
Nonetheless, the relationship between aging and human progenitor cell numbers and function is poorly studied. Although several reports have reported depletion of circulating progenitor cells with age, the mechanism underlying this relationship is not known. One mechanistic explanation entails exhaustion of BM stores from which circulating progenitors are thought to arise. In addition, many of these studies entailed analysis of patients with advanced chronic (vascular) disease, which might affect progenitor cell stores and not reflect the aging process itself.
We sought to determine whether or not aging is associated with depletion of circulating and marrow-resident progenitors in a relatively healthy human population. We assessed these progenitors on the basis of a functional assay (ALDH activity) as well as based on cell surface expression of several surface markers. Selection of progenitors on the basis of ALDH activity identifies a population of cells with capacity for multilineage differentiation, including hematopoietic (7,8,10–12,14,17–19), neuronal (9,13,20), mesenchymal (16), and endothelial (16,21) progenitors. The cell surface markers CD34, CD133, and VEGFR-2 have been used to identify both hematopoietic and endothelial progenitor cell content (22–28). In our estimation, the use of these markers identifies populations of cells with subsets capable of differentiation, under proper culture conditions, into a variety of cell types, with a preponderance of these cells representing predominantly hematopoietic precursors. The use of ALDH, a property that may be inherent to progenitor cells in general, offers a methodology to assay overall progenitor cell content.
We found use of VEGFR-2 as a cell surface marker ineffectual as the number of marrow cells expressing this marker was small.
Consistent with studies that have shown a decline in circulating progenitors with age (29–34), we demonstrate declines in the numbers of circulating CD34+ and CD133+–CD34+, with strong trends toward a similar loss of CD133+ cells, as well as progenitors defined on the basis of ALDH activity, a property inherent to a variety of progenitor cell types (7,16,19,20). Indeed, the relationship we observe in our current study is remarkably consistent with results observed in patients with coronary artery disease, in which the levels and age-related decline in circulating ALDHbr cells mirrors what is observed in this current study (6).
In contrast to this observation, we failed to observe a relationship between age and marrow-resident progenitor cell content as determined using similar techniques.
These findings suggest that, at least in a relatively healthy patient population without significant chronic vascular injury, (a) aging does not result in a depletion in the numbers of marrow-resident progenitor cells and (b) the loss of circulating progenitors is not reflective of underlying marrow-resident progenitor cell stores. The former observation has possible implications for the use of autologous sources of progenitor cells for cell therapy applications.
Although this result may be surprising, it is consistent with what is observed in several models studying healthy animals (2–4). In addition, these observations are congruent with the known remarkable replicative capacity of marrow, which is capable of reconstituting an ablated hematopoietic system even in serial transplantation experiments (35), as well as a recent study comparing BM from older (average age 57 years) to younger (average age 23 years) volunteers. In this study, Taraldsrud and colleagues (36) report nonsignificant differences in the numbers of CD34+ and CD133+ in marrow of these volunteers, although the number of these cells in patients suffering an acute infarction was dramatically decreased. These authors did not sample circulating progenitors but did find that the number of “primitive” progenitors as determined based on lack of CD38 expression was higher in older patients.
These findings are consistent in that aging does not appear to be associated with a numerical loss of marrow progenitor cell stores. A more likely explanation for the loss of progenitor cell–mediated reparative capacity is an age-related impairment in progenitor cell function. Multiple animal models have suggested that progenitor cells from older animals display more qualitative than quantitative differences (4,37–42). In a mouse model of atherosclerosis, impaired progenitor cell–mediated vascular repair was observed in elderly mice, but marrow content of progenitor cells remained intact (33), whereas age-related deficits in progenitor cell homing (43) and differentiation capacity (38,39) have also been noted.
Similar age-related declines in human progenitor cell function have also been intimated. For instance, the number of granulocyte–macrophage colony-forming units increases from youth to early age (3,4); however, the ability of colonies to generate additional granulocyte–macrophage colony-forming units declined during this age period (44).
In the therapeutic arena, progenitor cell function has been reported to play a greater role than progenitor cell numbers in determining the efficacy of cell therapy after myocardial infarction (29,45), and human progenitor cells have impaired functional capacity in patients suffering from chronic cardiovascular health conditions (46,47).
Based on the quantity of the samples we obtained, we were unable to independently assess marrow progenitor cell function by performing migration or senescence assays; however, it would be of great interest to assess in patients without suspected marrow pathology the effect of age on various functional parameters.
Our observations may imply that other mechanism(s) are responsible for the loss of circulating progenitor cells. Possible mechanisms include (a) a loss of peripherally expressed stimuli (chemotactic or growth factors) prompting progenitor cell mobilization or (b) a decline in the responsiveness of marrow-resident cells to such stimuli. To discriminate between these alternatives, the assessment of levels of various chemokines and metalloproteinases implicated in mobilization of progenitor cells from the BM is of great interest. In the case of the first possibility, the administration of a proper “cocktail” of such progenitor cell–mobilizing factors might be expected to overcome the impaired expression of such agents in the elderly, whereas the latter possibility points to an age-related decline in progenitor cell responsiveness, which may underlie impaired reparative capacity.
Limitations
This work has several limitations. BM sampling was performed using samples obtained from the femoral head and canal. Active marrow in humans has been reported to decrease with age, and in an elderly population, hematopoietic activity is restricted to the pelvic, sternal, and vertebral marrow (48). Marrow obtained from femoral samples may not reflect progenitor cell content in other more active bone stores. The age-related decline that is observed in hematopoiesis outside the most active areas; however, would be expected to result in a great age-related decline in marrow progenitor cell content in long bones such as the femur, inconsistent with our results.
We assayed for progenitor cells based on both a functional property of progenitor cells (ALDH activity) and the expression of cell surface markers CD133, CD34, and VEGFR-2. Our results replicate previous observations of an age-related decline in circulating progenitor cells numbers, an observation made with multiple progenitor cell identification techniques; however, primitive progenitors may not express these markers. It may be that depletion of such earlier progenitors is responsible for the age-related decline, an observation made in a mouse model of aging (33).
Patients enrolled in our study had indications for hip replacement surgery and may represent neither healthy controls nor patients with other clinical conditions. The applicability of these results to patients without arthritis or other indications for joint replacement is unknown. We found that these patients lacked a significant number of comorbidities, and the prevalence of vascular disease, either peripheral or coronary disease, was limited to a small proportion (∼5%) of the population. Although the effect of arthritis on marrow progenitor cell content cannot be discounted, we failed to observe any trend toward and age-related loss of progenitor cells, suggesting that a confounder would have a significant impact on our observations. In addition, although our ability to detect a meaningful difference given on our sample size is small, we observed no significant differences in the marrow progenitor cell content as a function of indication for orthopedic surgery.
These results may not apply in conditions characterized by chronic ongoing injury, such as advanced vascular disease. In this regard, it would be useful to replicate these studies using BM samples from patients with advanced vascular disease. One such condition involves patients undergoing open cardiac surgery, in which sternal samples may be sampled at the time of sternotomy. The applicability of these results to such patients is paramount if we are to understand the interrelationship between chronic disease and age-related progenitor cell decline.
CONCLUSIONS
The relationship between aging and progenitor cell–mediated repair has received great interest. We have demonstrated in a relatively healthy patient population who had BM sampling at time of orthopedic surgery that circulating, but not marrow-resident, progenitors are depleted as a function of age. This finding suggests that mechanism(s) in addition to depletion of marrow stores are operative in age-mediated declines in progenitor cell–mediated repair. This finding has important implications for the use of autologous marrow for progenitor cell therapy and suggests that progenitor-mediated repair may be augmented if the age-related decline in the regulation of progenitor cell egress and mobilization from the marrow can be identified and overcome.
FUNDING
This study was supported in part by NHLBI grant K-18 HL081419-01A1 (T.J.P.), as well as a Duke Pepper Older Americans Independence Center (OAIC) Research Career Development Program in Aging Research (5P30AG028716).
References
- 1.de Haan G, Van Zant G. Dynamic changes in mouse hematopoietic stem cell numbers during aging. Blood. 1999;93:3294–3301. [PubMed] [Google Scholar]
- 2.Chen J, Astle CM, Harrison DE. Genetic regulation of primitive hematopoietic stem cell senescence. Exp Hematol. 2000;28:442–450. doi: 10.1016/s0301-472x(99)00157-5. [DOI] [PubMed] [Google Scholar]
- 3.de Haan G, Nijhof W, Van Zant G. Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood. 1997;89:1543–1550. [PubMed] [Google Scholar]
- 4.Morrison S, Wandycz A, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 5.Bagnara G, Bonsi L, Strippoli P, et al. Hemopoiesis in healthy old people and centenarians: well-maintained responsiveness of cd34+ cells to hemopoietic growth factors and remodeling of cytokine network. J Gerontol A Biol Sci Med Sci. 2000;55:B61–B66. doi: 10.1093/gerona/55.2.b61. [DOI] [PubMed] [Google Scholar]
- 6.Povsic T, Zavodni K, Kelly F, et al. Circulating endogenous progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol. 2007;53:2243–2248. doi: 10.1016/j.jacc.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 7.Storms RW, Trujillo AP, Springer JB, et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc Natl Acad Sci U S A. 1999;96:9118–9123. doi: 10.1073/pnas.96.16.9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storms RW, Safford K, Rice H, Colvin OM, Smith CA. Aldehyde dehydrogenase is expressed by primitive cd34+ hematopoietic progenitors. Biol Blood Marrow Transplant. 2003;9:18. [Google Scholar]
- 9.Cai J, Cheng A, Luo Y, et al. Membrane properties of rat embryonic multipotent neural stem cells. J Neurochem. 2004;88:212–226. doi: 10.1046/j.1471-4159.2003.02184.x. [DOI] [PubMed] [Google Scholar]
- 10.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104:1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 11.Storms RW, Green PD, Safford KM, et al. Distinct hematopoietic progenitor compartments are delineated by the expression of aldehyde dehydrogenase and cd34. Blood. 2005;106:95–102. doi: 10.1182/blood-2004-09-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hess DA, Wirthlin L, Craft TP, et al. Selection based on cd133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corti S, Locatelli F, Papadimitriou D, et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 2006;24:975–985. doi: 10.1634/stemcells.2005-0217. [DOI] [PubMed] [Google Scholar]
- 14.Christ O, Lucke K, Imren S, et al. Improved purification of hematopoietic stem cells based on their elevated aldehyde dehydrogenase activity. Haematologica. 2007;92:1165–1172. doi: 10.3324/haematol.11366. [DOI] [PubMed] [Google Scholar]
- 15.Gentry T, Deibert E, Foster SJ, Haley R, Kurtzberg J, Balber AE. Isolation of early hematopoietic cells, including megakaryocyte progenitors, in the ALDH-bright cell population of cryopreserved, banked UC blood. Cytotherapy. 2007;9:569–576. doi: 10.1080/14653240701466347. [DOI] [PubMed] [Google Scholar]
- 16.Gentry T, Foster S, Winstead L, Deibert E, Fiordalisi M, Balber A. Simultaneous isolation of human BM hematopoietic, endothelial and mesenchymal progenitor cells by flow sorting based on aldehyde dehydrogenase activity: implications for cell therapy. Cytotherapy. 2007;9:259–274. doi: 10.1080/14653240701218516. [DOI] [PubMed] [Google Scholar]
- 17.Hess DA, Wirthlin L, Craft TP, Herrbrich PE, Lahey R, Eades WC, Creer MH, Nolta JA. Selection based on cd133 and high aldehyde dehydrogenase activity isolates long-term reconstituting human hematopoietic stem cells. Blood. 2006;107:2162–2169. doi: 10.1182/blood-2005-06-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierre-Louis O, Clay D, Grange PBdl, et al. Dual SP/ALDH functionalities refine the human hematopoietic Lin-CD34+CD38-stem/progenitor cell compartment. Stem Cells. 2009;27:2552–2562. doi: 10.1002/stem.186. [DOI] [PubMed] [Google Scholar]
- 19.Fallon P, Gentry T, Balber AE, et al. Mobilized peripheral blood SSClo ALDHbr cells have the phenotypic and functional properties of primitive haematopoietic cells and their number correlates with engraftment following autologous transplantation. Br J Haematol. 2003;122:99–108. doi: 10.1046/j.1365-2141.2003.04357.x. [DOI] [PubMed] [Google Scholar]
- 20.Corti S, Locatelli F, Papadimitriou D, et al. Transplanted ALDHhiSSClo neural stem cells generate motor neurons and delay disease progression of nmd mice, an animal model of SMARD1. Hum Mol Genet. 2006;15:167–187. doi: 10.1093/hmg/ddi446. [DOI] [PubMed] [Google Scholar]
- 21.Capoccia BJ, Robson DL, Levac KD, et al. Revascularization of ischemic limbs after transplantation of human bone marrow cells with high aldehyde dehydrogenase activity. Blood. 2009;113:5340–5351. doi: 10.1182/blood-2008-04-154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojakowski W, Tendera M, Michalowska A, et al. Mobilization of cd34/cxcr4+, cd34/cd117+, c-met+ stem cells, and mononuclear cells expressing early cardiac, muscle, and endothelial markers into peripheral blood in patients with acute myocardial infarction. Circulation. 2004;110:3213–3220. doi: 10.1161/01.CIR.0000147609.39780.02. [DOI] [PubMed] [Google Scholar]
- 23.Valgimigli M, Rigolin GM, Fucili A, et al. Cd34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 24.Theiss HD, David R, Engelmann MG, et al. Circulation of CD34+ progenitor cell populations in patients with idiopathic dilated and ischaemic cardiomyopathy (DCM and ICM) Eur Heart J. 2007;28:1258–1264. doi: 10.1093/eurheartj/ehm011. [DOI] [PubMed] [Google Scholar]
- 25.Scheubel RJ, Zorn H, Silber R-E, et al. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 26.Redondo S, Hristov M, Gordillo-Moscoso AA, Ruiz E, Weber C, Tejerina T. High-reproducible flow cytometric endothelial progenitor cell determination in human peripheral blood as CD34+/CD144+/CD3-lymphocyte sub-population. J Immunol Methods. 2008;335:21–27. doi: 10.1016/j.jim.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating CD34+ cells identifies a population of functional endothelial precursors. Hemost Thromb Vasc Biol. 2000;95:952–958. [PubMed] [Google Scholar]
- 28.Grundmann F, Scheid C, Braun D, et al. Differential increase of CD34, KDR/CD34, CD133/CD34 and CD117/CD34 positive cells in peripheral blood of patients with acute myocardial infarction. Clin Res Cardiol. 2007;96:621–627. doi: 10.1007/s00392-007-0543-7. [DOI] [PubMed] [Google Scholar]
- 29.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 31.Kunz G, Liang G, Cuculi G, et al. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152:190–195. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Lehrke S, Mazhari R, Durand DJ, et al. Aging impairs the beneficial effect of granulocyte colony-stimulating factor and stem cell factor on post-myocardial infarction remodeling. Circ Res. 2006;99:553–560. doi: 10.1161/01.RES.0000238375.88582.d8. [DOI] [PubMed] [Google Scholar]
- 33.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, et al. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- 34.Shaffer RG, Greene S, Arshi A, Supple G, Bantly A, Moore JS, Mohler ER., III Flow cytometric measurement of circulating endothelial cells: the effect of age and peripheral arterial disease on baseline levels of mature and progenitor populations. Cytometry B Clin Cytom. 2006;70B:56–62. doi: 10.1002/cyto.b.20085. [DOI] [PubMed] [Google Scholar]
- 35.Harrison DE, Astle CM, Delaittre J. Loss of proliferative capacity in immunohemopoietic stem cells is caused by serial tranplantation rather than aging. J Exp Med. 1978;147:1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taraldsrud E, Grøgaard HK, Solheim S, et al. Age and stress related phenotypical changes in bone marrow CD34+ cells. Scand J Clin Lab Invest. 2009;69:79–84. doi: 10.1080/00365510802419447. [DOI] [PubMed] [Google Scholar]
- 37.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 38.Spangrude G, Brooks D, Tumas D. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1016. [PubMed] [Google Scholar]
- 39.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Zant G, de Haan G, Rich I. Alternatives to stem cell renewal from a developmental viewpoint. Exp Hematol. 1997;25:187–192. [PubMed] [Google Scholar]
- 41.Van Zant G, Liang Y. The role of stem cells in aging. Exp Hematol. 2003;31:659–672. doi: 10.1016/s0301-472x(03)00088-2. [DOI] [PubMed] [Google Scholar]
- 42.Vergaillie C. Hematopoietic stem cells for transplantation. Nat Immunol. 2002;3:314–317. doi: 10.1038/ni0402-314. [DOI] [PubMed] [Google Scholar]
- 43.Morrison S, Wandycz A, Akashi K, Globerson A, Weissman IL. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 44.Marley SB, Lewis JL, Davidson RJ, et al. Evidence for a continuous decline in haemopoietic cell function from birth: application to evaluating bone marrow failure in children. Br J Haematol. 1999;106:162–166. doi: 10.1046/j.1365-2141.1999.01477.x. [DOI] [PubMed] [Google Scholar]
- 45.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 46.Heeschen C, Lehmann R, Honold J, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 47.Kissel CK, Lehmann R, Assmus B, et al. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 48.Harstock R, Smith E, Ketter C. Normal variation with aging of the amount of hematopoietic tissue in bone marrow from anterior iliac crest. Am J Clin Pathol. 1965;43:325–333. doi: 10.1093/ajcp/43.4.326. [DOI] [PubMed] [Google Scholar]