Abstract
The purpose of this study was to evaluate changes of very low-density lipoprotein (VLDL) components in hepatic blood (HB) from 5 nonlactating nonpregnant cows fasted from days 0 to 3 and subsequently refed to day 10 and, in addition, to assess those of other lipoproteins. Increased phospholipid concentrations in each lipoprotein after the start of fasting suggested their availability for the surface lipids of lipoproteins. Although the VLDL-triglyceride (TG) concentration in HB from all cows increased on day 1, the value on day 4 became similar to that on day 0. However, the concentration on day 10 was significantly increased. In all cows, the decreased ratio of the VLDL-TG concentration in HB to the non-esterified fatty acids (NEFA) concentration in portal blood (PB) on day 4 appeared to reflect relatively decreased secretion of TG as VLDL by NEFA excessively mobilized to the liver via PB. The markedly increased ratio on day 10 was considered to contribute to the improvement of hepatic lipidosis.
Résumé
L’objectif de la présente étude était d’évaluer les changements des composantes des lipoprotéines de très faible densité (VLDL) dans le sang hépatique (HB) chez 5 vaches non-gestantes n’étant pas en lactation et mises au jeûne du jour 0 au jour 3 et par la suite nourries jusqu’au jour 10, et également pour évaluer ceux des autres lipoprotéines. Des augmentations de concentration des phospholipides dans chaque lipoprotéine suite au début du jeûne suggéraient leur disponibilité pour les lipides de surface des lipoprotéines. Bien que la concentration de VLBL-triglycéride (TG) dans les HB de toutes les vaches ait augmenté au jour 1, la valeur au jour 4 est devenue similaire à celle du jour 0. Toutefois, la concentration au jour 10 était significativement augmentée. Chez toutes les vaches, le ratio diminué de la concentration VLDL-TG dans les HB par rapport à la concentration des acides gras non-estérifiés (NEFA) dans le sang portal (PB) au jour 4 a semblé refléter une diminution relative de la sécrétion de TG étant donné que les VLDL des NEFA sont excessivement mobilisées au foie via le PB. L’augmentation marquée du ratio au jour 10 était considérée contribuer à l’amélioration de la lipidose hépatique.
(Traduit par Docteur Serge Messier)
In cows, hepatic lipidosis is known to be closely linked to the occurrence of periparturient diseases, such as ketosis, left displacement of the abomasum, milk fever, and retained placenta (1–3). The direct factor in the induction of hepatic lipidosis is negative energy balance (NEB), attributable to stress around calving, decreased dry matter intake, hormonal imbalance, the start of lactation, or a combination of these conditions (4,5). In a cow falling into NEB, a great quantity of nonesterified fatty acids (NEFA) is mobilized from adipose tissue to the liver via the portal vein (PV), facilitating fatty acid metabolism in the liver, oxidation in mitochondria, and export of triglycerides (TG) as very low-density lipoprotein (VLDL) to the hepatic vein (HV). However, when excessive inflow exceeds the metabolic capacity of the liver, accumulation of TG in the liver occurs and fatty liver is consequently induced (4,6). Ruminants, however, are susceptible to fatty liver since they essentially have low competence for VLDL secretion from the liver (7,8).
We recently reported that fatty liver in cows could be induced by fasting the animals for 4 d (9). The pathophysiological features of fatty liver in the cow can be effectively assessed by combining the 4-day-fasting experiment with the over-the-wire-catheterization method (9), which can collect both portal blood (PB) and hepatic blood (HB). In this study, a markedly increased NEFA concentration in PB was observed during fasting, and the concentration became similar to the baseline value after refeeding (9). Liver TG content increased dramatically in fasted cows by the end of the fast (day 4) and returned to initial value on day 10. From these phenomena, we can hypothesize that the concentration of VLDL secreted from the liver to the HV is impaired by elevated NEFA in PB in the development of hepatic lipidosis during fasting, and that after refeeding the concentration of VLDL is increased for the recovery from hepatic lipidosis. The purpose of the study reported here was to test the above-mentioned hypothesis by evaluating changes of component concentrations of VLDL in HB from cows fasted and refed, and to further assess changes in other lipoprotein fractions.
Details of this study design have been reported previously (9). Briefly, 5 Holstein cows (non-pregnant and non-lactating, mean body weight 660 kg) were fasted for 4 d (day 0 to 3). Over-the-wire catheters were installed in the PV and HV in the liver to collect venous blood on day −1. After fasting, cows were refed until day 10. All cows were maintained in tie-stall barns under the Laboratory Animal Control Guidelines of Rakuno Gakuen University, which basically conform with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health in the USA (NIH publication No. 86-23, revised 1996).
Blood samples from the HV and PV were collected in the morning (before feeding) on days 0, 1, 4, and 10. Sera collected from HV were used immediately for lipoprotein separation without freezing.
Lipoprotein separation was performed based on the method described previously (10), using a fixed-angle rotor (TLA-110; Beckman Coulter, Fullerton, California, USA) in an ultracentrifuge (OPTIMA TLX; Beckman Coulter) at 16°C. Chylomicrons (CM; 117 000 × g for 10 min, d < 0.95), VLDL (657 000 × g for 2 h and 55 min, d < 1.006), low-density lipoprotein (LDL; 657 000 < g for 4 h and 20 min, d < 1.063), and high-density lipoprotein (HDL; 657 000 < g for 7 h and 15 min, d < 1.21) were prepared.
The concentrations of TG, phospholipids (PL), cholesteryl esters (CE), and free cholesterol (FC) in lipoprotein fractions were determined using commercial kits (Wako Pure Chemicals, Osaka, Japan) (10). The CE concentration was calculated by subtracting the FC concentration from that of TC. Protein concentration was measured using the method by Lowry et al (11). Lecitin:cholesterol acyltransferase (LCAT) activity was determined using a commercial kit (Dai-ichi Pure Chemicals, Tokyo, Japan).
All statistical analyses were performed using computer software (SPSS version 17.0; SPSS, Chicago, Illinois, USA). The data were analyzed by repeated measures [analysis of variance (ANOVA)]. The significance of differences between the means on day 0 for prefasting and the means at selected sampling days was evaluated by Dunnett’s test. Values were expressed as mean ± standard error.
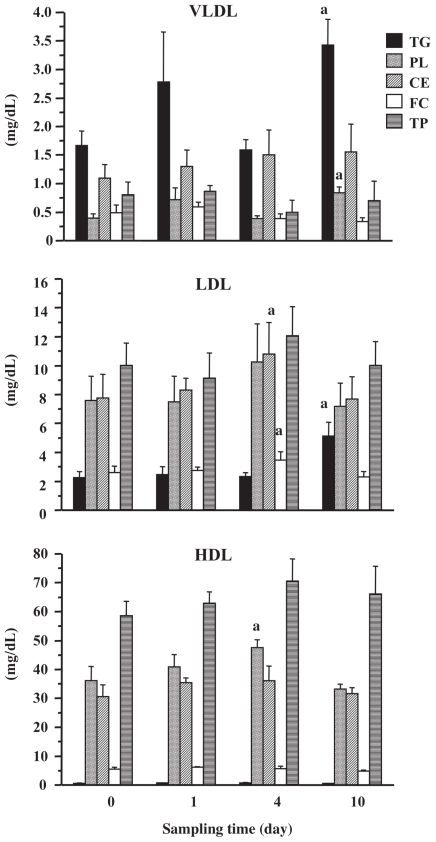
The concentrations of various components of each lipoprotein fraction in serum obtained from HB are presented in Figure 1. The concentrations of VLDL-TG and VLDL-PL in all cows were higher on day 1 compared with those on day 0, although the difference was not statistically significant. Thereafter, the concentrations returned to the baseline values. However, both concentrations were significantly increased on day 10 (P < 0.05) when they were more than 2-fold those on day 0. The VLDL-CE concentration was somewhat increased on days 1, 4, and 10. Compared with the concentration on day 0, there was a significant (P < 0.05) increase in the concentration of LDL-TG on day 10. The concentrations of LDL-CE and LDL-FC on day 4 were significantly (P < 0.05) higher than those on day 0. Total protein concentrations of the VLDL and LDL fractions were not significantly changed. The HDL-PL concentration was significantly (P < 0.05) higher on day 4 than that on day 0. The concentrations of HDL-CE and HDL-TP were elevated on days 1 and 4 compared with day 0. The concentrations of components in the CM fraction were considerably lower than those in the others (data not shown), which was consistent with a previous report (10) and indicated the difficulty of comparison. Therefore, data for the CM fraction were excluded from this study.
Figure 1.
Lipid and total protein concentrations in lipoprotein fractions obtained from hepatic blood.
VLDL — very low-density lipoprotein; LDL — low-density lipoprotein; HDL — high-density lipoprotein.
a P < 0.05, compared with values on day 0. Fasting was started on day 0 to day 3 and refeeding was done on day 4 after sampling.
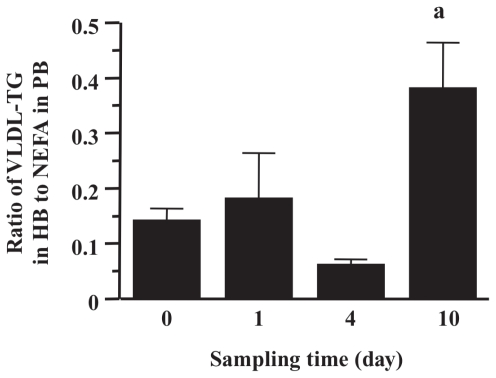
The ratio of the VLDL-TG concentration in HB to the NEFA concentration in PB (9) is shown in Figure 2. In all cows, the ratio on day 1 was increased compared with that of day 0, but reduced on day 4 (to less than half of that on day 1). The value on day 10 was significantly increased (P < 0.01) (approximately 4-fold that on day 0).
Figure 2.
Changes of concentration ratio of very low-density lipoprotein-triglyceride (VLDL-TG) (mg/dL) in hepatic blood (HB) to non-esterified fatty acids (NEFA) (mg/dL) in portal blood (PB). Data on NEFA were obtained from our previous report (9). In NEFA, the unit (mEq/L) was converted to mg/dL by multiplying the coefficient (28.25).
a P < 0.01, compared with values on day 0.
The LCAT activities in HB were higher on day 1 (721 ± 34 U) and on day 4 (776 ± 25 U) than on day 0 (668 ± 48 U). The activity on day 10 (637 ± 39 U) was similar to that on day 0.
It is possible that the increased VLDL-TG concentration in HB from all cows on day 1 was attributable to the elevation of VLDL synthesis and secretion followed by NEFA mobilized to the liver. However, it is also possible that the competence of the synthesis, secretion, or both of VLDL could not be maintained until day 4 because VLDL-TG concentrations in all cows at that time dropped to the baseline value in spite of the continued increase in NEFA concentration in PB. On day 10, VLDL-TG and LDL-TG concentrations were markedly increased. In sheep with experimentally induced fatty liver it was reported that serum VLDL and LDL concentrations are elevated, purportedly because of an increased rate of VLDL turnover (8). The significant increases of VLDL-TG and LDL-TG found in this study may reflect elevated transportation of accumulated TG from the liver to peripheral tissue to improve hepatic lipidosis. This speculation supports the dramatic decrease in liver TG content from day 4 to day 10 (9). It is difficult to believe that the increase in VLDL-TG represents an accumulation of lipoproteins in the blood due to a decreased rate of VLDL turnover, because liver function and liver TG content returned to normal on day 10. The major apolipoprotein in VLDL and LDL is apoliprotein B-100 (apoB-100) (10). Because the protein concentrations in both lipoprotein fractions did not change significantly through the experiment period, it was suggested that the synthesis of apoB-100 in the liver was not influenced by 4-day fasting. This phenomenon may indicate that the level of liver apoB-100 mRNA for the transcriptional modulation (12) was not impaired under fasting-induced NEB.
The HDL-CE concentration was significantly increased on day 4, which may have been due to a slight increase in LCAT, the enzyme responsible for esterification of cholesterol (13) activity, in HB. It is believed that the elevated protein concentration in HB at this time indicates an increase in the concentration of apoliprotein A-I (10), the predominant apoprotein in HDL for activating LCAT (13). The slight increases in VLDL-CE and the significant increase in LDL-CE on day 4 are not thought to be attributable to the transport from HDL by cholesteryl ester transfer protein (CETP), because CETP activity has been not detected in the cow (14). Therefore, these increases may indicate a decreased rate of VLDL turnover. On the other hand, Van den Top et al (15) reported that total and free cholesterol concentrations in the VLDL, LDL, and HDL fractions and plasma LCAT activity in cows decreased immediately after calving. The reason for the inconsistency in both results was considered to be associated with the difference of the severity of fatty liver attributable to experimental protocol.
The increases in PL concentrations of VLDL, LDL, and HDL fractions after fasting was started, suggests that PL are available as surface lipids in lipoproteins at that time (8), and further supports a previous report that cows with naturally occurring fatty liver had lower liver PL concentrations than those with fasting-induced fatty liver (16).
The ratio of the VLDL-TG concentration in HB to the NEFA concentration in PB increased on day 1 and decreased to the baseline on day 4 (Figure 2). This decrease was observed in all cows, which may indicate that TG secretion is overwhelmed by a large quantity of NEFA mobilized to the liver. Hepatic uptake of NEFA was reported to be proportional to increased NEFA transport to the liver without a change in hepatic extraction of NEFA in sheep (17), dogs (18), and cows (19). Accordingly, the excessive inflow of NEFA on day 4 exceeded the metabolic capacity for TG export from the liver, resulting in a 2.6-fold increase in liver TG content (9). In addition, the ratio on day 10 was increased because the VLDL-TG concentration in HB increased. Since the value was approximately 4-fold that on day 0, it was suggested that VLDL-TG secretion from the liver had a wide range. Therefore, the fact that the increased ratio on day 1 was considerably lower than that on day 10 indicated that the process of TG secretion from the liver could not work sufficiently, which may be partially expressed by lipotoxicity in hepatic cells due to mobilized NEFA (20).
In conclusion, the changes of components in VLDL and other lipoproteins were associated with the development of fasting-induced hepatic lipidosis and its recovery. The ratio of the VLDL-TG concentration in HB to the NEFA concentration in PB decreased with the development of hepatic lipidosis, which is expressed in an in vivo experiment where cows are susceptible to fatty liver because of low competence for VLDL secretion from the liver. The increased ratio on day 10 is considered to be the contribution of VLDL utilized in the recovery from hepatic lipidosis.
Acknowledgment
This study was partially supported by a Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (14560253). The authors thank Drs. R.R. Grummer and J.A.A. Piere, Department of Dairy Science, University of Wisconsin, for their valuable advice.
References
- 1.Oikawa S, Katoh N. Reduced concentrations of apolipoproteins B-100 and A-I in serum from cows with retained placenta. Can J Vet Res. 1997;61:312–314. [PMC free article] [PubMed] [Google Scholar]
- 2.Oikawa S, Katoh N, Kawawa F, Ono Y. Decreased serum apolipoprotein B-100 and A-I concentrations in cows with ketosis and left displacement of the abomasums. Am J Vet Res. 1997;58:121–125. [PubMed] [Google Scholar]
- 3.Oikawa S, Katoh N. Decreases in serum apolipoprotein B-100 and A-I concentrations in cows with milk fever and downer cows. Can J Vet Res. 2002;66:31–34. [PMC free article] [PubMed] [Google Scholar]
- 4.Gerloff BJ, Herdt TH, Emery RS. Relationship of hepatic lipidosis to health and performance in dairy cattle. J Am Vet Med Assoc. 1986;88:845–850. [PubMed] [Google Scholar]
- 5.Reid IM. Incidence and severity of fatty liver in dairy cows. Vet Rec. 1980;107:281–284. doi: 10.1136/vr.107.12.281. [DOI] [PubMed] [Google Scholar]
- 6.Herdt TH. Fatty liver in dairy cows. Vet Clin North Am Food Anim Pract. 1988;4:269–287. doi: 10.1016/s0749-0720(15)31048-3. [DOI] [PubMed] [Google Scholar]
- 7.Pullen DL, Liesman JS, Emery RS. A species comparison of lover slice synthesis and secretion of triacylglycerol from nonesterified fatty acids in media. J Anim Sci. 1990;68:1395–1399. doi: 10.2527/1990.6851395x. [DOI] [PubMed] [Google Scholar]
- 8.Herdt TH, Wensing T, Haagsman HP, van Golde LMG, Breukink HJ. Hepatic triacylglycerol synthesis during a period of fatty liver development in sheep. J Anim Sci. 1988;66:1997–2013. doi: 10.2527/jas1988.6681997x. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed T, Oikawa S, Iwasaki Y, et al. Metabolic profiles and bile acid extraction rate in the liver of cows with fasting-induced hepatic lipidosis. J Vet Med A. 2004;51:113–118. doi: 10.1111/j.1439-0442.2004.00614.x. [DOI] [PubMed] [Google Scholar]
- 10.Uchida E, Katoh N, Takahashi K. Induction of fatty liver in cows by ethionine administration and concomitant decreases of serum apolipoproteins B-100 and A-I concentrations. Am J Vet Res. 1992;53:2035–2042. [PubMed] [Google Scholar]
- 11.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 12.Bernabucci U, Ronchi B, Basirico L, et al. Abundance of mRNA of apolipoprotein B100, apolipoprotein E, and microsomal triglyceride transfer protein in liver. J Dairy Sci. 2004;87:2881–2888. doi: 10.3168/jds.S0022-0302(04)73418-9. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa H, Oikawa S, Oohashi T, Katoh N. Decreased serum lecithin: Cholesterol acyltransferase activity in spontaneous cases of fatty liver in cows. Vet Res Commun. 1997;21:1–8. doi: 10.1023/b:verc.0000009695.02015.20. [DOI] [PubMed] [Google Scholar]
- 14.Guyard-Dangremont V, Desrumaux C, Gambert P, Lallemant C, Lagrost L. Phospholipid and cholesteryl ester transfer activities in plasma from 14 vertebrate species. Relation to atherogenesis susceptibility. Comp Biochem Physiol B. 1998;120:517–525. doi: 10.1016/s0305-0491(98)10038-x. [DOI] [PubMed] [Google Scholar]
- 15.Van den Top AM, Van Tol A, Jansen H, Geelen MJH, Beynen AC. Fatty liver in dairy cows post partum is associated with decreased concentration of plasma triacylglycerols and decreased activity of lipoprotein lipase in adipocytes. J Dairy Res. 2005;72:129–137. doi: 10.1017/s0022029905000774. [DOI] [PubMed] [Google Scholar]
- 16.Herdt TH, Liesman BJ, Gerloff BJ, Emery RS. Reduction of serum triglycerol-rich lipoprotein concentrations in cows with hepatic lipidosis. Am J Vet Res. 1983;44:293–296. [PubMed] [Google Scholar]
- 17.Katz ML, Bergman EN. Hepatic and portal metabolism of glucose, free fatty acids, and ketone bodies in the sheep. Am J Physiol. 1969;216:953–960. doi: 10.1152/ajplegacy.1969.216.4.953. [DOI] [PubMed] [Google Scholar]
- 18.Basso LV, Havel RJ. Hepatic metabolism of free fatty acids in normal and diabetic dogs. J Clin Invest. 1970;49:537–547. doi: 10.1172/JCI106264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid IM, Collins RA, Baird GD, Roberts CJ, Symonds HW. Lipid production rates and the pathogenesis of fatty liver in fasted cows. J Agric Sci Camb. 1979;93:253–256. [Google Scholar]
- 20.Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, Unger RH. Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: Impairment in adipocyte-beta-cell relationship. Proc Natl Acad Sci. 1994;91:10878–10882. doi: 10.1073/pnas.91.23.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]