Abstract
A clinical field trial was performed to determine the effectiveness of an autogenous Salmonella Typhimurium bacterin compared with a commercial live S. Choleraesuis vaccine in pigs. The association between Salmonella shedding and weight gain was also investigated. Nine cohorts of weaned pigs, (330 to 350 pigs per cohort), were randomly assigned to 1 of 3 treatment groups (injection with S. Typhimurium bacterin, vaccination via water with S. Choleraesuis vaccine, or a control group receiving no vaccine). In each cohort, the average daily gain was calculated for a selected pen throughout the production stage. Pen (pooled) fecal samples were collected bi-weekly and cultured. The odds of Salmonella shedding in both vaccinated groups was higher than in the control group (P < 0.05). The prevalence of Salmonella shedding declined overall as pigs aged (P = 0.04). However, the control pigs showed the smallest decrease in Salmonella shedding over the entire production stage, while prevalence of Salmonella shedding in the vaccinated groups decreased twice as much as the control group over the entire production stage. Salmonella Typhimurium var. Copenhagen DT104, S. Cerro, and S. Agona, which had been isolated on the study farm previously, were recovered from pigs in this study. Shedding of S. Typhimurium var. Copenhagen decreased over time in both vaccine treatment groups. On the other hand, S. Cerro shedding rate was lower in the control pigs compared with vaccinated pigs and S. Agona could be recovered only from the samples collected from S. Choleraesuis vaccinated pigs. The pigs from pens with a higher Salmonella recovery rate experienced slower growth compared with pigs from pens where Salmonella was not isolated. This latter finding indicates that there might be an economic incentive for producers to try to control endemic salmonellosis if effective programs could be developed.
Résumé
Un essai clinique a été réalisé afin de comparer l’efficacité d’une bactérine autogène de Salmonella Typhimurium à un vaccin vivant commercial de S. Choleraesuis. L’association entre l’excrétion de Salmonella et le gain de poids a également été étudiée. Neuf cohortes de porcs sevrés (330 à 350 porcs par cohorte) ont été réparties de manière aléatoire à un des 3 groupes de traitement (injection avec la bactérine de S. Typhimurium, vaccination via l’eau avec un vaccin S. Choleraesuis, ou un groupe témoin ne recevant aucun vaccin). Dans chaque cohorte, le gain moyen quotidien a été calculé pour un enclos sélectionné durant toute la période de production. Des échantillons de fèces pris dans les parcs ont été récoltés bi-hebdomadairement et mis en culture. Les probabilités d’excrétion de Salmonella dans les deux groupes vaccinés étaient plus élevées que dans le groupe témoin (P < 0,05). La prévalence d’excrétion de Salmonella a diminué à mesure que les porcs vieillissaient (P = 0,04). Toutefois, les porcs témoins ont présenté la plus petite réduction dans l’excrétion de Salmonella durant toute la période de production, alors que la prévalence d’excrétion de Salmonella dans les groupes vaccinés durant toute la période de production a diminué deux fois plus que le groupe témoin. Salmonella Typhimurium var. Copenhagen DT104, S. Cerro et S. Agona, qui avaient été isolés préalablement sur la ferme à l’étude, ont été isolés à partir de porcs dans cette étude. L’excrétion de S. Typhimurium var. Copenhagen a diminué dans le temps chez les deux groupes d’animaux vaccinés. Par contre, le taux d’excrétion de S. Cerro était plus faible chez les porcs du groupe témoin comparativement aux porcs vaccinés et S. Agona n’a été retrouvé qu’à partir des échantillons prélevés des animaux vaccinés avec S. Choleraesuis. Les porcs provenant des enclos avec un taux plus élevé d’isolement de Salmonella présentaient une croissance ralentie comparativement aux porcs provenant des enclos où on ne retrouva pas de Salmonella. Cette dernière trouvaille indique qu’il y aurait un incitatif financier pour les producteurs à tenter de limiter la salmonellose endémique si des programmes efficaces pouvaient être développés.
(Traduit par Docteur Serge Messier)
Introduction
Salmonella continue to be important foodborne pathogens with a significant economic impact (1). Exposure to Salmonella either by direct contact with infected pigs or through consumption of contaminated pork, is an important mode of infection in humans (2). In addition to the public health issue, Salmonella have the potential to cause clinical disease in pigs (3) resulting in a significant economic impact on swine production.
It is necessary to take the presence of Salmonella on swine farms as a serious issue and apply appropriate practical intervention strategies. Immunization appears to be one of the most promising approaches for control (4–8). Salmonella Choleraesuis vaccines are commercially available and may provide cross-protection against other Salmonella serovars as well (9); however, S. Choleraesuis does not appear to be a common pathogen in Ontario swine herds any longer (10–11).
In contrast, Salmonella Typhimurium var. Copenhagen has become the most frequent serovar recovered on Ontario swine farms (11), and yet it only occasionally causes clinical disease (12). More frequently S. Typhimurium is present without the herdsman noticing any clinical signs (13). There is little information regarding the economic impact of S. Typhimurium infection in pigs, particularly in herds with subclinical infection. Although live S. Typhimurium vaccines have been reported to protect pigs against Salmonella shedding in experimental challenge studies (14–15), there are no commercial S. Typhimurium vaccines available for use in swine.
The objectives of the present study were i) to determine if an autogenous S. Typhimurium var. Copenhagen vaccine or a commercial live S. Choleraesuis vaccine can reduce the prevalence of Salmonella shedding in market weight hogs under field conditions, ii) to investigate whether or not Salmonella vaccination can improve growth performance, and iii) to determine if Salmonella shedding affects weight gain in pigs.
Materials and methods
Study design and sample collection
The trial was conducted on one farrow-to-finish pig operation with a history of clinical and sub-clinical salmonellosis. Previously, Salmonella Typhimurium var. Copenhagen DT104 and Salmonella Cerro had been recovered from fecal samples collected from grower-finisher pens on this operation (10–11). In addition, Salmonella Agona had been isolated from specimens from clinical cases of salmonellosis on this farm (Farzan and Friendship, unpublished data). The biosecurity measures on this farm included, but were not limited to, shower-in/shower-out for visitors and farm workers, all-in/all-out management of rooms with washing and disinfection between fills, and single-source supply of replacement gilts.
Nine cohorts of weaned pigs, approximately 350 pigs in each group, were randomly assigned to 1 of 3 treatment groups. Group 1 — intramuscular injection with an autogenous S. Typhimurium bacterin (4 cohorts); group 2 — in-water administration of a modified-live S. Choleraesuis vaccine (Argus SC/ST, Intervet CanadaCorp, Kirkland, Quebec) (3 cohorts); group 3 — an untreated control group (3 cohorts). In each cohort, the weaned pigs were housed in a 4-pen room (stage-1 nursery) for 2 to 3 wk, after which the pigs were moved into a 6-pen room (stage-2 nursery). In each cohort, one pen was randomly selected in the nursery stage and 30 pigs were ear-tagged and weighed. After 4 to 7 wk, the pigs were moved into 12-pen rooms (grower-finisher stage) and kept there until marketing. The tagged pigs were weighed again when marketing. Pooled-fecal samples were collected from manure found on the pen floor prior to vaccination, weekly from each nursery pen, and bi-weekly from grower-finisher pens.
Salmonella identification
The presence of Salmonella in the fecal samples was tested at the Laboratory Service Division, University of Guelph according to MFHPB-20 (16) and MFLP-84 (17) protocol. Briefly, a 10% suspension of fecal samples in 0.85% saline was prepared and 1 mL transferred into 10 mL of Buffer Peptone Water (BPW). The pre-enrichment mixtures were incubated at 35°C for 18 to 24 h, then 1 mL of culture was reacted with 20 μL of anti-Salmonella beads (Dynabeads; Invitrogen Corporation, Carlsbad, California, USA). The beads were washed twice in 1 mL of PBST (phosphate buffered saline containing 0.01% Tween 20, pH 7.4), and concentrated in 100 μL of PBST using an automated immunomagnetic separator (Invitrogen Corporation). The 100 μL concentrates were transferred into 10 mL of Rappaport-Vassiliadis Soya Peptone Broth (RVS) (Oxoid, Nepean, Ontario) and incubated at 42 ± 0.5°C for 18 to 24 h. A loopful from the RVS cultures was plated on brilliant green sulfapyridine (BGS) and bismuth sulfite (BS) agars and incubated for 24 ± 2 h at 35 ± 0.5°C. Confirmation of Salmonella isolates was performed by serological and biochemical means using triple sugar iron agar (TSI), lysine iron agar (LIA), urea slants, and Salmonella O antiserum Poly A-I and Vi slide agglutination tests (Difco, Detroit, Michigan, USA). The isolates were submitted to the Reference Laboratory for Salmonellosis, Laboratory for Foodborne Zoonoses (LFZ), Public Health Agency of Canada, Guelph for serotyping and phagetyping.
Preparation of autogenous vaccine
The autogenous vaccine was prepared at Gallant Custom Laboratories, Cambridge, Ontario using a S. Typhimurium var. Copenhagen DT104 strain previously isolated from the study farm (11). Briefly, 1000 mL of trypticase soy broth (TSB) was inoculated with a single colony of the S. Typhimurium var. Copenhagen DT104 and incubated at 37°C aerobically for 6 h using agitation. In order to determine the number of colony forming units (CFU/mL) serial dilutions of bacterial cultures in isotonic saline (0.9% NaCl) buffer were prepared and 0.1 mL of diluted cultures were plated on XLT-4 plates and incubated at 37°C overnight. The 1000 mL TSB culture was centrifuged and the pellet was washed with PBS buffer containing 0.45% formalin. The wash and centrifugation steps were repeated 5 times. The bacterial cells were suspended in alhydrogel in a ratio 1:3 (antigen: adjuvant) to obtain 108 CFU/mL bacterin. The vaccine sterility was controlled by plating on blood agar and Salmonella selective media. To test the safety of the vaccine, a dose of 2 mL was intramuscularly injected into 2 weaned pigs and the pigs were observed for 1 wk.
Data analysis
Data were entered into a spreadsheet (Microsoft Excel 2003; Microsoft, Redmond, Washington, USA) and imported into statistics software (Stata 9.1 Intercooled for Windows XP; StataCorp, College Station, Texas, USA). A Generalized Linear Latent and Mixed Models (GLLAMM) was used to compare the presence of Salmonella, as well as prevalence of most frequent serotypes and phagetypes in the pooled fecal samples collected from the pens in 3 groups. A repeated measurement index was included as random effect into the model to take the correlation between weekly and bi-weekly testing into account. A mixed linear regression method with pen as random effect was applied to compare the average daily gain in the vaccinated and the control pigs. Since the average starting weight of pigs did differ in 3 cohorts, it was included in the model. Sex and age of pigs were considered for initial inclusion in the regression analysis. In order to investigate the impact of Salmonella shedding regardless of vaccination, the average daily gain was regressed versus the recovery rate of Salmonella from the pens where the ear-tagged pigs were kept. The presence of confounding and interaction, between weight of pigs at weaning and age of pigs at sampling, with the main effect was investigated in each model.
Results
Salmonella shedding
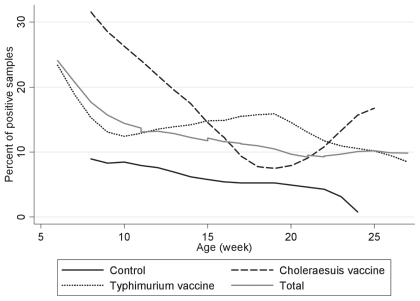
In total, 653 pooled pen samples were collected from stage-1 nursery (20 samples), stage-2 nursery (201 samples), and finisher (432) rooms. Salmonella were cultured from 85 (13%) of the samples. The number of Salmonella-positive samples in each group is shown in Table I. The Salmonella recovery rate differed among the 3 groups before and after vaccination with the lowest in the control group (5.9%) and the highest in the S. Choleraesuis vaccinated group (19.0%). In the multilevel analysis, the odds of Salmonella shedding in both vaccinated groups was higher than in the control group (Table II). The S. Choleraesuis vaccinated pigs were more likely [OR = 5.2 (1.8, 15.5)] to shed Salmonella compared with control pigs. Similarly the odds of Salmonella shedding was 3.3 (1.1, 9.9) greater in the pigs that were injected with autogenous vaccine versus control pigs. In multivariable analysis while controlling for vaccination, the prevalence of Salmonella shedding declined overall when pigs aged (P = 0.04). Overall, the recovery of Salmonella decreased from 24.5% at weaning to 10.0% at marketing (Figure 1, Table II). The control pigs showed the smallest decrease (8.0%) in Salmonella shedding over the entire production stage with decreasing from 8.8% at weaning to 0.8% at marketing age (Figure 1). In contrast, prevalence of Salmonella shedding in the vaccinated groups decreased twice as much as the control group over the entire production stage. Prevalence of Salmonella shedding in the S. Typhimurium vaccinated group declined from 24% prior to vaccination to 8.5% in marketing-age pigs (Figure 1). Salmonella shedding among the S. Choleraesuis vaccinated pigs declined from 32.0% prior to vaccination to 16.0% at marketing age (Figure 1).
Table I.
Culture of Salmonella from pooled manure samples collected from pens housing pigs assigned to 1 of 3 treatments: S. Choleraesuis live oral vaccine, S. Typhimurium autogenous bacterin given intramuscularly, and non-vaccinated controls
| Number of positive samples/total (%) | ||||
|---|---|---|---|---|
| Treatment group | Nursery 1 | Nursery 2 | Finisher | Total |
| S. Choleraesuis vaccine | Not sampled | 14/75 (18.7) | 30/156 (19.2) | 44/231(19.0) |
| S. Typhimurium vaccine | 4/20 (20.0) | 8/78 (10.3) | 19/156 (12.2) | 31/254 (12.2) |
| Control | Not sampled | 4/48 (8.3) | 6/120 (5.0) | 10/168 (5.9) |
| Total | 4/20 (20.0) | 26/201 (12.9) | 55/432 (12.7) | 85/653 (13.0) |
Table II.
The impact of vaccination and age of pigs on Salmonella shedding in the vaccinated and control pigs analyzed by Generalized Linear Latent and Mixed Models
| Odds ratio | Standard error | 95% Confidence interval | P-value | |
|---|---|---|---|---|
| S. Choleraesuis vaccine | 5.2 | 2.9 | 1.8, 15.5 | 0.003 |
| S. Typhimurium vaccine | 3.3 | 1.8 | 1.1, 9.9 | 0.037 |
| Control Reference | ||||
| Age (week) | 0.9 | 0.0 | 0.8, 0.99 | 0.04 |
Figure 1.
Salmonella shedding trends among vaccinated and control pigs over the entire production stage.
Salmonella serovars
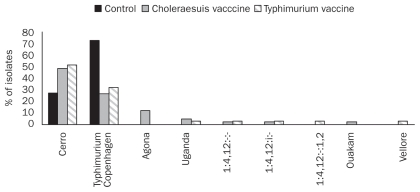
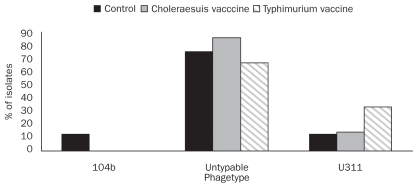
Nine different Salmonella serovars were identified over the entire period of the trial. S. Cerro was the most frequent serovar isolated followed by S. Typhimurium var. Copenhagen (Figure 2). Salmonella Typhimurium var. Copenhagen was isolated from 72.7% of samples collected from unvaccinated pigs, while it could only be cultured from 26.8% and 32.3% of samples collected from oral and autogenous vaccinated pigs, respectively (Figure 2). On the other hand, S. Cerro shedding rate was lower in the control pigs compared with vaccinated pigs in that it was isolated from 27.3% of samples in the control pigs versus 48.8% and 51.6% of samples collected from pigs that received oral live S. Choleraesuis, and injectable autogenous killed vaccine, respectively. Salmonella Agona could be recovered only from the samples collected from S. Choleraesuis vaccinated pigs. Salmonella Typhimurium var. Copenhagen phagetypes isolated from pigs in 3 groups are shown in Figure 3. Three phagetypes were identified among the 31 S. Typhimurium isolates including DT104b (1 isolate), U311 (6 isolates), and Untypable (UT) (24 isolates). No DT104 phagetypes were identified and the only 1 DT104b isolate was recovered from the control pigs.
Figure 2.
Salmonella serovars isolated from fecal samples collected from vaccinated and control pigs.
Figure 3.
Salmonella Typhimurium var. Copenhagen phagetypes isolated from fecal samples collected from vaccinated and control pigs.
Average daily weight gain
In total, 251 pigs were ear-tagged in the 9 cohorts and housed in separate pens during the nursery and finishing stages. Salmonella was cultured from 5 (10.4%), 7 (13.5%), and 2 (6.5%) of the samples from pens housing S. Choleraesuis vaccinated, S. Typhimurium vaccinated, and non-vaccinated controls, respectively. The average daily gain in these 3 groups was determined as 736.5 g, 647.9 g, and 762.6 g for S. Choleraesuis vaccinated, S. Typhimurium vaccinated, and non-vaccinated controls, respectively. In the multilevel analysis, the control pigs that had the lowest average of Salmonella shedding showed best growth performance compared with the vaccinated groups (Table III). In order to investigate the impact of Salmonella shedding regardless of vaccination, the average daily gain was regressed versus the recovery rate of Salmonella from the pens where the ear-tagged pigs were kept. The multilevel analysis showed that the pigs from pens with a higher Salmonella recovery rate were deemed to have a lower average daily gain (P = 0.059) (Table IV).
Table III.
The effect of Salmonella-vaccination on average daily weight gain in pigs
| Group | Coefficient (g) | Standard error | 95% Confidence interval | P-value |
|---|---|---|---|---|
| S. Choleraesuis vaccine | −26.6 | 13.5 | −53.0, 0.27 | 0.048 |
| S. Typhimurium vaccine | −90.8 | 16.2 | −122.5, −59.0 | < 0.001 |
| Control Reference | ||||
| Weight at weaning (kg) | 4.7 | 2.1 | 0.7, 8.7 | 0.022 |
| Intercept | 694.5 | 31.2 | 633.3, 755.8 | < 0.001 |
Table IV.
The effect of Salmonella shedding on average daily weight gain in pigs
| Parameter | Coefficient (g) | Standard error | 95% Confidence interval | P-value |
|---|---|---|---|---|
| Salmonella-positive samples (%)a | −3.0 | 1.6 | −6.1, 0.4 | 0.059 |
| Weight at weaning (kg) | 10.5 | 1.8 | 7.0, 14.0 | < 0.001 |
| Intercept | 608.3 | 34.5 | 540.7, 675.9 | < 0.001 |
Percent of Salmonella-positive samples collected from pens where the ear-tagged pigs were kept.
Discussion
Salmonella Typhimurium var. Copenhagen has become the most frequent serovar on Ontario swine farms (10–11). There is no S. Typhimurium vaccine registered for use in pigs in Canada but S. Choleraesuis vaccines are commercially available. Salmonella Choleraesuis modified live vaccine was reported to cross-protect against other Salmonella serovars (9). Husa et al (5) have recently conducted a controlled challenge trial on 2 different modified live S. Choleraesuis vaccines, including Argus (SC/ST, Intervet) and Enterisol SC-54 (Boehringer Ingelheim Vetmedica, St. Joseph, Missouri, USA). They have shown that the enteric lesion and clinical scores in the pigs vaccinated with Argus were similar to the control pigs and that the vaccine could not reduce Salmonella shedding after the animals were challenged with S. Typhimurium. In addition, the growth rate was lower in the Argus-vaccinated pigs compared with controls in the Husa et al (5) trial. Yet, the effectiveness of the S. Choleraesuis vaccine and its protection against other serovars under farm conditions remains unknown. Nevertheless, because of the significant labor required to inject vaccines into nursery pigs, compliance may be a problem in the case of autogenous bacterins and S. Choleraesuis modified-live vaccine may be preferred due to being easier to administer. The primary goal of the present study was to investigate the effectiveness of a commercial oral live S. Choleraesuis vaccine compared with an autogenous S. Typhimurium bacterin to control Salmonella shedding in the market weight pigs under field conditions. Neither vaccination approach proved to be better than non-vaccinated controls to reduce Salmonella sp. shedding. However, as this was a field trial, there were factors that could not be easily controlled for and as such, interpretation of the data was difficult. The prevalence of Salmonella shedding was different among the treatment groups prior to vaccination in that it was the lowest in the control group. Vaccination was associated with a significant decrease in shedding from nursery to market; however, this could have been because the level was high to begin with. It has previously been shown that younger pigs are more likely to shed Salmonella than finisher pigs (18); therefore, our estimate of vaccine effectiveness may be biased because the decrease in shedding might be due to age. In order to take this into account “age of pig at the sampling date” was included into the multivariable analysis.
The vaccination was likely done after pigs had been exposed and that efficacy might have been improved had the pigs been vaccinated sooner. At least one study suggests that it might be prudent to establish a vaccination strategy for pregnant sows to control Salmonella in their piglets (19) followed by another vaccination at weaning age.
Salmonella vaccination strategy using the live attenuated strains administrated orally has been suggested as the most effective approach (20), as oral immunization can stimulate local gut immunity (21) inducing mucosal IgA secretion in the gut (22) that is important in protection. The oral Salmonella live vaccines can also induce cell-mediated immunity that may play a major role in protecting pigs because Salmonella are facultative intracellular pathogens and may avoid humoral immune response in the intracellular environment (23). However, despite a lack of protection for pigs against Salmonella shedding, the S. Choleraesuis vaccine proved to be easily administered in the drinking water, and this approach was readily acceptable by the producer.
Salmonella Typhimurium var. Copenhagen DT104, S. Cerro, and S. Agona, which had been previously isolated on the study farm (10–11), could be recovered from pigs in this study. Although neither vaccination approach proved to be better than non-vaccinated controls to reduce Salmonella sp. shedding, both vaccines were associated with a decline in shedding of S. Typhimurium var. Copenhagen. The failure of the S. Choleraesuis vaccine to reduce Salmonella Agona shedding, however, might be due to lack of cross-protection. Although S. Choleraesuis vaccine was reported to cross-protect against S. Typhimurium (9), it did not protect pigs against shedding other members of serogroup B (S. Agona) in the present study. Salmonella Cerro, a member of serogroup K with somatic antigen O18, was the most frequent serovar recovered from both vaccinated groups suggesting that neither S. Typhimurium (sero-group B; antigen O4) nor S. Choleraesuis (serogroup C1; antigen O6, 7) cross-protect the pigs against a member of serogroup K, such as S. Cerro with somatic antigen O18. On the other hand, the pigs vaccinated with S. Typhimurium did not appear to shed Salmonella Agona and since S. Typhimurium and S. Agona are members of serogroup B carrying O4, cross-protection between these serovars might be effective.
These findings indicate that the most logical approach to Salmonella vaccination would be to try to develop a multivalent Salmonella vaccine containing multi-serogroup somatic antigens that could be administered in the drinking water. In addition to making the product easy to use, it is also likely that oral immunization could induce local gut immunity and cell-mediated immunity.
Another significant finding of this study is that pigs that appeared clinically healthy but were found to be shedding Salmonella grew slower than pigs not shedding Salmonella. This suggests that there is an economic cost to subclinical Salmonella infection. Fraser et al (24) have shown that experimental infection with Salmonella Typhimurium did not affect the average daily gain in pigs while infection with Salmonella Choleraesuis reduced the average daily gain. Husa et al (5) have recently conducted an experimental trial and shown that the growth rate was lower in the Argus-vaccinated pigs compared to unvaccinated control pigs. However, to the best of our knowledge, the present study is the first report on the impact of Salmonella shedding on weight gain in pigs under field conditions. These findings indicate that the cost of intervention strategies might be compensated by improved weight gain in the absence of clinical disease. Therefore, there would be an economic incentive for producers to institute the control measures if a practical and effective control program could be devised.
Acknowledgments
The authors acknowledge the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) — Food Safety Research Program and Ontario Pork for funding the project. We thank Dr. Jackie Gallant from Gallant Laboratory Inc. for preparing the autogenous vaccine; Dr. Susan Lee, Carlos Leon-Velarde, and Nathaniel Larson from Laboratory Service Division at University of Guelph for Salmonella culturing; and Linda Cole, Betty Wilkie, Ketna Mistry, and Ann Perets of the Salmonella Typing Laboratory from Laboratory for Foodborne Zoonoses, Public Health Agency of Canada. The authors also thank the pig producer (BT) who participated in the project.
References
- 1.Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berends BR, Van Knapen F, Mossel DA, et al. Impact on human health of Salmonella spp. on pork in The Netherlands and the anticipated effects of some currently proposed control strategies. Int J Food Microbiol. 1998;44:219–229. doi: 10.1016/s0168-1605(98)00121-4. [DOI] [PubMed] [Google Scholar]
- 3.Griffith RW, Schwartz KJ, Meyerholz DK. Salmonella. In: Straw EB, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Publ; 2006. pp. 739–754. [Google Scholar]
- 4.Denagamage TN, O’Connor AM, Sargeant JM, et al. Efficacy of vaccination to reduce Salmonella prevalence in live and slaughtered swine: A systematic review of literature from 1979 to 2007. Foodborne Pathog Dis. 2007;4:539–549. doi: 10.1089/fpd.2007.0013. [DOI] [PubMed] [Google Scholar]
- 5.Husa JA, Edler RA, Walter DH, et al. A comparison of the safety, cross-protection, and serologic response associated with two commercial oral Salmonella vaccines in swine. J Swine Health Prod. 2009;17:10–21. [Google Scholar]
- 6.Kelly SM, Bosecker BA, Curtiss R., 3rd Characterization and protective properties of attenuated mutants of Salmonella Choleraesuis. Infect Immun. 1992;60:4881–4890. doi: 10.1128/iai.60.11.4881-4890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy MJ, Yancey RJ, Sanchez MS, et al. Attenuation and immunogenicity of Deltacya Deltacrp derivatives of Salmonella Choleraesuis in pigs. Infect Immun. 1999;67:4628–4636. doi: 10.1128/iai.67.9.4628-4636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roof MB, Doitchinoff DD. Safety, efficacy, and duration of immunity induced in swine by use of an avirulent live Salmonella Choleraesuis-containing vaccine. Am J Vet Res. 1995;56:39–44. [PubMed] [Google Scholar]
- 9.Maes D, Gibson K, Trigo E, et al. Evaluation of cross-protection afforded by a Salmonella Choleraesuis vaccine against Salmonella infections in pigs under field conditions. Berl Munch Tierarztl Wochenschr. 2001;114:339–341. [PubMed] [Google Scholar]
- 10.Farzan A, Friendship RM, Cook A, et al. Occurrence of Salmonella, Campylobacter, Yersinia enterocolitica, Escherchia coli O157, and Listeria monocytogenes in swine. Zoonoses and Public Health. 2009 doi: 10.1111/j.1863-2378.2009.01248.x. (Epub ahead of print 2009 Jul 23) [DOI] [PubMed] [Google Scholar]
- 11.Farzan A, Friendship RM, Dewey CE, et al. A longitudinal study of the Salmonella status on Ontario swine farms within the time period 2001–2006. Foodborne Pathog Dis. 2008;5:579–588. doi: 10.1089/fpd.2007.0074. [DOI] [PubMed] [Google Scholar]
- 12.Lubrick C, Friendship RM, Farzan A. Recurring outbreaks of salmonellosis in a swine finisher barn. Proc The 40th AASV Annual Meeting; Dallas, Texas. March 7–10, 2009. [Google Scholar]
- 13.Wood RL, Pospischil A, Rose R. Distribution of persistent Salmonella Typhimurium infection in internal organs of swine. Am J Vet Res. 1989;50:1015–1021. [PubMed] [Google Scholar]
- 14.Springer S, Lindner T, Steinbach G, et al. Investigation of the efficacy of a genetically-stabile live Salmonella Typhimurium vaccine for use in swine. Berl Munch Tierarztl Wochenschr. 2001;114:342–345. [PubMed] [Google Scholar]
- 15.Lumsden JS, Wilkie BN, Clarke RC. Resistance to fecal shedding of Salmonellae in pigs and chickens vaccinated with an aromatic-dependent mutant of Salmonella Typhimurium. Am J Vet Res. 1991;52:1784–1787. [PubMed] [Google Scholar]
- 16.D’Aoust JY, Purvis U. Compendium of Analytical Methods. Ontario, Ottawa: Research Division, Health Protection Branch, Public Health Agency of Canada; 1998. [Last accessed June 30, 2010]. Isolation and identification of Salmonella from foods; HPB Method MFHPB-20. Full document. Available from http://www.hc-sc.gc.ca/fn-an/res-rech/analy-meth/microbio/volume2/mfhpb20-01_e.html. [Google Scholar]
- 17.Shaw SJ, Burton W, Nundy DC. Compendium o f Analytical Methods. Ontario, Ottawa: Research Division, Health Protection Branch, Public Health Agency of Canada; 2001. [Last accessed July 9, 2010]. Identification of Salmonella Species by Dynabeads Anti Salmonella: MFLP-84. Full document. Available from http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/res-rech/mflp84-eng.pdf. [Google Scholar]
- 18.Rosendal T. MSc thesis. University of Guelph; 2007. Salmonella epidemiology in finisher pigs and the effect of two treatment strategies on the occurrence of Salmonella. [Google Scholar]
- 19.Roesler U, Heller P, Waldmann KH, et al. Immunization of sows in an integrated pig-breeding herd using a homologous inactivated Salmonella vaccine decreases the prevalence of Salmonella Typhimurium infection in the offspring. J Vet Med B Infect Dis Vet Public Health. 2006;53:224–228. doi: 10.1111/j.1439-0450.2006.00951.x. [DOI] [PubMed] [Google Scholar]
- 20.Haesebrouck F, Pasmans F, Chiers K, et al. Efficacy of vaccines against bacterial diseases in swine: What can we expect? Vet Microbiol. 2004;100:255–268. doi: 10.1016/j.vetmic.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Letellier A, Messier S, Lessard L, et al. Host response to various treatments to reduce Salmonella infections in swine. Can J Vet Res. 2001;65:168–172. [PMC free article] [PubMed] [Google Scholar]
- 22.Mittrucker HW, Kaufmann SH. Immune response to infection with Salmonella Typhimurium in mice. J Leukoc Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 23.Lindberg AA, Robertson JA. Salmonella Typhimurium infection in calves: Cell-mediated and humoral immune reactions before and after challenge with live virulent bacteria in calves given live or inactivated vaccines. Infect Immun. 1983;41:751–757. doi: 10.1128/iai.41.2.751-757.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraser JN, Davis BL, Skjolaas KA, et al. Effects of feeding Salmonella enterica serovar Typhimurium or serovar Choleraesuis on growth performance and circulating insulin-like growth factor-I, tumor necrosis factor-alpha, and interleukin-1beta in weaned pigs. J Anim Sci. 2007;85:1161–1167. doi: 10.2527/jas.2006-482. [DOI] [PubMed] [Google Scholar]