Abstract
Background
The vein of Marshall (VOM) is a left atrial (LA) vein that contains autonomic innervation and triggers of AF. Its location coincides with areas usually ablated during pulmonary vein (PV) antral isolation (PVAI).
Objectives
To delineate the safety and ablative effects of ethanol infusion in the VOM during catheter ablation of atrial fibrillation (AF).
Methods
Patients undergoing PVAI (n=14) were consented for adjunctive VOM ethanol infusion. In 10/14 patients, the VOM was cannulated with an angioplasty wire and balloon. Echocardiographic contrast was injected in the VOM under echocardiographic monitoring. Two infusions of 100% ethanol (1cc each) were delivered via the angioplasty balloon in the VOM. LA bipolar voltage maps were created before and after ethanol infusion. Radiofrequency ablation times required to isolate each PV and other procedural data were compared with those of 10 age-, sex-, AF type- and LA size-matched controls undergoing conventional PVAI.
Results
The VOM communicated with underlying myocardium, as shown by echocardiographic contrast passage into the LA. There were no acute complications related to VOM ethanol infusion, which led to the creation of a low-voltage area in the LA measuring 10.6±7.6 cm2 and isolation of the left inferior PV in 4/10 patients. Radiofrequency ablation time required to achieve isolation of the left inferior PV was reduced (2.2±4 min vs 11.4±10.3 min in controls, p<0.05).
Conclusions
VOM ethanol infusion is safe in humans, decreases radiofrequency ablation time in the left inferior PV, and may have a role as an adjunct to PVAI.
Keywords: vein of Marshall, ethanol, ablation, alcohol, atrial fibrillation
Introduction
The vein of Marshall (VOM) is an atrial branch of the coronary sinus that has been implicated in the pathogenesis of atrial fibrillation (AF), both as a source of focal ectopic beats that trigger AF, (1-6) as well as a source of parasympathetic(7) and sympathetic(8) innervations that modulate electrical properties of atrial tissue and contribute to AF maintenance.(9) These properties make the VOM an attractive target during ablation of AF. Furthermore, the location of the VOM in the left atrium (LA), running in the epicardial aspect of the lateral ridge (thick pectinate muscle between the left pulmonary veins and the LA appendage), and running along the postero-lateral LA towards the annulus (coronary sinus) coincides with LA regions usually ablated during catheter ablation of AF.(10)
We have developed a technique to retrogradely cannulate the VOM and have demonstrated that selective ethanol infusion in the VOM achieves rapid ablation of LA tissues.(11) Here we describe the adjunctive effects of VOM ethanol infusion on pulmonary vein antral isolation (PVAI) in patients subjected to standard radiofrequency catheter ablation of AF.
Methods
Patients
The clinical research protocol was approved by the Institutional Review Committee of the Methodist Hospital, and all subjects were given detailed information and signed an informed consent form. Fourteen patients with symptomatic, drug refractory AF (9 paroxysmal, 5 chronic persistent), undergoing clinically indicated ablation of AF, were included in the study. We completed the protocol of VOM cannulation and ethanol infusion in 10 patients. In order to assess the role of VOM ethanol infusion in facilitating radiofrequency ablation to achieve pulmonary vein isolation, we selected as controls 10 patients undergoing conventional PVAI without the VOM procedure. Control patients were individually matched (paired) with the VOM patient group by age (±5 years), sex, LA volume (± 20 cc) mm and classification of AF.
EP study and VOM cannulation
Patients underwent a baseline electrophysiological study including His bundle electrogram recording and coronary sinus electrogram recording. Coronary sinus cannulation was obtained by inserting a 7F sheath via the right internal jugular vein (Rapido CS-EH, Boston Scientific, St. Paul, Minnesota). A balloon occlusion coronary sinus venogram was then performed to assess the presence or absence of the VOM, which was identified as a branch of the coronary sinus directed posteriorly and superiorly. If present, subselective cannulation of the VOM was attempted with the use of a left internal mammary artery (LIMA) angiographic guide catheter or a subselective catheter for coronary sinus branch cannulation (Rapido IC-90, Boston Scientific), while injecting radiographic contrast to verify engagement in the VOM. Once engagement was obtained, an angioplasty wire (BMW, Abbott, Abbott Park, Illinois) was advanced into the VOM as far as possible to secure cannulation. A pre-loaded angioplasty balloon (8 mm length, 2 mm nominal diameter) was then advanced into the proximal VOM, inflated and a selective VOM venogram was obtained by injecting contrast into the balloon lumen. It was then left engaged in the VOM. Double trans-septal access to the LA was then obtained and both an ablation catheter (Thermocool, Biosense-Webster, Diamond Bar, CA) and a duodecapolar circular catheter (Lasso, Biosense-Webster or Reflexion Spiral, Daig )were advanced into the LA. An Acunav (Siemens) phased-array intracardiac echocardiographic catheter was used to guide the trans-septal puncture and the position of the catheters in the LA. A baseline three-dimensional voltage map was created using either the NavX (St Jude Medical) or Carto (Biosense-Webster) systems. We then verified the stability of the angioplasty balloon in the proximal VOM, inflated it, and delivered angiographic contrast through the balloon lumen to verify its selective engagement in the VOM, and assessed any leakage of angiographic contrast back in the coronary sinus. Under intracardiac echocardiographic guidance, or three-dimensional transesophageal echocardiography, 1 cc of echocardiographic contrast agent (Definity ®, Lantheus Medical Imaging, N. Billerica, MA) was injected in the lumen of the VOM to assess the communication of the VOM with the LA. We then performed two separate injections of 1 cc 100% ethanol administered over 2 minutes each, 2 minutes apart. Continuous arterial blood pressure and echocardiographic monitoring was performed during injections. Mixed venous ethanol level was measured after ethanol injections. Repeat VOM angiograms were performed to verify the integrity of the vein.
After ethanol delivery, a repeat three-dimensional voltage map of the LA was performed and PVAI was performed as part of the routine catheter ablation of AF using radiofrequency energy with the Thermocool catheter. A duodecapolar circular catheter was positioned in the ostium of each pulmonary vein, and radiofrequency ablation was performed proximal to the circular catheter in the antral region. Positioning of the duodecapolar circular catheter in the pulmonary vein ostia and antral regions was verified by intracardiac echocardiography. Radiofrequency ablation time required to achieve pulmonary vein isolation (elimination of pulmonary vein potentials recorded from the circular catheter) was quantified for each vein. For consistency in the quantification, radiofrequency ablation lesions applied in the carina of ispilateral pulmonary veins were counted as part the superior vein. In some patients, the Artisan robotic catheter manipulation system (Hansen, Mountain View, CA) was used to perform the ablation (See Table 1). As judged by the operator, additional ablation lesions were delivered in the posterior wall and roof of the LA, mitral annulus, and areas of complex and fractionated potentials.(12)
Table.
Patient characteristics and procedural data in patients subjected to VOM ethanol infusion (VOM) versus controls.
| Variable | VOM (n=10) | Control (n=10) | P Value |
|---|---|---|---|
|
Clinical characteristics
| |||
| Age (years) | 57.1 | 57.8 | 0.86 |
| Male sex (no., %) | 9 (90%) | 9 (90%) | 1 |
| Persistent AF/Paroxysmal AF | 5/5 | 5/5 | 1 |
| Duration of AF (months) | 36.3±20.4 | 36.9±19.8 | 0.94 |
| Antiarrhythmic drugs tried (no.) | 1±0.47 | 1.3±0.48 | 0.19 |
| Previous electrical cardioversion (no.) | 1.4±0.96 | 1.2±0.78 | 0.61 |
|
| |||
|
Structural heart disease
| |||
| Congestive heart failure (no., %) | 2 (20%) | 2 (20%) | 1 |
| LA volume (cc.) | 81.5±30.9 | 80.9±26.6 | 0.9 |
| Left ventricular ejection fraction (%) | 57.9±15 | 56.5±13.3 | 0.44 |
|
| |||
|
Procedural parameters
| |||
| Pulmonary vein antral isolation | 100% | 100% | 1 |
| Ablation of complex fractionated | 5 | 5 | 1 |
| potentials (no. of patients) | |||
| Mitral isthmus line (no of patients) | 8 | 8 | 1 |
| Total procedure time (min) | 259±57.5 | 212±26.6 | 0.02 |
| Total fluoroscopy time (min) | 50.7±13.4 | 48.9±10.3 | 0.75 |
| VOM procedure time (min) | 49±10.3 | ||
| VOM fluoroscopy time (min) | 8.4±3.8 | ||
| Hansen system used (no., %) | 6 (60%) | 5 (50%) | 0.52 |
| Total radiofrequency ablation time (min) | 95.4±39.3 | 116.37±21.8 | 0.13 |
Statistical analyses
Quantitative parameters between VOM patients and matched controls were compared using paired Student’s t test. Qualitative parameters were compared using X2 or Fisher’s exact test where appropriate. A p value of less than 0.05 was considered significant.
Results
Patient characteristics
Table 1 summarizes the patient characteristics. Of the 14 patients with symptomatic, drug refractory AF, that consented for the study, 4 were excluded: the VOM was absent in 2 patients and not amenable to cannulation in 2 patients. A small dissection in the coronary sinus wall was created in one of them, which prompted discontinuation of the procedure, but had no clinical or hemodynamic consequences. We completed the protocol of VOM cannulation and ethanol infusion in 10/14 patients (5 with paroxysmal AF and 5 with chronic persistent AF). Ten control patients were selected from our procedural data base for comparison of procedural parameters. Controls were paired with study patients with no significant differences in age, AF type, AF duration, and other relevant parameters (Table 1).
VOM characteristics
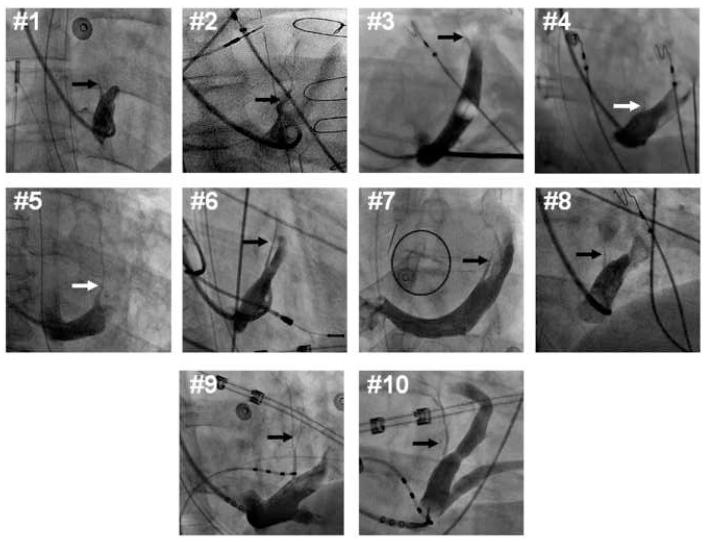
The VOM was identified as a posteriorly-directed vein branch of the coronary sinus in the right anterior oblique fluoroscopic projection and superiorly-directed in the left anterior oblique projection. Its length, and branching patterns were variable. Figure 1 shows the VOM course in 10 patients as visualized in subselective venograms obtained with either a LIMA or a Rapido catheter. Cannulation was relatively easy, achieved by rotating either of these two catheters posteriorly in the right anterior oblique projection, and probing the roof of the coronary sinus with an angioplasty wire. In one of the failed cannulation cases, a ventricular branch of the coronary sinus had its take-off at the same level as the VOM, and the shoulder of the Rapido catheter kept engaging into this ventricular branch, preventing an adequate reach to the roof of the coronary sinus with the catheter tip. In another failed case, the small size and abruptly posterior take-off of the VOM prevented advancement of the angioplasty wire.
Figure 1.
Vein of Marshall subselective venograms in each patient. Right anterior oblique fluoroscopic projections (except for patient #7, left anterior oblique projection) of contrast injection through a LIMA angiographic guide, or balloon occlusion catheter. The vein of Marshall (arrows) and its branches are readily identifiable as a branch of the coronary sinus directed superiorly and posteriorly.
Procedural impact
The VOM ethanol infusion procedure included right internal jugular access, coronary sinus cannulation and venogram, subselective VOM venogram, and cannulation with the angioplasty wire and balloon, VOM echocardiographic contrast injection, VOM ethanol infusion and repeat three-dimensional voltage mapping of the LA. Combined together, the added procedural time averaged 49 ± 10.3 min with an added fluoroscopy time of 8.4 ± 3.8 min.
VOM echocardiographic contrast injection
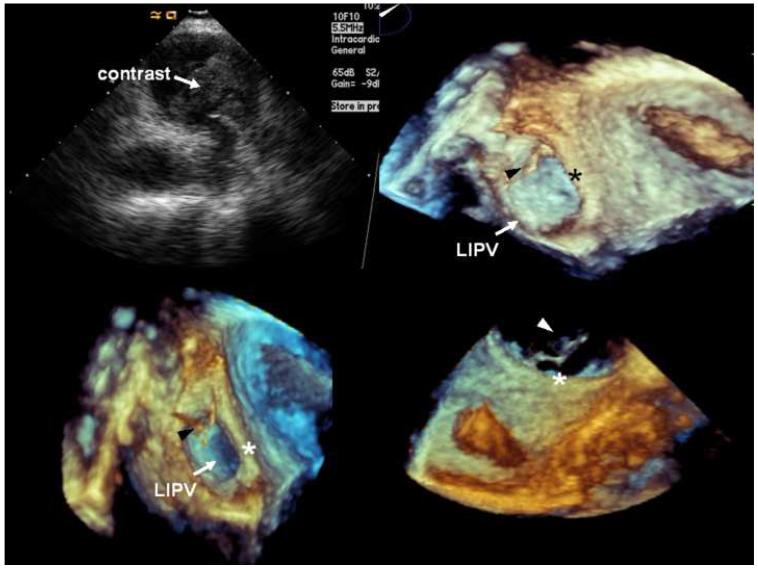
VOM venograms showed branching of the VOM into smaller atrial branches that are consistent with connection with atrial tissue and a true venous function. Selective injection of echocardiographic contrast in the VOM showed consistent contrast appearance in the LA cavity, suggesting retrograde contrast passage from the VOM through venules and capillaries into atrial tissue and the atrial cavity. Imaging with intracardiac echocardiography focusing in the left pulmonary veins showed earliest contrast appearance in the carina between left superior and left inferior pulmonary vein (Figure 2), demonstrating a connection of the VOM with myocardial tissue and thus communication from the vein into myocardial capillaries and cardiomyocytes and into the LA cavity via Thebesian channels. Three dimensional transesophageal echocardiography demonstrated the earliest appearance of echocardiographic contrast in the lateral ridge of the LA, between the mitral annulus and the left inferior pulmonary vein (Figure 2).
Figure 2.
Echocardiographic monitoring of contrast injection in the VOM. A, Intracardiac echocardiographic snapshot of the left pulmonary veins, showing contrast appearance in the carina. B, C, and D, Three-dimensional transesophageal echocardiography snapshots focusing in the lateral wall of the LA, left lateral ridge (*), and left inferior pulmonary vein (LIPV). The first appearance of echocardiographic contrast agent (arrowheads) was detected in the lateral ridge, anterior to the left inferior pulmonary vein.
Tissue ablation by ethanol injection
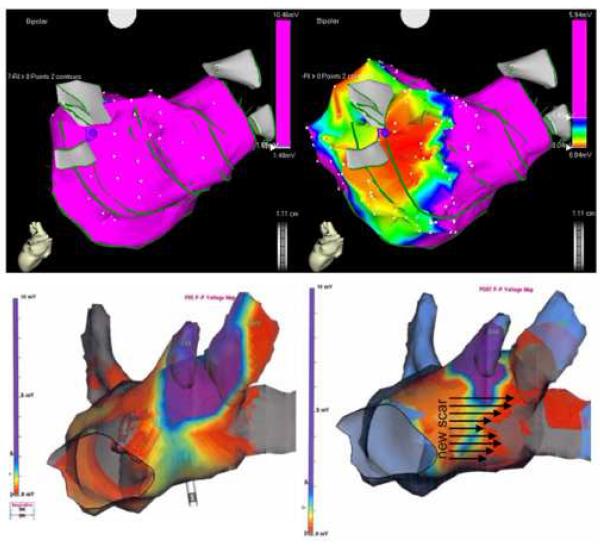
Ethanol infusion (2 injections of 1 cc over 2 minutes, 2 minutes apart) was well tolerated. Other than occasional atrial extrasystoles, there were no hemodynamic or clinical consequences of ethanol infusion. Repeat bipolar voltage maps of the LA after ethanol infusion revealed the creation of a new low-voltage area measuring 10.6±7.6 cm2, using a low voltage threshold of 0.05 mV. In all cases, the ablated areas had been previously healthy with bipolar voltage amplitudes exceeding 1 mV (borderline 0.5 to 1 mV regions were present in patients that had ongoing AF during mapping). The low-voltage area corresponded to the anatomical location of the VOM and its branches: lateral wall of the LA, between the coronary sinus and the left-sided pulmonary veins, and anterior to them. Figure 3 shows two examples of voltage maps pre- and post ethanol infusion, with the new low voltage areas created by ethanol ablation. Although ethanol extravasation and damage to neighboring tissues beyond the VOM is possible, repeat VOM angiograms after ethanol infusion did not show obliteration of the vein or contrast extravasation.
Figure 3.
Examples of three-dimensional bipolar voltage maps before (left) and after (right) VOM ethanol infusion. Minor differences in the LA geometry are due to increased density of the post infusion mapping. Maps were obtained with either the CARTO (top), or the NavX (bottom) systems. Top, posterior view of the LA, showing a large area of low bipolar voltage amplitude (red color, <0.04 mV) in the areas corresponding to the left pulmonary vein antrum. Bottom, left lateral view of bipolar voltage maps another patient (obtained during AF, hence the voltage amplitudes below 1 mV), showing an new area of low voltage (gray color, 0 mV), located anterior to the left pulmonary veins.
Pulmonary vein isolation
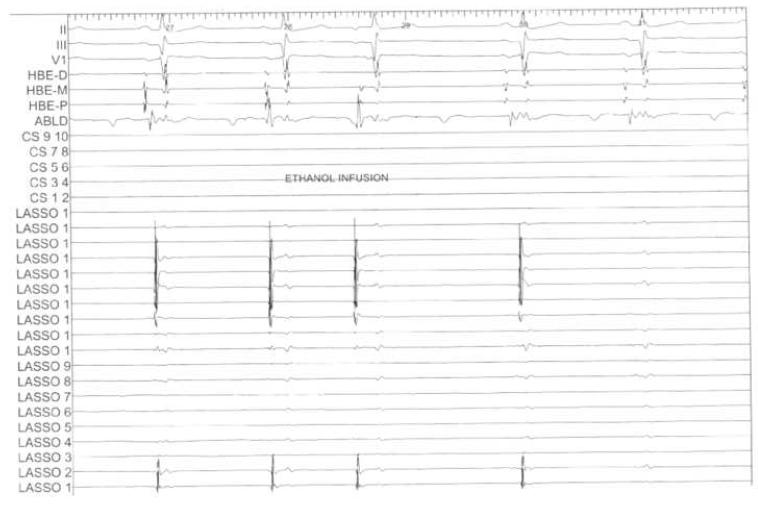
Following ethanol infusion, we proceeded with PVAI as guided by the electrograms recorded by the circular duodecapolar catheter. In 4 patients, no radiofrequency ablation was required to isolate the left inferior pulmonary vein, and in 2 patients, isolation required less than 1 minute of radiofrequency application (in the posterior aspect of the left inferior pulmonary vein antrum). Figure 4 shows pulmonary vein potentials recorded from the left inferior pulmonary vein during ethanol infusion. An atrial extrasystole was present during infusion, and then there was complete elimination of all pulmonary vein potentials. In all 10 patients, the radiofrequency ablation time required to achieve isolation of the left inferior pulmonary vein was 2.2±4 min. In 2 patients, the left superior pulmonary vein was isolated as well. However, the effect on left superior pulmonary vein radiofrequency ablation time was less consistent, totaling 15.7±15.6 min. Compared with control patients subjected to conventional ablation, the left inferior pulmonary vein ablation time was significantly reduced by VOM ethanol infusion (2.2±4 min vs 11.4±10.3 min, p<0.05).
Figure 4.
Intracardiac and left inferior pulmonary vein electrograms during VOM ethanol infusion. Top, selected surface ECG leads. HBE, His bundle electrograms. CS, coronary sinus electrograms. LASSO, circular duodecapolar signals recorded from the left inferior pulmonary vein ostium. During ethanol infusion an atrial extrasystole is seen (third beat). By the fifth beat, complete elimination of all pulmonary vein potentials is seen.
Complications and follow-up
There were no acute complications attributable to ethanol infusion. Ethanol levels in mixed venous blood were undetectable in all patients. One patient developed a hemopericardium during radiofrequency ablation at the roof of the left pulmonary vein antrum using the Hansen robotic system, and pericardiocentesis was sufficient to stabilize the patient. This hemopericardium occurred 47 minutes after ethanol infusion, which had been otherwise well tolerated. However, we cannot demonstrate the absence a mechanistic contribution of ethanol infusion. After 6.7±2.1 months of follow-up (range 5-11 months), there were no significant post-procedure complications. Three patients experienced self-limited pleuritic chest pain in both the VOM group and the control patient group. In two patients in the VOM group, pleuritic chest pain prompted hospitalization and diagnostic evaluations with transthoracic echocardiogram and computerized tomography of the chest that were normal in both cases. Transthoracic or transesophegeal echocardiograms performed at three months post procedure did not reveal any new abnormalities in any patient, including new left ventricular abnormalities. One patient presented with atypical atrial flutter and required mapping and ablation: a macroreentrant flutter involving the right pulmonary vein antrum was successfully ablated. Two patients had recurrent AF, both had preexisting chronic AF and underwent a repeat procedure with successful restoration of sinus rhythm. Successful maintenance of sinus rhythm (8/10 patients) did not differ between the two patient groups.
Discussion
We present a feasibility study of VOM ethanol infusion as an adjunctive procedure during catheter ablation of AF. Our results indicate that VOM ethanol infusion is feasible, safe, and achieves rapid ablation of LA tissue. Furthermore, our data supports the utility of the procedure in isolating the left pulmonary veins, particularly the left inferior.
Safety of ethanol infusion
Ethanol infusion for the treatment of hypertrophic cardiomyopathy has been associated with complications derived from: 1) unintended collateral damage to tissue perfused by the infused vessel (septal branch of the left anterior descending coronary artery), such as heart block; 2) the consequences of scar generation, such as ventricular tachycardia;(13) or 3) spillage of ethanol in unintended arterial branches. The analogies between septal ethanol ablation and ethanol infusion in the VOM are limited: VOM infusion is physiologically retrograde, and spilled ethanol drains via the coronary sinus into the right atrium to be diluted to non-damaging concentrations. Similarly, ethanol infused selectively in the VOM passes through myocardial tissue into the LA cavity, where it is diluted. Although VOM venograms performed before and after VOM ethanol infusion did not show contrast extravasation (supporting the integrity of the VOM vascular wall during the procedure), we cannot rule out microscopic ethanol dissection into the subepicardial region contributing to the tissue ablation. Additionally, no systemic effects were detected at the doses tested (total 2 cc).
Vein of Marshall as a true atrial vein
The vascular connections between the VOM and atrial tissue are unclear. It is commonly understood that the left cardinal venous system becomes atretic during embryological development, and its connection to the coronary sinus becomes the ligament of Marshall. Even if the ligament is patent as the VOM, whether the VOM provides any venous drainage function to the LA is unclear. Our study clearly shows an anatomical communication between the VOM, and underlying atrial tissue. Although the VOM was not uniformly present (12/14), we show that when present, it serves a true venous function that communicated with the underlying myocardium and is therefore a viable route for the delivery of therapeutic agents to the LA. Echocardiographic demonstration of contrast passage into the LA cavity supports such transmyocardial transport, and highlights the relevance of a slow ethanol infusion to allow for rapid dilution as it reaches the systemic circulation.
Mechanistic rationale
The ligament of Marshall is known to contain sympathetic(8) and parasympathetic(7) innervations that have been implicated in the genesis of atrial ectopic rhythms(14,15) and AF in animal models(16) and in humans.(4,6,9,17) Therefore, it is a logical target for ablation in the treatment of AF. Besides the local LA tissue ablation with VOM ethanol infusion, we have also previously shown in dogs that ethanol infusion via the VOM can selectively abolish vagal innervation of the LA, by blunting vagally-mediated decreases in effective refractory periods in the LA.(11) Besides its mechanistic roles in AF, the VOM lies in a region of the LA that is normally targeted during catheter ablation of AF. It courses in the epicardial aspect of the lateral ridge(10), which is a thick pectinate muscle that can be difficult to ablate. The VOM may provide connections between the pulmonary veins and the LA,(18) not amenable to ablation by standard endocardial circumferential pulmonary ve in antral ablation. Indeed, it has been noted that pulmonary vein potentials may persist in the carina even after circumferential antral pulmonary vein isolation.(19) It is reasonable to speculate that the ligament/vein of Marshall may provide such connections, which would be selectively targeted by VOM ethanol infusion.
Clinical implications
Procedurally, minimizing instrumentation time in the LA –the source of potential embolic complications in the systemic circulation- is attractive. Ethanol infusion in the VOM achieves tissue ablation in the LA from a purely right-sided approach that does not require anticoagulation by itself, and may reduce the LA ablation time. In this study we show that radiofrequency ablation time to achieve left inferior pulmonary vein isolation was dramatically reduced by VOM ethanol infusion. Overall, total radiofrequency ablation times in the VOM group were not significantly different to those of the control group, likely due to the large variations in total ablation times in the VOM group, caused by the patient heterogeneity (chronic and paroxysmal AF, large variations in LA size) and by variations in the extent of tissue ablated by ethanol. Total procedure times were longer in patients subjected to VOM ethanol infusion. We believe the main reason for procedure prolongation stems from the research protocol that mandated two voltage maps of the LA – pre- and post ethanol infusion- to delineate the extent of tissue ablated. In a practical clinical setting this redundancy would not be necessary. Thus, if VOM ethanol infusion were to be performed prior to LA access, followed by ablation of the remaining pulmonary vein antra, we expect that overall procedure times could be reduced, given the decrease in the need for radiofrequency ablation required to reach procedural endpoints.
Radiofrequency ablation in the posterior wall of LA can lead to collateral esophageal damage during ablation,(20-22), due to the close proximity (within millimeters(23)) of the esophagus. Although such anatomical relationship is highly variable, in autopsy specimens the majority of the contact with the esophagus was with the mid posterior LA (40%) or the left PVs (40%).(23) In a computer tomography study, the border of the esophagus in contact with the posterior LA was closer to the left (10±6 mm) than to the right PV (25±8 mm),(24) supporting the idea that the esophagus is most commonly close to the left PVs than to the right PVs, as confirmed by others.(25,26) In that regard, VOM ethanol infusion – an intervention that reduces ablation in the left pulmonary vein and posterior LA- may conceivably reduce the incidence of esophageal damage.
There are however, important potential limitations of this technique. First, the VOM maynot be amenable to cannulation. Second, as used in this study, the technique allows no control on the lesion size, which was determined by the size of the VOM and presumably influenced by the ethanol dose.
Conclusions
Targeting the VOM and its neighboring tissues is supported by mechanistic data and by the anatomical coincidence of the VOM with routinely ablated tissue. VOM ethanol infusion is feasible and safe in humans, and decreases radiofrequency ablation time required to isolate the left inferior pulmonary vein. Further studies to delineate the potential role of VOM ethanol infusion during catheter ablation of AF are warranted.
Acknowledgments
Financial Support: The Methodist Hospital Research Institute
Abbreviations used
- AF
atrial fibrillation
- LA
left atrium, left atrial
- PV
pulmonary vein
- PVAI
pulmonary vein antral isolation
- VOM
vein of Marshall
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hwang C, Karagueuzian HS, Chen PS. Idiopathic paroxysmal atrial fibrillation induced by a focal discharge mechanism in the left superior pulmonary vein: possible roles of the ligament of Marshall. J Cardiovasc Electrophysiol. 1999;10:636–48. doi: 10.1111/j.1540-8167.1999.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 2.Katritsis D, Ioannidis JP, Anagnostopoulos CE, et al. Identification and catheter ablation of extracardiac and intracardiac components of ligament of Marshall tissue for treatment of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2001;12:750–8. doi: 10.1046/j.1540-8167.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 3.Polymeropoulos KP, Rodriguez LM, Timmermans C, Wellens HJ. Images in cardiovascular medicine. Radiofrequency ablation of a focal atrial tachycardia originating from the Marshall ligament as a trigger for atrial fibrillation. Circulation. 2002;105:2112–3. doi: 10.1161/01.cir.0000016168.49833.ce. [DOI] [PubMed] [Google Scholar]
- 4.Katritsis D, Giazitzoglou E, Korovesis S, Paxinos G, Anagnostopoulos CE, Camm AJ. Epicardial foci of atrial arrhythmias apparently originating in the left pulmonary veins. J Cardiovasc Electrophysiol. 2002;13:319–23. doi: 10.1046/j.1540-8167.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin WS, Tai CT, Hsieh MH, et al. Catheter ablation of paroxysmal atrial fibrillation initiated by non-pulmonary vein ectopy. Circulation. 2003;107:3176–83. doi: 10.1161/01.CIR.0000074206.52056.2D. [DOI] [PubMed] [Google Scholar]
- 6.Kurotobi T, Ito H, Inoue K, et al. Marshall vein as arrhythmogenic source in patients with atrial fibrillation: correlation between its anatomy and electrophysiological findings. J Cardiovasc Electrophysiol. 2006;17:1062–7. doi: 10.1111/j.1540-8167.2006.00542.x. [DOI] [PubMed] [Google Scholar]
- 7.Ulphani JS, Arora R, Cain JH, et al. The ligament of Marshall as a parasympathetic conduit. Am J Physiol Heart Circ Physiol. 2007;293:H1629–35. doi: 10.1152/ajpheart.00139.2007. [DOI] [PubMed] [Google Scholar]
- 8.Kim DT, Lai AC, Hwang C, et al. The ligament of Marshall: a structural analysis in human hearts with implications for atrial arrhythmias. J Am Coll Cardiol. 2000;36:1324–7. doi: 10.1016/s0735-1097(00)00819-6. [DOI] [PubMed] [Google Scholar]
- 9.Kamanu S, Tan AY, Peter CT, Hwang C, Chen PS. Vein of Marshall activity during sustained atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:839–46. doi: 10.1111/j.1540-8167.2006.00516.x. [DOI] [PubMed] [Google Scholar]
- 10.Cabrera JA, Ho SY, Climent V, Sanchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J. 2008;29:356–62. doi: 10.1093/eurheartj/ehm606. [DOI] [PubMed] [Google Scholar]
- 11.Valderrábano M, Chen HR, Sidhu J, Rao L, Ling Y, Khoury DS. Retrograde Ethanol Infusion in the Vein of Marshall: Regional Left Atrial Ablation, Vagal Denervation, and Feasibility in Humans. Circ Arrhythmia Electrophysiol. 2009;2:50–56. doi: 10.1161/CIRCEP.108.818427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–53. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Sorajja P, Valeti U, Nishimura RA, et al. Outcome of alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Circulation. 2008;118:131–9. doi: 10.1161/CIRCULATIONAHA.107.738740. [DOI] [PubMed] [Google Scholar]
- 14.Scherlag BJ, Yeh BK, Robinson MJ. Inferior interatrial pathway in the dog. Circ Res. 1972;31:18–35. doi: 10.1161/01.res.31.1.18. [DOI] [PubMed] [Google Scholar]
- 15.Doshi RN, Wu TJ, Yashima M, et al. Relation between ligament of Marshall and adrenergic atrial tachyarrhythmia. Circulation. 1999;100:876–83. doi: 10.1161/01.cir.100.8.876. [DOI] [PubMed] [Google Scholar]
- 16.Wu TJ, Ong JJ, Chang CM, et al. Pulmonary veins and ligament of marshall as sources of rapid activations in a canine model of sustained atrial fibrillation. Circulation. 2001;103:1157–63. doi: 10.1161/01.cir.103.8.1157. [DOI] [PubMed] [Google Scholar]
- 17.Makino M, Inoue S, Matsuyama TA, et al. Diverse myocardial extension and autonomic innervation on ligament of Marshall in humans. J Cardiovasc Electrophysiol. 2006;17:594–9. doi: 10.1111/j.1540-8167.2006.00375.x. [DOI] [PubMed] [Google Scholar]
- 18.Tan AY, Chou CC, Zhou S, et al. Electrical connections between left superior pulmonary vein, left atrium, and ligament of Marshall: implications for mechanisms of atrial fibrillation. Am J Physiol Heart Circ Physiol. 2006;290:H312–22. doi: 10.1152/ajpheart.00369.2005. [DOI] [PubMed] [Google Scholar]
- 19.Udyavar AR, Chang SL, Tai CT, et al. The important role of pulmonary vein carina ablation as an adjunct to circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol. 2008;19:593–8. doi: 10.1111/j.1540-8167.2008.01182.x. [DOI] [PubMed] [Google Scholar]
- 20.Pappone C, Oral H, Santinelli V, et al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004;109:2724–6. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 21.Ghia KK, Chugh A, Good E, et al. A nationwide survey on the prevalence of atrioesophageal fistula after left atrial radiofrequency catheter ablation. J Interv Card Electrophysiol. 2008 doi: 10.1007/s10840-008-9307-1. [DOI] [PubMed] [Google Scholar]
- 22.Cummings JE, Schweikert RA, Saliba WI, et al. Brief communication: atrial-esophageal fistulas after radiofrequency ablation. Ann Intern Med. 2006;144:572–4. doi: 10.7326/0003-4819-144-8-200604180-00007. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Quintana D, Cabrera JA, Climent V, Farre J, Mendonca MC, Ho SY. Anatomic relations between the esophagus and left atrium and relevance for ablation of atrial fibrillation. Circulation. 2005;112:1400–5. doi: 10.1161/CIRCULATIONAHA.105.551291. [DOI] [PubMed] [Google Scholar]
- 24.Lemola K, Sneider M, Desjardins B, et al. Computed tomographic analysis of the anatomy of the left atrium and the esophagus: implications for left atrial catheter ablation. Circulation. 2004;110:3655–60. doi: 10.1161/01.CIR.0000149714.31471.FD. [DOI] [PubMed] [Google Scholar]
- 25.Tsao HM, Wu MH, Higa S, et al. Anatomic relationship of the esophagus and left atrium: implication for catheter ablation of atrial fibrillation. Chest. 2005;128:2581–7. doi: 10.1378/chest.128.4.2581. [DOI] [PubMed] [Google Scholar]
- 26.Cury RC, Abbara S, Schmidt S, et al. Relationship of the esophagus and aorta to the left atrium and pulmonary veins: implications for catheter ablation of atrial fibrillation. Heart Rhythm. 2005;2:1317–23. doi: 10.1016/j.hrthm.2005.09.012. [DOI] [PubMed] [Google Scholar]