Abstract
The aim of this study was to validate 2 commercially available enzyme-linked immunosorbent assays (ELISAs) for adiponectin in dogs, 1 canine-specific and 1 originally designed for measurements in humans. Intra-assay and interassay precision was evaluated by multiple measurements in canine serum samples, and assay accuracy was indirectly determined by linearity under dilution. Interference caused by hemolysis and lipemia was also studied. Both assays were subsequently used for measuring adiponectin concentrations in clinically healthy dogs and those with different grades of obesity. The intra-assay and inter-assay precision was less than 7.5% and 13.5% in serum samples with low and high adiponectin concentrations, respectively. Lipemia and hemolysis did not affect the results of any of the assays. Both assays were able to differentiate lean dogs from those that were overweight or obese on the basis of the measured adiponectin concentrations. From these results it can be concluded that canine adiponectin concentrations can be measured reliably by means of the 2 ELISAs evaluated in this study.
Résumé
L’objectif de la présente étude était de valider deux trousses commerciales d’épreuve immuno-enzymatique (ELISA) pour mesurer l’adiponectine chez les chiens, une spécifique à l’espèce canine et une développée au départ pour utilisation chez l’humain. La précision intra- et inter-essais a été évaluée par des mesures multiples d’échantillons de sérum canin, et la précision de l’épreuve a été mesurée indirectement par la linérarité après dilution. L’interférence causée par l’hémolyse et la lipémie a également été examinée. Les deux épreuves ont par la suite été utilisées pour mesurer les concentrations d’adiponectine chez des chiens cliniquement en santé et d’autres avec différents grades d’obésité. La précision intra-essai et inter-essai était respectivement de moins de 7,5 % et 13,5 % dans des échantillons de sérum avec des concentrations faibles et élevées d’adiponectine. La lipémie et l’hémolyse n’ont pas affecté les résultats des deux trousses. Les deux épreuves étaient en mesure de différencier les chiens maigres de ceux avec un surplus de poids ou obèses à partir des concentrations d’adiponectine mesurées. À partir de ces résultats il peut être conclu que les concentrations d’adiponectine canines peuvent être mesurées de manière fiable à l’aide des deux trousses ELISA évaluées dans cette étude.
(Traduit par Docteur Serge Messier)
Introduction
Adiponectin is a cytokine produced in adipocytes that reduces plasma glucose concentrations and increases fatty acid oxidation (1–3). It also inhibits the inflammatory process of arteriosclerosis by suppressing the expression of adhesion molecules on endothelial cells (4). The serum concentration of adiponectin decreases with body fat accumulation and is considered a biomarker for obesity in humans (5,6). In addition, levels of adiponectin decrease with resistance to insulin (7,8), type 2 diabetes (9,10), dyslipidemia (11), and abnormalities of the coronary arteries (4,12). In dogs, adiponectin has gained attention recently after reports that its concentrations are lower in obese dogs than in dogs with a normal body condition score (BCS) (13,14).
Most of the published studies in animal species, including dogs, have used multispecies radioimmunoassay (RIA) for adiponectin quantification (14–17). Use of enzyme-linked immunosorbent assay (ELISA) instead of RIA has several advantages, such as nonexposure to radioisotopes, easy integration into laboratory functions, and rapid turnaround (18).
Although different ELISA methods are commercially available for adiponectin measurement, to our knowledge no validation studies of their use in dogs have been published. However, adiponectin is highly homologous among different species, with 87% homology in the case of humans and dogs (13,14). The objective of this study was to validate 2 commercially available ELISAs for adiponectin in dogs, 1 canine-specific and 1 originally designed for measurement in humans, the postulation being that the latter could be used to quantify canine adiponectin. For the validation, the analytical performance of the assays was determined. In addition, the effects of hemolysis and lipemia on the assays, as well as the ability of the assays to differentiate low and high adiponectin concentrations, corresponding to obesity and optimal BCS, respectively, were studied.
Materials and methods
Dogs and samples
Dogs with potentially high and low serum adiponectin concentrations were selected for the study. The client-owned dogs, of different sexes, breeds, and ages, had presented at private clinics in southern Spain for routine checkups between January and July 2008. None had evidence of acute or chronic disease (except for obesity) from physical and clinical examination or from routine hematologic and biochemical analysis. Assessment of their nutritional status was based on a 5-scale BCS: 1 — thin; 2 — underweight; 3 — optimal (lean); 4 — overweight; 5 — obese (19). Serum specimens with a potentially high adiponectin concentration were obtained from dogs with an optimal BCS; specimens with a potentially low concentration were obtained from dogs that were overweight or obese. Of the 30 dogs selected, 22 were overweight or obese [12 females (6 neutered) and 10 males (1 neutered); BCS > 3; body weight, 3.2 to 50 kg; age, 1 to 16 y; mixed breeds]; the other 8 were of optimal weight for health (4 females and 4 males; BCS = 3; body weight, 5 to 18.9 kg; age, 1.2 to 7 y; mixed breeds).
Blood samples were collected the morning after an overnight fast of at least 12 h by puncture of the jugular or saphenous vein and placed in tubes containing a clotting accelerator (TapVal; Aquisel, Barcelona, Spain). Samples were centrifuged at 2000 × g for 10 min at room temperature to obtain serum, which was stored in aliquots in plastic vials at −20°C. On the day of analysis, the aliquots were brought to room temperature and thoroughly vortexed before the adiponectin measurement.
Adiponectin analysis
Canine-specific assay
The commercially available canine sandwich ELISA (Canine Adiponectin ELISA Kit; Millipore, St. Charles, Missouri, USA) used in our study has the following sequence of steps: 1) capture of canine adiponectin by polyclonal antibodies against adiponectin; 2) washing away of unbound materials; 3) binding of antibodies with horseradish peroxidase (HRP); 4) quantification of immobilized antibody–enzyme conjugates by monitoring of HRP activity in the presence of the substrate 3,3′,5,5′-tetramethyl-benzidine (TMB); and 5) addition of an acidic solution (0.3 M HCl) to stop the reaction. The enzyme activity is measured spectrophotometrically by increased absorbance at 450 nm, corrected for the absorbance at 590 nm, in a microtiter plate reader (PowerWave XS; Bio-Tek Instruments, Winooski, Vermont, USA). Since the increase in absorbance is directly proportional to the amount of captured canine adiponectin, the concentration in the studied serum sample can be calculated from the reference curve generated in the same assay with reference standards of known concentrations of canine adiponectin. The calibration curve was established by duplicate determination of calibrators.
High-sensitivity assay of human adiponectin
This assay (Human Adiponectin ELISA, High Sensitivity Kit; BioVendor–Laboratorni medicina, Modrice, Czech Republic) has the following sequence of steps: 1) capture of adiponectin by polyclonal antibodies against adiponectin; 2) washing away of unbound materials; 3) binding of antibodies with HRP; 4) washing away of unbound materials; 5) reaction of HRP-conjugated antibodies with a hydrogen peroxide/TMB substrate; and 6) addition of an acidic solution (0.2 M H2SO4) to stop the reaction. The enzyme activity is measured spectrophotometrically at 450 nm in a PowerWave XS microtiter plate reader. A standard curve is constructed from duplicate determination of calibrators by plotting absorbance values against the adiponectin concentrations of the calibrators, and the concentrations in studied samples are determined by means of this standard curve.
Species-specific standards are highly recommended to achieve similar affinity of antiserum against standards and samples (20), and the use of serum samples as a standard is preferred in many cases instead of purified protein (it is more economical, yields a more suitable physiological matrix, and can ensure a consistent, long-lasting supply of standard material) (20–22). A canine serum sample with the adiponectin concentration determined by the canine-specific adiponectin ELISA was evaluated as a standard for curve calibration of the human adiponectin ELISA.
For this purpose, 12 canine samples were analyzed with the assay for human adiponectin and the use of 2 different standard curves: the original curve from the kit (human standard), which has an upper value of 150 ng/mL, and a curve prepared from a canine serum sample with an adiponectin concentration of 30 μg/mL as measured by the commercially available canine-specific ELISA. This canine serum sample was serially diluted with dilution buffer, and a standard curve was constructed with a range of 0.137 to 30 μg/mL. This canine standard was used for the remainder of the analytical and clinical validation of the human assay.
Analytical validation
For analytical validation of both methods the following parameters were calculated.
Precision
The intra-assay coefficient of variation (CV) was calculated after analysis of 2 samples of low adiponectin concentration and 2 of high concentration 5 times in a single assay run. The inter-assay CV was determined by analyzing the same samples in 5 separate runs, carried out on different days.
Accuracy
Owing to the lack of a reference method or commercially available certified reference material for canine adiponectin, which would be optimal for testing accuracy (23), accuracy of the assays was evaluated indirectly by linearity under dilution (24). Two canine serum samples with high adiponectin concentrations were serially diluted with the corresponding sample diluent provided with each assay.
Limit of detection
This was calculated on the basis of data from 11 replicate determinations of the zero standard (dilution buffer) as the mean value plus 2 standard deviations.
All samples used for repetitive analysis were frozen in aliquots, and only the vials needed for each run were used, to avoid possible changes due to repetitive thawing and freezing.
Effects of hemolysis and lipemia
To investigate the effects of hemolysis and lipemia on the assays, 2 canine serum samples were mixed with different concentrations of hemoglobin or lipid solution, and each preparation was run in duplicate.
A fresh hemolysate was prepared by adding distilled water to packed saline-washed canine erythrocytes from a healthy dog. The concentration of hemoglobin in the hemolysate was determined with the Vet ABC Animal Blood Counter (ABX Diagnostics, Montpellier, France) and adjusted to 200 g/L by adding assay buffer to produce a stock solution. The stock solution was serially diluted with sample diluent buffer, and 10 μL of each dilution was added to 2 samples of 90 μL of canine serum with high and low adiponectin concentrations. The final hemoglobin concentrations were 8, 4, 2, 1, 0.5, and 0.0 g/L (10 μL of sample diluent buffer was added to the samples to give a 0.0 g/L concentration), which would correspond to slight hemolysis (0.5 g/L), moderate hemolysis (1 and 2 g/L), and marked hemolysis (4 and 8 g/L).
A commercial fat emulsion (Lipofundina 20%; Braun Medical, Barcelona, Spain) with a triglyceride concentration of 200 g/L was serially diluted with sample diluent buffer, and 10 μL of each dilution was added to 2 samples of 90 μL of canine serum with high and low adiponectin concentrations. Homogeneity was achieved by vortexing. The final triglyceride concentrations were 5, 2.5, 1.25, 0.625, and 0.3125 g/L, which would correspond to slight lipemia (0.3125 and 0.625 g/L), moderate lipemia (1.25 and 2.5 g/L), and marked lipemia (5 g/L).
Statistical methods
Arithmetic means, medians, and intra-assay and interassay CVs were calculated by means of routine descriptive statistical procedures and software [Excel (Microsoft, Redmond, Washington, USA) and GraphPad Prism (GraphPad Software, San Diego, California, USA)]. Differences between results obtained with human and canine standards and comparison of the results for lean dogs with those for overweight or obese dogs obtained with the assay for human adiponectin were studied by use of Student’s t-test with a significance level of P < 0.05. In addition, Bland–Altman plots were used to evaluate the clinical agreement between results obtained in the ELISA for human adiponectin with 2 different standards and to reveal any bias. Bias was computed as the mean difference between scores for the 2 methods. Values for 95% limits of agreement (LOA) were computed as the mean difference ± 1.96 standard deviations of the difference. Linearity under dilution was accomplished by ordinary linear regression analysis comparing the measured levels of adiponectin with the expected levels. Interferograms were prepared to show the differences in adiponectin concentration when hemoglobin or lipid was added. On the graphs the X-axes show increasing concentrations of hemoglobin or lipid; the Y-axes show the percentage change in adiponectin [Vf(Vo) × 100]. Additionally, regression analysis was applied in the comparison of results of the canine and human ELISAs to derive regression data. The significance level used in each case was P < 0.05.
Results
Assay characteristics
Canine-specific ELISA
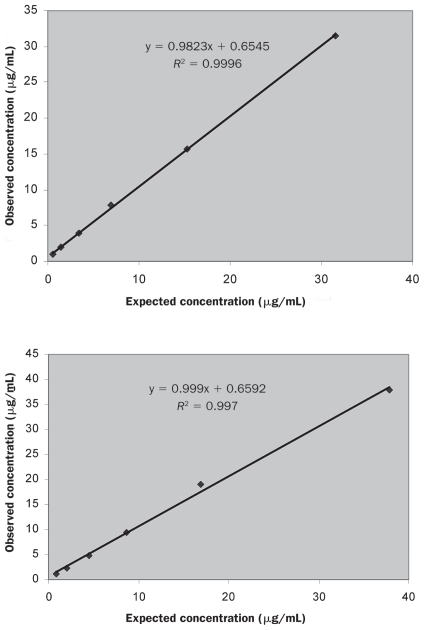
The time needed to perform the assay was 4 h. The intra-assay and inter-assay CVs were 4.40% and 13.31% for samples with low adiponectin concentrations and 6.26% and 8.52% for samples with high concentrations (Table I). The linearity under dilution of 2 canine samples is shown in Figure 1: dilution of the samples with different adiponectin concentrations resulted in linear regression equations with correlation coefficients of 0.999 and 0.997. The detection limit was 0.098 (mean, 0.019; standard deviation, 0.039) μg/mL.
Table I.
Intra-assay and inter-assay coefficients of variation (CVs) in the adiponectin concentration of serum samples with high and low values [mean and standard deviation (s) for 2 samples] as determined by a canine-specific enzyme-linked immunosorbent assay (ELISA) and an ELISA originally designed to measure the adiponectin concentration in human serum
| ELISA | Comparison | Number of samples | Obtained in present study | Reported by manufacturer | |||
|---|---|---|---|---|---|---|---|
| Adiponectin concentration (μg/mL) |
CV (%) | Mean adiponectin concentration (μg/mL) | CV (%) | ||||
| Mean | s | ||||||
| Canine-specific | Intra-assay | 2 | 20.20 | 1.26 | 6.26 | 13.39 | 2.86 |
| 2 | 7.11 | 0.31 | 4.40 | 1.58 | 4.21 | ||
| Inter-assay | 2 | 25.81 | 2.20 | 8.52 | 13.47 | 0.46 | |
| 2 | 5.59 | 0.74 | 13.31 | 1.73 | 8.97 | ||
| Human | Intra-assay | 2 | 18.58 | 1.39 | 7.45 | 13.75 | 4.2 |
| 2 | 5.45 | 0.135 | 2.48 | 6.59 | 6.7 | ||
| Inter-assay | 2 | 18.54 | 1.76 | 9.52 | 19.08 | 9.5 | |
| 2 | 5.52 | 0.59 | 10.60 | 6.41 | 7.6 | ||
Figure 1.
Linearity under dilution of 2 canine serum samples with different adiponectin concentrations measured with the canine-specific adiponectin enzyme-linked immunosorbent assay (ELISA).
High-sensitivity ELISA of human adiponectin
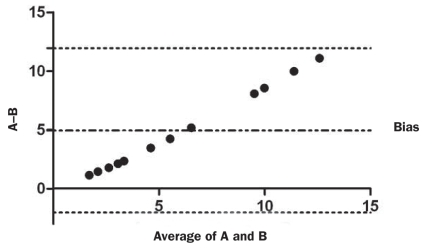
In the evaluation of canine serum as a standard for curve calibration in this assay, the use of canine standards resulted in significantly higher adiponectin values (P μ 0.001) than the use of human standards for the same 12 canine serum samples. In addition, Bland–Altman analysis indicated a proportional bias (Figure 2). The mean difference between adiponectin values obtained with the canine and human standards was 4.95 (95% LOA, −2.04 to 11.95) μg/mL.
Figure 2.
Bland–Altman plot illustrating the 95% limits of agreement (LOA) for adiponectin values (μg/mL) determined by the ELISA for human adiponectin with canine (A) and human (B) standards in 12 canine blood samples. The top and bottom horizontal dotted lines represent the 95% values (calculated as the mean difference ± 1.96 × the standard deviation of the difference); the middle line represents the mean difference (bias).
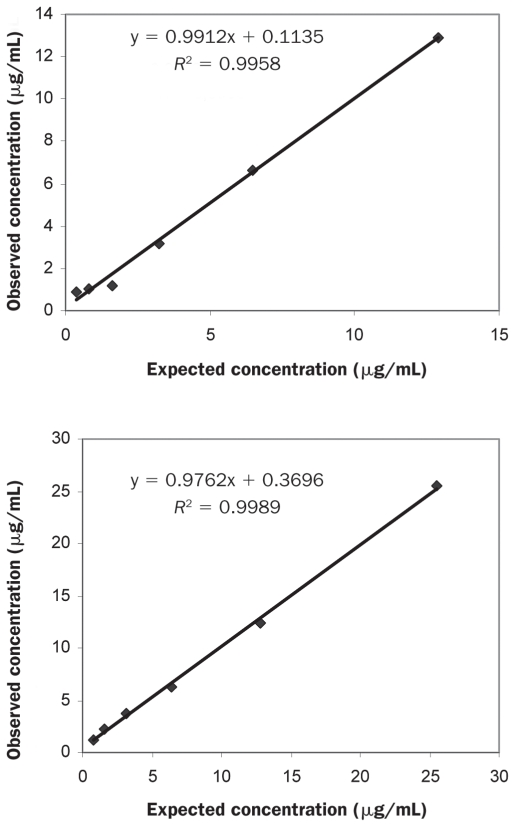
The time needed to perform the assay was 2.5 h. The intra-assay and inter-assay CVs were 2.48% and 10.60% for samples with low adiponectin concentrations and 7.45% and 9.52% for samples with high concentrations (Table I). The linearity under dilution of 2 canine samples is shown in Figure 3: dilution of the samples with different adiponectin concentrations resulted in linear regression equations with correlation coefficients of 0.996 and 0.999. The detection limit was 0.74 (mean, 0.49; standard deviation, 0.13) μg/mL.
Figure 3.
Linearity under dilution of 2 canine serum samples with different adiponectin concentrations measured with the ELISA originally designed to measure the adiponectin concentration in human serum.
Effects of hemolysis and lipemia on adiponectin analysis
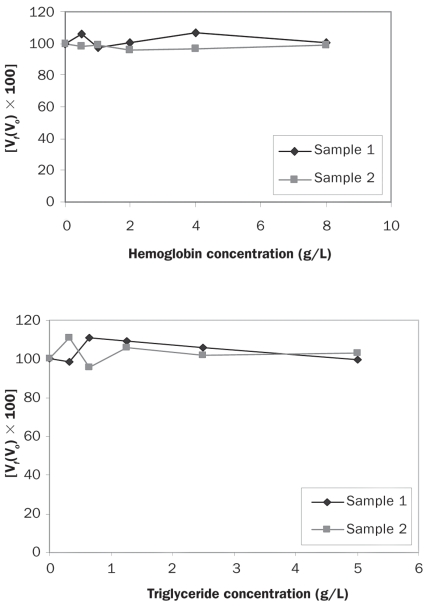
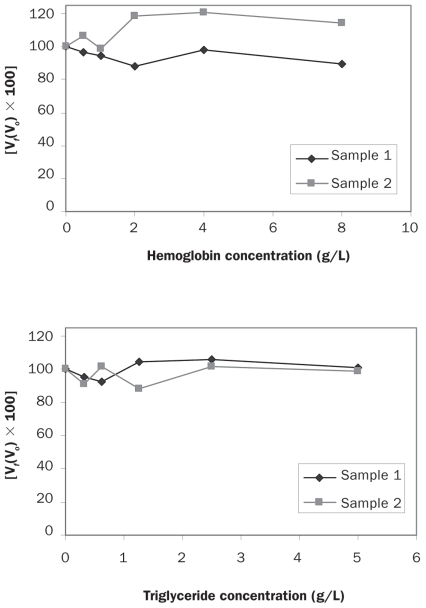
The different degrees of hemolysis and lipemia tested in this study did not affect the measured levels of adiponectin in either the canine-specific assay or the high-sensitivity assay of human adiponectin, as shown by the interferograms presented in Figures 4 and 5, respectively.
Figure 4.
Interferograms showing the effect of hemoglobin and triglyceride on adiponectin determinations by the canine-specific ELISA. The Y-axes show the percentage change in adiponectin concentration for a given concentration of hemoglobin or triglyceride. Vf — final value; Vo — original value.
Figure 5.
Interferograms showing the effect of hemoglobin and triglyceride on adiponectin determinations by the ELISA for human adiponectin.
Ability of the assays to differentiate lean and obese dogs
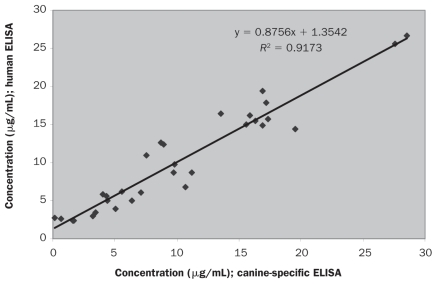
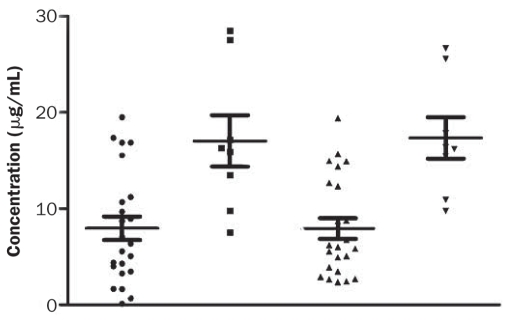
The adiponectin concentration in the 30 serum samples from the client-owned dogs ranged from 0.12 to 28.52 μg/mL as determined by the canine-specific ELISA and 2.37 to 26.69 μg/mL as determined by the ELISA for human adiponectin. Regression analysis (Figure 6) demonstrated high correlation (r = 0.9173) between the results with the 2 methods. With both methods, the samples from the overweight or obese dogs showed significantly lower adiponectin concentrations (P < 0.005 and 0.0005, respectively) than the samples collected from the lean dogs (Figure 7).
Figure 6.
Regression analysis of the adiponectin concentrations determined by the 2 ELISAs.
Figure 7.
Serum adiponectin concentrations in lean dogs (those of optimal body condition score) determined by the canine-specific (▪) and human (▾) ELISAs and in overweight or obese dogs determined by the canine-specific (•) and human (▴) ELISAs.
Discussion
There are numerous methods to determine grade of obesity in dogs, but most of them are complicated, expensive, or time-intensive (25). So in recent years it has become of great interest to find new, rapid, and reliable methods to diagnose and monitor obesity in this species. Adiponectin is decreased in obese dogs and can be used as an obesity marker (13,14). But the measurement of adiponectin concentrations is not very common in companion animal practices, which often use RIA kits. These kits have the inherent disadvantages of the need to have special installations and to dispose of radioactive wastes, as well as being labor-intensive and having a long processing time. The development of adiponectin assays based on the ELISA technique represents substantial progress from the technologic, ecologic, and economic viewpoints (18), but the commercially available ELISA methods have not been validated for use in dogs. Validation studies must ensure that analytical methods are able to detect the corresponding analyte, provide repeatedly accurate results, and discriminate between healthy and diseased animals (26).
This study validated 2 different ELISA kits for adiponectin measurement in dogs: 1 canine-specific and 1 designed for measuring adiponectin in humans. The use of a canine serum sample with a known adiponectin concentration measured by the canine-specific assay to calibrate the human adiponectin ELISA was evaluated. A proportional bias was detected between the human and canine standards. Although the reasons for this bias are not clear and should be further investigated, from a practical point of view this bias could indicate that the use of a canine serum sample as a standard might contribute to better differentiation between low and high adiponectin concentrations in overweight or obese and lean dogs, respectively.
In this study similar CVs were obtained in the 2 assays. However, except for the intra-assay CV for low adiponectin values in the human assay, the CVs obtained with both ELISA kits for intra-assay and interassay precision were higher than those reported by the manufacturers. A murine RIA kit previously used for quantification of adiponectin in dogs gave a higher intra-assay CV (9.5%) but a slightly lower interassay CV (9.7%) than the kits in our study (14). Nevertheless, both ELISA kits appear to be suitable for measuring canine adiponectin concentrations, as the CVs did not exceed 15%, the limit of the analytical objective performance standard for precision (27).
The accuracy of the adiponectin ELISAs was indirectly evaluated by linearity under dilution. This procedure is an alternative used with canine adiponectin measurements when no reference method or commercially available certified reference material is available (24,28). Regression analysis revealed high accuracy of both assays: r = 0.09989 and 0.9958 for the canine-specific ELISA and r = 0.997 and 0.9996 for the ELISA for measurement in humans. The canine-specific assay had a lower limit of detection (0.098 μg/mL) than the assay for human adiponectin (0.74 μg/mL), which could suggest that the canine assay detects low adiponectin concentrations more efficiently.
Veterinary clinical pathology laboratories usually have to deal with hemolytic or lipemic serum. In dogs, hemolysis can be attributed to various diseases or to complications at the time of blood sampling. Lipemia is usually related to recent ingestion or endocrine diseases related to obesity. For these reasons it is important to know the influence of hemolysis and lipemia on adiponectin values in dogs. We found that concentrations up to 5 g/L of triglycerides and up to 8 g/L of hemoglobin did not cause differences in the adiponectin values in the 2 assays, which suggests that lipemia and hemolysis would not influence the adiponectin values measured in canine samples with either of the assays used in our study. These results agree with those of previous reports on the same high-sensitivity ELISA for human adiponectin and a latex particle-enhanced turbidimetric immunosorbent assay for human adiponectin: neither method showed significant interference of hemoglobin concentrations up to 500 mg/dL (18,29). In addition, it was reported that triglyceride levels up to 2000 formazin turbidity units did not alter adiponectin values obtained with the human assay used in our study (29). This could be of practical importance because obese dogs frequently have lipemic serum, and lipemia predisposes to hemolysis, so both interferences could often be present in obese dogs.
The results of the 2 assays evaluated in the present study showed high correlation (r = 0.9173) in regression analysis. However, this finding should be interpreted with caution because there may be a dependence between the methods since calibration material used for the human assay was established by the canine-specific ELISA (21).
With both assays, the overweight or obese dogs in this study had significantly lower serum adiponectin concentrations than the lean dogs, as has been reported previously (13,14). The mean concentrations in the 2 groups of dogs were higher than those reported by another group of investigators (14), who found an adiponectin concentration in normal dogs of 1.22 μg/mL, but lower than those reported by others (13), who found means of 16.8 ± 3.5 μg/mL and 33.4 ± 2.9 μg/mL in obese and lean dogs, respectively. The differences can be attributed to different methods used in the studies.
The main limitations of the validation performed in this study are that neither method was compared with a reference method for adiponectin measurement in dogs (but, to the authors’ knowledge, such a method does not exist) and that spiking recovery tests with reference materials of known adiponectin concentrations were not performed (because canine adiponectin is not commercially available). However, the linearity under dilution, which in cases of lack of a reference method or reference materials could be an estimation of accuracy, provided very high correlations, indicating that both methods could measure adiponectin in a linear and proportional way.
In summary, the 2 commercially available ELISAs for adiponectin determination in dogs that were validated in this study exhibit analytical characteristics that allow their use in the laboratory, with adequate precision, linearity under dilution, and ability to discriminate between high and low adiponectin values, corresponding to lean and overweight or obese dogs, respectively. It is expected that validation of these 2 assays will increase the analytical options of veterinary laboratories for canine adiponectin measurement and will contribute to a wider use of adiponectin as a marker of obesity and related diseases in canine clinical practice.
References
- 1.Fruebis J, Tsao TS, Javorschi S, et al. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamauchi T, Kamon J, Waki H, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 4.Ouchi N, Kihara S, Arita Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Comm. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 6.Chandran M, Ciaraldi T, Phillips SA, Henry RR. Adiponectin: More than just another fat cell hormone? Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 7.Weyer C, Funahashi T, Tanaka S, et al. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86:1930–1935. doi: 10.1210/jcem.86.5.7463. [DOI] [PubMed] [Google Scholar]
- 8.Kondo H, Shimomura I, Matsukawa Y, et al. Association of adiponectin mutation with type 2 diabetes: A candidate gene for the insulin resistance syndrome. Diabetes. 2002;51:2325–2328. doi: 10.2337/diabetes.51.7.2325. [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 10.Hotta K, Funahashi T, Bodkin NL, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 11.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–2769. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 12.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–141. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 13.Ishioka K, Omachi A, Sagawa M, et al. Canine adiponectin: cDNA structure, mRNA expression in adipose tissues and reduced plasma levels in obesity. Res Vet Sci. 2006;80:127–132. doi: 10.1016/j.rvsc.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Brunson BL, Zhong Q, Clarke KJ, et al. Serum concentrations of adiponectin and characterization of adiponectin protein complexes in dogs. Am J Vet Res. 2007;68:57–62. doi: 10.2460/ajvr.68.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Mustonen AM, Pyykönen T, Nieminen P. Adiponectin and peptide YY in the fasting blue fox (Alopex lagopus) Comp Biochem Physiol A Mol Integr Physiol. 2005;140:251–256. doi: 10.1016/j.cbpb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Kearns CF, McKeever KH, Roegner V, Brady SM, Malinowski K. Adiponectin and leptin are related to fat mass in horses. Vet J. 2006;172:460–465. doi: 10.1016/j.tvjl.2005.05.002. Epub 2005 Jun 29. [DOI] [PubMed] [Google Scholar]
- 17.Lin HV, Kim JY, Pocai A, et al. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes. 2007;56:1969–1976. doi: 10.2337/db07-0127. [DOI] [PubMed] [Google Scholar]
- 18.Risch L, Hoefle G, Saely C, et al. Evaluation of two fully automated novel enzyme-linked immunosorbent assays for the determination of human adiponectin in serum. Clin Chim Acta. 2006;373:121–126. doi: 10.1016/j.cca.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Lund EM, Armstrong PJ, Kirk CA, Kolar LM, Klausner JS. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc. 1999;214:1336–1341. [PubMed] [Google Scholar]
- 20.Eckersall PD. Acute phase proteins as markers of inflammatory lesions. Comp Haematol Int. 1995;5:93–97. [Google Scholar]
- 21.Tecles F, Martinez-Subiela S, Petrucci G, Gutierrez-Panizo C, Ceron JJ. Validation of a commercially available human immunoturbidimetric assay for haptoglobin determination in canine serum samples. Vet Res Commun. 2007;31:23–36. doi: 10.1007/s11259-006-3397-y. [DOI] [PubMed] [Google Scholar]
- 22.Bence LM, Addie DD, Eckersall PD. An immunoturbidimetric assay for rapid quantitative measurement of feline alpha-1-acid glycoprotein in serum and peritoneal fluid. Vet Clin Pathol. 2005;34:335–340. doi: 10.1111/j.1939-165x.2005.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 23.Jensen AL, Kjelgaard-Hansen M. Method comparison in the clinical laboratory. Vet Clin Pathol. 2006;35:276–285. doi: 10.1111/j.1939-165x.2006.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 24.Jensen AL. Validation of diagnostic tests in haematology laboratories. In: Feldman BF, Zinkl JG, Jain NC, editors. Schalm’s Veterinary Hematology. 5th ed. Ames, Iowa: Blackwell Publishing; 2006. pp. 20–28. [Google Scholar]
- 25.German AJ. The growing problem of obesity in dogs and cats. J Nutr. 2006;136:1940S–1946S. doi: 10.1093/jn/136.7.1940S. [DOI] [PubMed] [Google Scholar]
- 26.Tecles F, Fuentes P, Martínez-Subiela S, Parra MD, Munoz A, Ceron JJ. Analytical validation of commercially available methods for acute phase proteins quantification in pigs. Res Vet Sci. 2007;83:133–139. doi: 10.1016/j.rvsc.2006.10.005. Epub 2006 Dec 1. [DOI] [PubMed] [Google Scholar]
- 27.Guidance for Industry: Bioanalytical Method Validation. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Veterinary Medicine; 2001. [Last accessed March 26, 2010]. Available from www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070107.pdf. [Google Scholar]
- 28.Stockham SL, Scott MA. Fundamentals of Veterinary Clinical Pathology. Ames, Iowa: Iowa State Univ Pr; 2002. pp. 13–17. [Google Scholar]
- 29.Nishimura A, Sawai T. Determination of adiponectin in serum using a latex particle-enhanced turbidimetric immunoassay with an automated analyzer. Clin Chim Acta. 2006;371:163–168. doi: 10.1016/j.cca.2006.03.008. [DOI] [PubMed] [Google Scholar]