Abstract
Objective
To establish the relationship between adherence and symptom control in adolescents and young adults with asthma.
Design
This was a telephone-interview study conducted as an ancillary project to the Childhood Asthma Management Program Continuation Study (CAMPCS).
Setting
The six monthly interviews were conducted from a single calling center in Denver, Colorado.
Participants
Included were 756 adolescent and young adult participants in CAMPCS, who upon entry into the original study were diagnosed to have mild to moderate asthma.
Outcome measures
Participants were queried about medication use and symptom control within each one-month interview window. Strategies adopted to improve self-report accuracy included use of repeat interviews, confidential reporting to staff unknown to the participants, and use of questions focused on recent behavior.
Results
Only participants who were consistently on or off ICS medication for the entire six-month study interval were included. Three groups of patients were contrasted: those not on ICS medication (n=420), those on ICS with High Adherence (at least 75% of medication taken, n=90), and those on ICS with Low/Medium adherence (less than 75% taken, n=148). Participants in the Low/Medium adherence group reported on average less symptom control and more variability in wheezing, awakening at night, missed activities, and beta2 agonist use over the 6-month period, although most in this group perceived their asthma to be under good control.
Conclusion
Despite extensive patient education and support, diminished ICS adherence was frequent and undermined symptom control in this group of adolescents and young adults with mild to moderate asthma.
Introduction
For patients with persistent asthma, all evidence-based guidelines direct that controller medications be used daily in order to control underlying inflammation. However, many patients will not reliably take daily controller medications over extended periods of time1, 2, 3, 4, 5 and typically use less than half the amount of medication prescribed.6, 7, 2 Patients have consistently indicated their desire to adjust medication level to their symptoms and cease medication use during asymptomatic periods.8 While treatment plans that limit the use of inhaled corticosteroids to periods of symptom exacerbation fall outside most asthma treatment guidelines, recent investigations have begun to examine the efficacy of symptom-based, patient-determined use of controller medication.9, 10
Clinical information about symptoms and information on asthma self-management must necessarily be obtained from the patient. However, the circumstance under which patients are queried may greatly influence the accuracy of patient-reported information. The accuracy of information declines rapidly when patients are asked to recall symptoms over long time intervals. 11, 12 Therefore, repeated surveys of recent events, or brief interval reporting, can provide increased accuracy.13 Using brief interval reporting through telephone interviews, this study surveyed adolescents and young adults about their level of adherence to asthma medications and associated symptom control.
METHODS
This study included a series of six monthly telephone interviews with adolescent and young adult participants in the second phase of the Childhood Asthma Management Program (CAMP). CAMP was a multicenter clinical trial initiated and sponsored by the National Heart, Lung, and Blood Institute to assess the long-term effects of asthma treatment on lung growth in children in eight North American cities (Albuquerque, Baltimore, Boston, Denver, St. Louis, San Diego, Seattle, and Toronto). Participants were randomized to one of three treatment conditions: (1) budesonide (an inhaled corticosteroid); (2) nedocromil (an inhaled, nonsteroid anti-inflammatory); or (3) as-needed beta-agonist without anti-inflammatory treatment. The CAMP Continuation Study (CAMPCS) followed CAMP as a 4.5-year observational study that continued to document disease progress. In the CAMPCS protocol, participants were interviewed twice by telephone and attended two study visits each year. Decisions about type and dose of medication were made by the child’s personal physician. This report includes information obtained through an ancillary study conducted during CAMPCS.
CAMP Subjects
Participants in CAMP met the criteria of mild to moderate asthma if, during 6 of the 12 months preceding trial entry, they (1) had asthma symptoms at least twice per week, (2) used an inhaled bronchodilator at least two times per week, and/or (3) used daily asthma medication. Participants were excluded if they had an FEV1 less than 65% predicted or an FEV1 PC20 to methacholine of greater than 12.5 mg/ml; had an intubation at any time in the past due to asthma; or had in the year prior to screening two or more hospitalizations or more than five oral steroid bursts. Between December 1993 and September 1995, a total of 1,041 children aged 5 to 12 (mean±SD = 8.9 ± 2.1 years, median = 8.9 years) were enrolled into the CAMP trial. Of these participants, 40.3% were female and 31.7% were racial or ethnic minority. Asthma severity, as assessed by a physician or nurse coordinator, was mild in 47.7% (n=494) and moderate in 52.3% (n=541) of participants. A patient asthma education program was standardized across all eight CAMP sites and presented in three sessions of approximately one hour each that included handouts, handbooks, and videos that addressed disease characteristics, peak flow monitoring, medications triggers, and asthma action plans.14 Of the 1041 original CAMP participants, 941 were enrolled in CAMPCS.
Interview study participants
Institutional Review Board approval for this ancillary telephone-interview study was obtained at each of the eight study sites, and informed consent was obtained from 756 participants to participate in the interviews.
Brief-interval telephone interview procedures
A series of six monthly telephone calls were made to each patient from the calling center located at National Jewish Medical and Research Center in Denver. All calls were conducted by one of two respiratory nurses who had no other contact with the CAMP study participants. The nurses began each call reminding respondents of the fact that the nurses were not from their local CAMP clinic and would not be communicating any of the participant’s responses to the CAMP staff. In all six calls, participants were questioned about recent medication prescriptions, adherence, symptoms, use of rescue medication, and health care utilization. Adherence questions were directed to ICS use, the predominant medication class in this cohort. Participants who were on and off medications during the study interval, including ICS and a leukotriene antagonist, were not included in the data set. This procedure was adopted to avoid confusion and focus adherence inquiry to medications prescribed consistently for the entire 6-month interval. Adherence questions focused on medication use during the previous day. Symptom questions were restricted to those occurring in the previous week (e.g., “In the last week, on how many days have you had wheezing or difficulty breathing when exercising?”). Thus, for each of the six interviews, participants reported the number of days in which each symptom occurred in the previous week. Four symptom questions inquired about the presence of specific asthma symptoms including wheezing while exercising, wheezing while not exercising, night awakening, and restricted activities. Participants also reported the number of days in which they used their beta2 agonist for rescue during the preceding week. Health care utilization questions focused on events occurring in the previous month and included queries about hospitalizations or visits to the health care provider or emergency department. Finally, participants were asked “For the past month, do you believe that your asthma has been under good control?”. The rationale for employing six brief adherence interviews reflects previously published evidence that self-report of adherence, which tends to significantly exaggerate actual adherence, will be more accurate under specific conditions that: (1) limit memory demands,15 (2) limit interpersonal discomfort,16 (3) focus on very specific, recent behaviors,17–19 and (4) repeat 24-hour interviews over 6 months13. While adherence behavior within a single 24-hour period may not be representative of the broader pattern of behavior, repetition of the 24-hour interviews over 4–8 occasions provides an accurate estimate of overall adherence. 20, 21, 22, 23, 24
Statistical analyses
Descriptive statistics were calculated for all variables, including medians, ranges, means, and standard deviations. Adherence levels for each participant were calculated from each interview by dividing the number of reported puffs used in the previous day by the prescribed amount. For example, a patient reporting that they took 2 puffs when 4 had been prescribed had 50% adherence on that day. These adherence levels were then averaged over the 6 interviews to create a single overall estimate of that participant’s adherence over the six-month period. While categorizations of adherence levels vary across studies, a convention of 75% adherence has been frequently employed 25, 7, 26 and was adopted as the separation between “Low/Medium” adherence <75%) and “High” adherence (>75%). Levene’s test was used to test for differences among group variances. Comparisons involving proportions or percentages were conducted by chi-square analysis. For these comparisons, symptom indices consisted of the percent of participants reporting the symptom in the week prior to the interview. Levene’s test was used to test for differences among group variances. All statistical analyses were performed using SPSS V.14 (SPSS, Inc., Chicago).
RESULTS
Baseline demographic and disease characteristics of the study cohort
Demographic and disease characteristics of the participants in this ancillary study (n=756) were very similar to the original cohort of CAMP participants (n=1041) and those in the full CAMPCS study (n=941) (Table 1). One third of participants were of an ethnic minority, and most were adolescents, with a mean age of 17.3 years (range = 13 to 22 years) at the point of informed consent.
Table 1.
Baseline demographic characteristics of current group, original CAMP group, and CAMPCS group
| Current group (N=756) | Original CAMP group (N=1041) | CAMPCS group (N=941) | |
|---|---|---|---|
| Age (yrs, mean±SD) | 8.8±2.1 | 8.9±2.1 | 8.9±2.1 |
| Ethnic (%) | |||
| Non-hispanic white | 68.2 | 68.1 | 68.6 |
| Non-hispanic black | 13.1 | 13.1 | 13.7 |
| Hispanic | 9.9 | 9.4 | 9.1 |
| Other | 7.4 | 8.8 | 7.9 |
| Gender (%) | |||
| male | 59.3 | 60.7 | 60.2 |
| Age of onset of asthma (yrs, mean±SD) | 3.0±2.4 | 3.1±2.6 | 3.1±2.5 |
| Severity of asthma at randomization (%) | |||
| mild | 47.7 | 47.8 | 47.9 |
| moderate | 52.3 | 52.1 | 52.2 |
Medication adherence
Of the 756 participants interviewed, 238 reported that they had a prescription for an ICS and available supply of their medication during the entire six-month period. The 78 participants who reported having an active ICS prescription on some interviews and not others, or 20 who were on a leukotriene antagonist, were not included in the analysis. The remaining 420 participants, each of whom reported that their physician had not prescribed a controller medication during the 6-month period, comprised the No ICS comparison group. Averaged across individual responses to the six interviews, mean adherence over the six month period 78.2% (SD= 39.0). Low/Medium adherence (<75%) was reported by 38% of participants, and High adherence (≥ 75%) was reported by 62%. Most of the 238 participants were on a single controller medication. Mean adherence levels did not differ between those participants on one controller vs. those on two or more controller medications (data not shown).
Symptom reporting
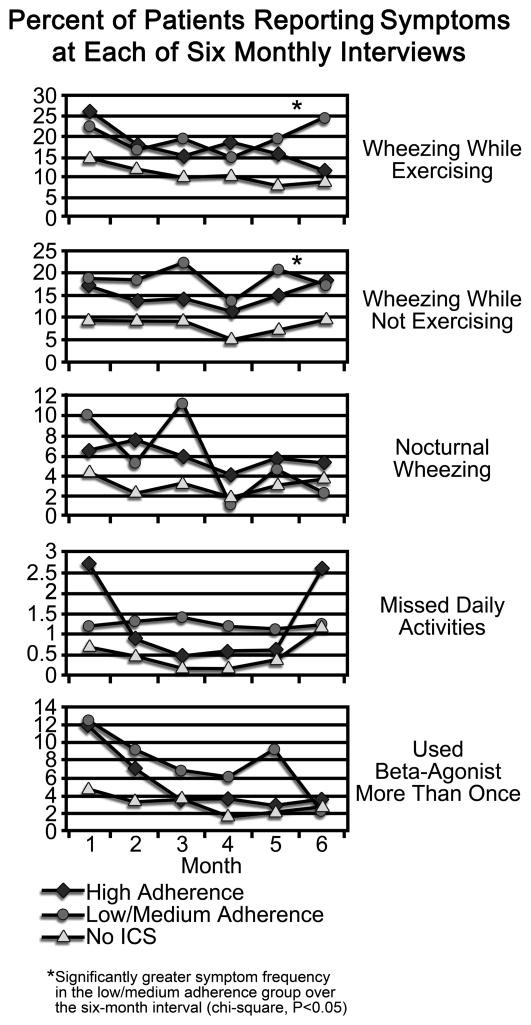
The percent of participants reporting symptoms in each of the six monthly telephone intervals is reflected in Figure 1. Across the group of 756 participants, wheezing, while exercising or not, occurred on average in 13.7% of participants within each 7-day reporting period. Across all 6 reporting periods, 4.2% experienced awakening with wheezing at least once. The presence of symptoms resulted in beta2 agonist use in 4.4% and resulted in missed activities in 1.7% of patients. Across the 6 months, the Low/Medium adherence group indicated more wheezing, with or without exercise, than those in the High adherence or No ICS groups (p<0.05).
FIGURE 1.
Percent of patients reporting symptoms or beta-agonist use at each of six monthly telephone interviews
To examine the variability of symptom scores across the six interviews, comparisons between the three groups were made for symptom score standard deviations for each symptom score. Standard deviations were significantly increased in the Low/Medium adherence group when compared to the High adherence and No ICS groups for wheezing while exercising (p=0.000) and while not exercising (p=0.000), waking at night (p=0.014), missing activities (0.001), and two or more uses of rescue medication (p=0.000).
Health care events and perceived control
At each interview, fewer than one percent of participants reported a hospitalization or emergency department visit and, within each interval, an average of 1.2% visited a health care provider for asthma care, with no differences separating the three groups. More than 96% of participants in all three groups agreed that their asthma was under “good control” across all 6 interviews.
DISCUSSION
Participants with reduced ICS adherence reported more wheezing than those with higher levels of adherence; over the six interviews, one in five participants in the Low/Medium adherence group experienced wheezing during the previous week. Further, in the Low/Medium adherence group, month-to-month variability of symptoms, including wheezing, awakening at night, missed activities, and beta2 agonist use, was twice that of the other two groups. The association between low adherence and inadequate control did not extend to increased need for hospitalization or visits to the physician or emergency department, all of which occurred rarely. Nonetheless, these data establish that in a group of adolescents and young adults with mild to moderate asthma who had received asthma education, diminished ICS adherence undermined symptom control. Few other studies have directly linked ICS adherence level to symptom control, and none have done so with adolescents. An administrative database study from a health maintenance organization revealed that decreasing adherence, as reflected in refill rates over three years in a group of 405 adults with asthma, was incrementally associated with increases in emergency department visits, requirement of oral steroid, and hospitalizations. Extrapolating from these data, the authors estimated that 48 of 80 hospitalizations may have been prevented had those patients been fully adherent to their ICS regimen.6
In this study, although 20% of those in the Low/Medium adherence group reported wheezing, only 1–2% reported that their asthma was not under “good control” in each of the six interviews. About 10% of the group with no controller medication reported wheezing, although less than 3% judged their control as inadequate. Given the extensive education and frequent study contacts provided to CAMPCS participants, these results suggest that despite awareness of available anti-inflammatory interventions participants are willing to accept the presence of some asthma symptoms in the course of their daily lives. Stronger symptom control in the No ICS group suggests less need for controller medication, although even in this group nearly 10% reported wheezing.
Numerous steps, including assurances of anonymity and focus on reporting adherence over a recent time interval (i.e., last week), were taken to decrease potential embarrassment and increase adherence reporting accuracy. Despite this, participants likely over-reported adherence. The mean reported adherence rate of 78% is higher than that seen in studies employing objective adherence measures. Further, the distribution was skewed upward with a relatively large proportion of participants reporting perfect (i.e., 100%) adherence. In studies comparing self report to objectively-measured adherence, self report over-estimated adherence by 50% in adults 2, 27 and children.5 Nonetheless, previous research also demonstrates that self-report correlates moderately with objective measures of adherence31 and is generally more accurate when conducted through a confidential interview.32 In a study of adherence in children with asthma, concordance between diary and electronic monitor data was 85% even though adherence recorded on diary cards was significantly over reported.28 Thus, while tending to over report positive behavior, many patients nonetheless categorize themselves with relative accuracy on a continuum of adherence. No sufficient alternative exists to measure and understand symptoms, pain, feelings, experiences and actions to manage the illness other than to ask patients. Consequently, patient self-report remains a necessary component of the assessment of disease activity and treatment success. Researchers and clinicians will continue to utilize patient reports, recognizing the limitations of self-report methodology while seeking approaches to minimize error from memory limitations or embarrassment.
Clinical implications
Adolescents and young adults with asthma underutilize daily controller medications and appear to tolerate some degree of asthma symptoms. Given that participants in the Childhood Asthma Management Program have received comprehensive asthma education and a high degree of caregiver contact exceeding that received in most clinical settings, it may be unrealistic to assume that most patients under less compelling circumstances will use controller medication consistent with current expert guidelines. Careful consideration of patient goals and preferences must be factored into physician decisions about inclusion of 365-day-a-year controller medication use in the prescribed treatment plan.
Acknowledgments
Supported by grant R01 HL70267 from the National Heart Lung and Blood Institute and General Clinical Research Centers grant M01-RR00051
Abbreviations
- ICS
Inhaled corticosteroid
- CAMP
Childhood Asthma Management Program
- CAMPCS
Childhood Asthma Management Program Continuation Study
- FEV1
Forced expiratory volume in one second
- FEV1 PC20
Provocative concentration of methacholine required to cause a 20% decrease in FEV1
- SD
Standard deviation
- ANOVA
Analysis of variance
References
- 1.Onyirimba F, Apter A, Reisine ST, et al. Direct clinician-to-patient feedback discussion of inhaled steroid use; its effect on adherence. Ann Allergy Asthma Immunol. 2003;90(4):411–415. doi: 10.1016/S1081-1206(10)61825-X. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan JA, Riekert KA, McCoy JV, et al. Corticosteriod use after hospital discharge among high-risk adults with asthma. Am J Respir and Crit Care Med. 2004;170:1281–1285. doi: 10.1164/rccm.200403-409OC. [DOI] [PubMed] [Google Scholar]
- 3.Walders N, Kopel SJ, Koinis-Mitchell D, McQuaid EL. Patterns of quick-relief and long-term controller medication use in pediatric asthma. J Pediatr. 2005;146:177–182. doi: 10.1016/j.jpeds.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 4.McQuaid E, Kopel S, Klein R, Fritz G. Medication adherence in pediatric asthma: reasoning, responsibility, and behavior. J Ped Psychol. 2003;28:323–333. doi: 10.1093/jpepsy/jsg022. [DOI] [PubMed] [Google Scholar]
- 5.Bender B, Annett R, Ikle D, DuHamel T, Rand C, Strunk R. Relationship between disease and psychological adaptation in children in the Childhood Asthma Management Program and their families. For the CAMP Research Group. Arch Ped Adolesc Med. 2000;154:706–713. doi: 10.1001/archpedi.154.7.706. [DOI] [PubMed] [Google Scholar]
- 6.Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteriods and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;(114):1288–1293. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Apter A, Reisine S, Affleck G, Barrows E, ZuWallack R. Adherence with twice-daily dosing of inhaled steroids. Am J Respir Crit Care Med. 1998;157:1810–1817. doi: 10.1164/ajrccm.157.6.9712007. [DOI] [PubMed] [Google Scholar]
- 8.Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence; responses to interview, focus groups, and questionnaires. Immunology and Allergy Clinics of North America. 2005;25:107–130. doi: 10.1016/j.iac.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Boushey HA, Sorkness CA, King TS, et al. Daily versus as-needed corticosteriods for mild persistent asthma. N Eng J Med. 2005;352:1519–1528. doi: 10.1056/NEJMoa042552. [DOI] [PubMed] [Google Scholar]
- 10.O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir and Crit Care Med. 2005;171:129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 11.Barsky A. Forgetting, fabricating, and telescoping: the instability of the medical history. Arch Intern Med. 2002;162(9):981–984. doi: 10.1001/archinte.162.9.981. [DOI] [PubMed] [Google Scholar]
- 12.Fransson P. Recall of pretreatment symptoms among men treated with radiotherapy for prostate cancer. Acta Oncol. 2005;44(4):355–361. doi: 10.1080/02841860510029806. [DOI] [PubMed] [Google Scholar]
- 13.Johnson S, Kelly M, Henreta J, Cunningham W, Tomer A, Silverstein J. A longitudinal analysis of adherence and health status in childhood diabetes. Journal an of Pediatric Psychology. 1992;17:537–553. doi: 10.1093/jpepsy/17.5.537. [DOI] [PubMed] [Google Scholar]
- 14.Szefler S, Oliver S, Bender B, Nelson H, Culkin C, Taggart V. Design and operation of a patient education control for the childhood asthma management program. Annals of Allergy, Asthma and Immunology. 1998;81:571–579. [PubMed] [Google Scholar]
- 15.Neath I. Contextual and distinctive processes and the serial position function. Journal of Memory & Language. 1993;32(6):820–840. [Google Scholar]
- 16.Hays R, Hayashi T, Stewart A. A five-team measure of socially desirable response set. Educational and Psychological Measurement. 1989;49:629–636. [Google Scholar]
- 17.Baxter S, Smith A, Litaker M, et al. Recency affects reporting accuracy of children’s dietary recalls. Ann Epidemiol. 2004;14:385–390. doi: 10.1016/j.annepidem.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Rudd P. Clinicians and patients with hypertension: unsettled issues about compliance. Am Heart J. 1995;130(3):572–579. doi: 10.1016/0002-8703(95)90368-2. [DOI] [PubMed] [Google Scholar]
- 19.Wiener L, Riekert K, Ryder C, wood L. Assessing medication adherence in adolescents with HIV when electronic monitoring is not feasible. AIDS Patient Care STDS. 2004;18:527–538. doi: 10.1089/apc.2004.18.527. [DOI] [PubMed] [Google Scholar]
- 20.Zabinski M, Daly T, Norman G, et al. Psychosocial correlates of fruit, vegetable, and dietary fat intake among adolescent boys and girls. J Am Diet Assoc. 2006;106(6):814–821. doi: 10.1016/j.jada.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Kabagambe E, Baylin A, Allan D, Siles X, Spiegelman D, Campos H. Application of the method of triads to evaluate the performance of food frequency questionnaires and biomarkers as indicators of long-term dietary intake. Am J Epidemiol. 2001;154(12):1126–1135. doi: 10.1093/aje/154.12.1126. [DOI] [PubMed] [Google Scholar]
- 22.Council NR. Assessment Using Food Consumption Surveys. 1986 [Google Scholar]
- 23.Johnson S, Freund A, Silverstein J, Hansen C, Malone J. Adherence-health status relationships in childhood diabetes. Health Psychol. 1990;9(5):606–631. [PubMed] [Google Scholar]
- 24.Council NR. Assessment Using Food Consumption Surveys. Washington, DC: National Academy Press; 1986. [Google Scholar]
- 25.Rand C, Wise R. Measuring adherence to asthma medication regimens. Am J Respir and Crit Care Med. 1994;149:S69–S76. doi: 10.1164/ajrccm/149.2_Pt_2.S69. [DOI] [PubMed] [Google Scholar]
- 26.Diette GB, Wu AW, Skinner EA, et al. Treatment patterns among adult patients with asthma. Arch Intern Med. 1999;159:2697–2704. doi: 10.1001/archinte.159.22.2697. [DOI] [PubMed] [Google Scholar]
- 27.Tashkin D, Rand C, Nicles M. A nebulizer chronolog to monitor compliance with inhaler use. Am J Med. 1991;91:335–365. doi: 10.1016/0002-9343(91)90260-5. [DOI] [PubMed] [Google Scholar]
- 28.Butz AM, Donithan M, Bollinger ME, Rand C, Thompson RE. Monitoring nebulizer use in children; comparison of electronic and asthma diary data. Ann Allergy Asthma Immunol. 2005;94(3):360–365. doi: 10.1016/S1081-1206(10)60988-X. [DOI] [PubMed] [Google Scholar]