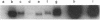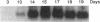Abstract
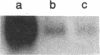
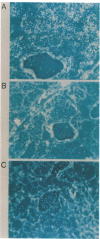
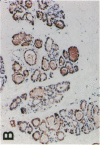
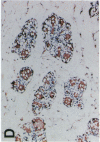
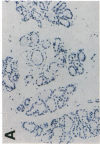
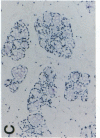
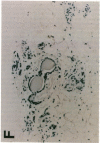
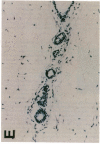
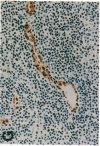
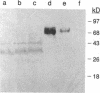
Glycosylation-dependent cell adhesion molecule 1 (GlyCAM 1) is a mucinlike endothelial glycoprotein that acts as an adhesive ligand for L selectin by presenting one or more O-linked carbohydrates to the lectin domain of this leukocyte cell surface selectin. The GlyCAM 1 glycoprotein has been previously shown to be expressed specifically by the endothelial cells of peripheral and mesenteric lymph nodes and in an unknown site in lung. Here we report that this protein is also expressed during lactation by mammary epithelial cells. Northern blot analysis has shown that the mRNA for GlyCAM 1 appears to be induced during pregnancy in a manner similar to that previously described for hormonally induced milk proteins. In situ hybridization analysis reveals that the site of GlyCAM 1 synthesis in the mammary gland is in the epithelial cells that produce these same milk proteins. Immunohistochemistry of mammary glands using antisera directed against GlyCAM 1 peptides demonstrates that these epithelial cells contain GlyCAM 1 protein, and that this protein is also found lumenally in the milk of the secreting mammary gland. Analysis of murine milk shows that immunoreactive GlyCAM 1 is found in the soluble whey fraction. Finally, labeling analysis of milk GlyCAM 1 has demonstrated that this form of the glycoprotein lacks the sulfate-modified carbohydrate that has recently been shown to be required for the ligand binding activity to L selectin. The nonsulfated mammary GlyCAM 1 is unable to interact with L selectin, consistent with the hypothesis that milk GlyCAM 1 has a different function than endothelial GlyCAM 1. These data thus suggest that milk GlyCAM 1 is a hormonally regulated milk protein that is part of the milk mucin complex. In addition, the finding that the mammary form of GlyCAM 1 contains different carbohydrate modifications than the endothelial form suggests that this glycoprotein may be a scaffold for carbohydrates that mediate functions in addition to cell adhesion.
Full text
PDF


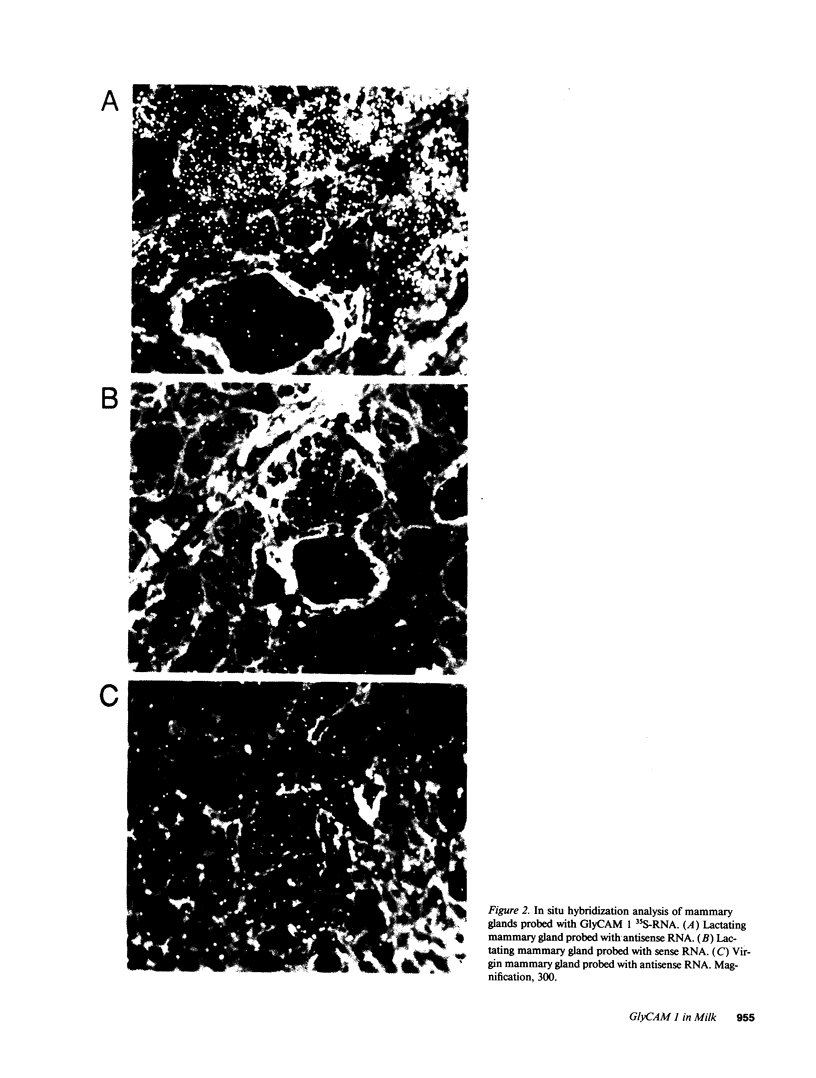
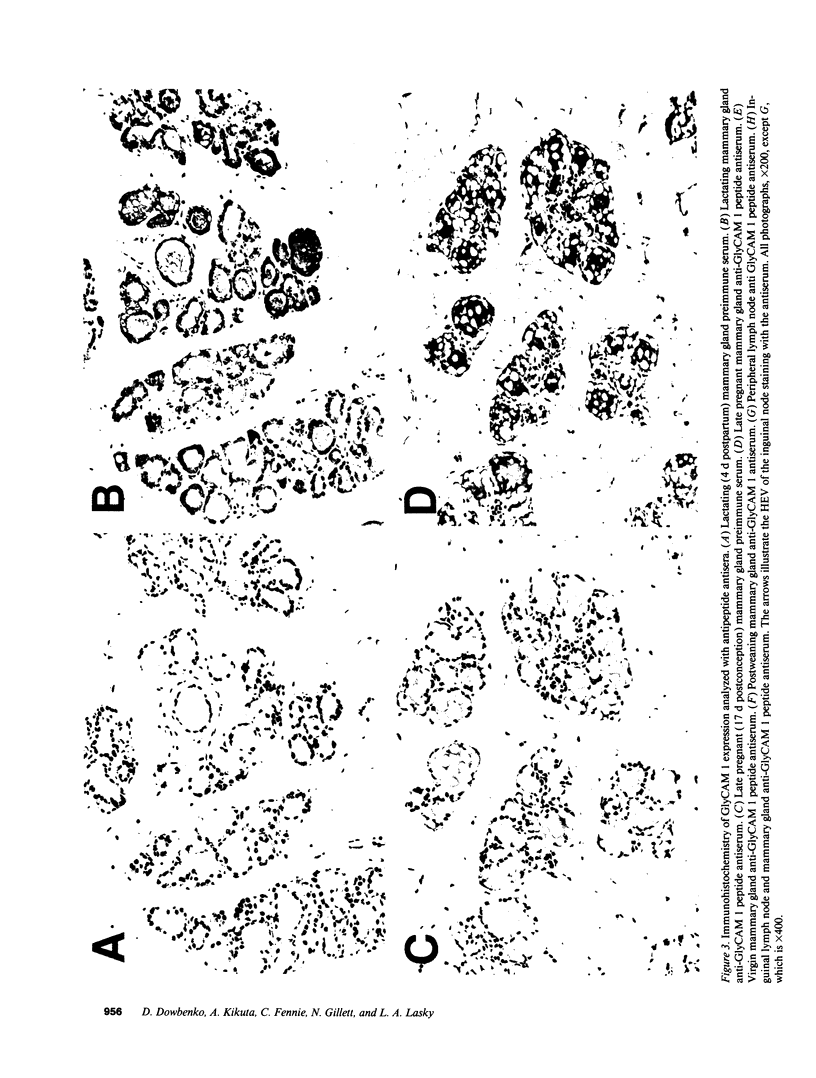
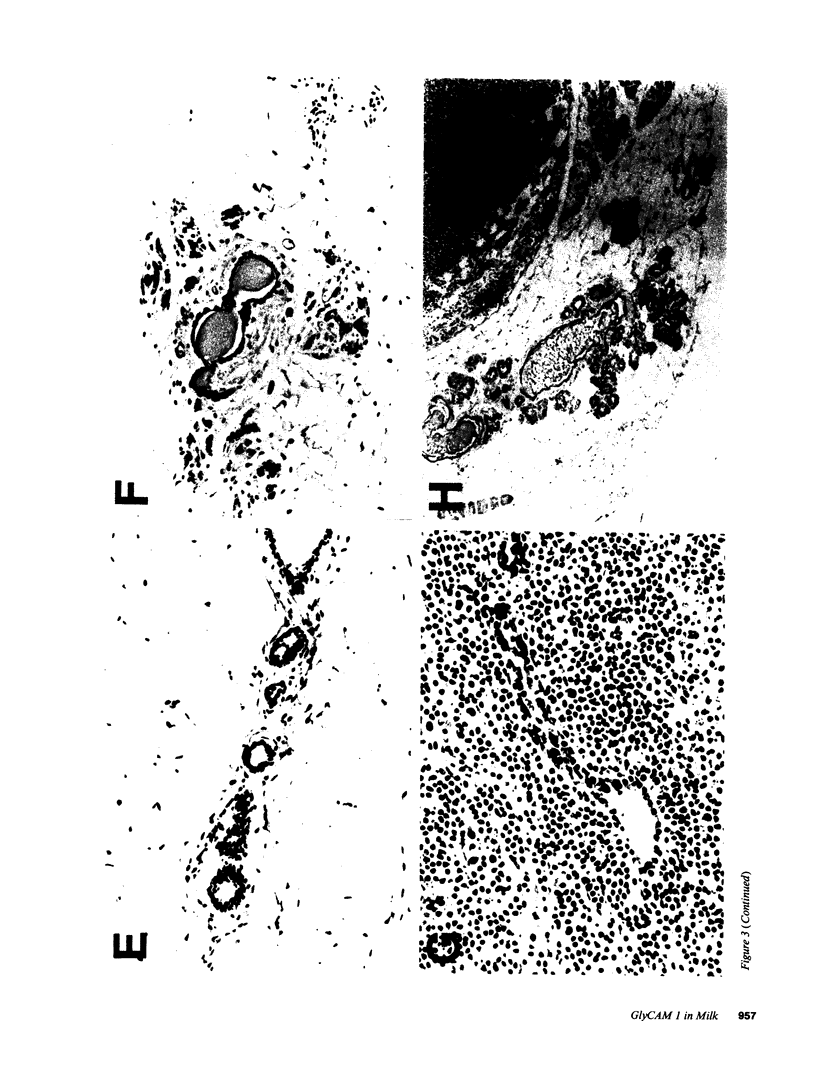



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P., Milsom D. W., Ford W. L. Migration of lymphocytes across specialized vascular endothelium. V. Production of a sulphated macromolecule by high endothelial cells in lymph nodes. J Cell Sci. 1982 Oct;57:277–292. doi: 10.1242/jcs.57.1.277. [DOI] [PubMed] [Google Scholar]
- Berg E. L., Robinson M. K., Warnock R. A., Butcher E. C. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991 Jul;114(2):343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustein M., Kraal G., Mebius R. E., Watson S. R. Identification of a soluble form of a ligand for the lymphocyte homing receptor. J Exp Med. 1992 Nov 1;176(5):1415–1419. doi: 10.1084/jem.176.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraway K. L., Hull S. R. Cell surface mucin-type glycoproteins and mucin-like domains. Glycobiology. 1991 Mar;1(2):131–138. doi: 10.1093/glycob/1.2.131. [DOI] [PubMed] [Google Scholar]
- Cyster J. G., Shotton D. M., Williams A. F. The dimensions of the T lymphocyte glycoprotein leukosialin and identification of linear protein epitopes that can be modified by glycosylation. EMBO J. 1991 Apr;10(4):893–902. doi: 10.1002/j.1460-2075.1991.tb08022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowbenko D., Andalibi A., Young P. E., Lusis A. J., Lasky L. A. Structure and chromosomal localization of the murine gene encoding GLYCAM 1. A mucin-like endothelial ligand for L selectin. J Biol Chem. 1993 Feb 25;268(6):4525–4529. [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florman H. M., Wassarman P. M. O-linked oligosaccharides of mouse egg ZP3 account for its sperm receptor activity. Cell. 1985 May;41(1):313–324. doi: 10.1016/0092-8674(85)90084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M. Leukosialin, a major O-glycan-containing sialoglycoprotein defining leukocyte differentiation and malignancy. Glycobiology. 1991 Sep;1(4):347–356. doi: 10.1093/glycob/1.4.347. [DOI] [PubMed] [Google Scholar]
- Gendler S. J., Burchell J. M., Duhig T., Lamport D., White R., Parker M., Taylor-Papadimitriou J. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S. J., Spicer A. P., Lalani E. N., Duhig T., Peat N., Burchell J., Pemberton L., Boshell M., Taylor-Papadimitriou J. Structure and biology of a carcinoma-associated mucin, MUC1. Am Rev Respir Dis. 1991 Sep;144(3 Pt 2):S42–S47. doi: 10.1164/ajrccm/144.3_pt_2.S42. [DOI] [PubMed] [Google Scholar]
- Greenwalt D. E., Lipsky R. H., Ockenhouse C. F., Ikeda H., Tandon N. N., Jamieson G. A. Membrane glycoprotein CD36: a review of its roles in adherence, signal transduction, and transfusion medicine. Blood. 1992 Sep 1;80(5):1105–1115. [PubMed] [Google Scholar]
- Greenwalt D. E., Mather I. H. Characterization of an apically derived epithelial membrane glycoprotein from bovine milk, which is expressed in capillary endothelia in diverse tissues. J Cell Biol. 1985 Feb;100(2):397–408. doi: 10.1083/jcb.100.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwalt D. E., Watt K. W., So O. Y., Jiwani N. PAS IV, an integral membrane protein of mammary epithelial cells, is related to platelet and endothelial cell CD36 (GP IV). Biochemistry. 1990 Jul 31;29(30):7054–7059. doi: 10.1021/bi00482a015. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Lagace R. E., Byrd J. C., Toribara N. W., Siddiki B., Fearney F. J., Lamport D. T., Kim Y. S. Molecular cloning of rat intestinal mucin. Lack of conservation between mammalian species. J Biol Chem. 1991 Nov 25;266(33):22733–22738. [PubMed] [Google Scholar]
- Harding S. E. The macrostructure of mucus glycoproteins in solution. Adv Carbohydr Chem Biochem. 1989;47:345–381. doi: 10.1016/s0065-2318(08)60417-5. [DOI] [PubMed] [Google Scholar]
- Hendriks H. R., Duijvestijn A. M., Kraal G. Rapid decrease in lymphocyte adherence to high endothelial venules in lymph nodes deprived of afferent lymphatic vessels. Eur J Immunol. 1987 Dec;17(12):1691–1695. doi: 10.1002/eji.1830171203. [DOI] [PubMed] [Google Scholar]
- Hobbs A. A., Richards D. A., Kessler D. J., Rosen J. M. Complex hormonal regulation of rat casein gene expression. J Biol Chem. 1982 Apr 10;257(7):3598–3605. [PubMed] [Google Scholar]
- Imai Y., Lasky L. A., Rosen S. D. Sulphation requirement for GlyCAM-1, an endothelial ligand for L-selectin. Nature. 1993 Feb 11;361(6412):555–557. doi: 10.1038/361555a0. [DOI] [PubMed] [Google Scholar]
- Imai Y., Singer M. S., Fennie C., Lasky L. A., Rosen S. D. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991 Jun;113(5):1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentoft N. Why are proteins O-glycosylated? Trends Biochem Sci. 1990 Aug;15(8):291–294. doi: 10.1016/0968-0004(90)90014-3. [DOI] [PubMed] [Google Scholar]
- Kawamura K., Satow H., Do Ik L., Sakai S., Takada S., Obinata M. Modulation of the transferred mouse 26K casein gene in mouse L cells by glucocorticoid hormone. J Biochem. 1987 Jan;101(1):103–110. doi: 10.1093/oxfordjournals.jbchem.a121880. [DOI] [PubMed] [Google Scholar]
- Laegreid A., Kolstø Otnaess A. B., Orstavik I., Carlsen K. H. Neutralizing activity in human milk fractions against respiratory syncytial virus. Acta Paediatr Scand. 1986 Sep;75(5):696–701. doi: 10.1111/j.1651-2227.1986.tb10276.x. [DOI] [PubMed] [Google Scholar]
- Larocca D., Peterson J. A., Urrea R., Kuniyoshi J., Bistrain A. M., Ceriani R. L. A Mr 46,000 human milk fat globule protein that is highly expressed in human breast tumors contains factor VIII-like domains. Cancer Res. 1991 Sep 15;51(18):4994–4998. [PubMed] [Google Scholar]
- Larocca D., Peterson J. A., Walkup G., Urrea R., Ceriani R. L. Cloning and sequencing of a complementary DNA encoding a Mr 70,000 human breast epithelial mucin-associated antigen. Cancer Res. 1990 Sep 15;50(18):5925–5930. [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Dowbenko D., Imai Y., Henzel W. J., Grimley C., Fennie C., Gillett N., Watson S. R., Rosen S. D. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992 Jun 12;69(6):927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Singer M. S., Yednock T. A., Dowbenko D., Fennie C., Rodriguez H., Nguyen T., Stachel S., Rosen S. D. Cloning of a lymphocyte homing receptor reveals a lectin domain. Cell. 1989 Mar 24;56(6):1045–1055. doi: 10.1016/0092-8674(89)90637-5. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Mebius R. E., Streeter P. R., Brevé J., Duijvestijn A. M., Kraal G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J Cell Biol. 1991 Oct;115(1):85–95. doi: 10.1083/jcb.115.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porchet N., Dufosse J., Audie J. P., Duperat V. G., Perini J. M., Nguyen V. C., Degand P., Aubert J. P. Structural features of the core proteins of human airway mucins ascertained by cDNA cloning. Am Rev Respir Dis. 1991 Sep;144(3 Pt 2):S15–S18. doi: 10.1164/ajrccm/144.3_pt_2.S15. [DOI] [PubMed] [Google Scholar]
- Roux M. E., McWilliams M., Phillips-Quagliata J. M., Weisz-Carrington P., Lamm M. E. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977 Nov 1;146(5):1311–1322. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satow H., Sakai S., Obinata M. Post-transcriptional control of 26 k casein genes during lactogenesis in mouse mammary glands. J Biochem. 1986 Jun;99(6):1639–1643. doi: 10.1093/oxfordjournals.jbchem.a135638. [DOI] [PubMed] [Google Scholar]
- Schmitt-Ney M., Happ B., Ball R. K., Groner B. Developmental and environmental regulation of a mammary gland-specific nuclear factor essential for transcription of the gene encoding beta-casein. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3130–3134. doi: 10.1073/pnas.89.7.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segrest J. P., De Loof H., Dohlman J. G., Brouillette C. G., Anantharamaiah G. M. Amphipathic helix motif: classes and properties. Proteins. 1990;8(2):103–117. doi: 10.1002/prot.340080202. [DOI] [PubMed] [Google Scholar]
- Sherblom A. P., Carraway K. L. A complex of two cell surface glycoproteins from ascites mammary adenocarcinoma cells. J Biol Chem. 1980 Dec 25;255(24):12051–12059. [PubMed] [Google Scholar]
- Vonderhaar B. K., Ziska S. E. Hormonal regulation of milk protein gene expression. Annu Rev Physiol. 1989;51:641–652. doi: 10.1146/annurev.ph.51.030189.003233. [DOI] [PubMed] [Google Scholar]
- Watson S. R., Imai Y., Fennie C., Geoffroy J. S., Rosen S. D., Lasky L. A. A homing receptor-IgG chimera as a probe for adhesive ligands of lymph node high endothelial venules. J Cell Biol. 1990 Jun;110(6):2221–2229. doi: 10.1083/jcb.110.6.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth N. S. Lactation in humans. Psychoneuroendocrinology. 1988;13(1-2):171–188. doi: 10.1016/0306-4530(88)90013-3. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Peterson J. A., Vonderfecht S. L., Fouts E. T., Midthun K., Newburg D. S. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992 Nov;90(5):1984–1991. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Willoughby R., Wee S. B., Miskuff R., Vonderfecht S. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J Clin Invest. 1987 Jan;79(1):148–154. doi: 10.1172/JCI112775. [DOI] [PMC free article] [PubMed] [Google Scholar]