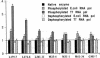Abstract
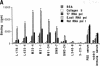
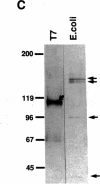
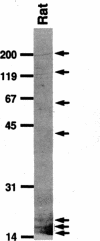
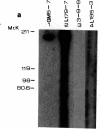
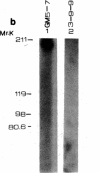
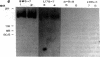
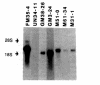
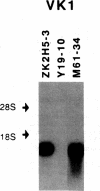
Autoantibodies against nuclear proteins like RNA polymerase I (RNA pol I) are produced in a number of rheumatic autoimmune diseases. Production of antibodies specific for the 190-kD subunit of RNA pol I appears to be characteristic in the patients with systemic sclerosis. Previous investigations have shown that the tight skin (TSK) mouse is an experimental model for systemic sclerosis. In the present study we show that the TSK mice produce high titers of anti-RNA pol I antibodies, both of IgM and IgG classes. To characterize the immunochemical properties of these antibodies we obtained a large panel of hybridomas from these mice. Analysis of these hybridomas revealed that clonal frequency of autoreactive B cells specific for RNA pol I are higher in the TSK mice that in the controls. mAbs obtained from the TSK mice were specific for the 190-kD subunit and cross-reacted with Escherichia coli and phage T7 RNA polymerases (155-, 150-, and 107-kD polypeptides). We have also demonstrated that these antibodies bind better to the phosphorylated enzymes. The anti-RNA pol I mAbs were divided into three groups in terms of their functional property. The first group of antibodies increased the catalytic activity of the enzyme whereas the antibodies of the second group inhibited the enzymatic activity. Competitive inhibition RIAs showed that these two groups of antibodies bound to distinct epitopes. The third group of antibodies was neutral and had no activity on the enzyme function. These results suggest that TSK mouse anti-RNA pol I antibodies recognize three or more conserved epitopes. To understand the molecular basis of the generation of such autoreactive antibodies we analyzed their V gene repertoire. Northern analysis of RNAs of 14 TSK hybridomas showed that the VH genes encoding these antibodies were mainly from VH J558 family. It is possible that these genes were derived from a single germline gene or from a set of related genes of a single subgroup.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baekkeskov S., Aanstoot H. J., Christgau S., Reetz A., Solimena M., Cascalho M., Folli F., Richter-Olesen H., De Camilli P., Camilli P. D. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature. 1990 Sep 13;347(6289):151–156. doi: 10.1038/347151a0. [DOI] [PubMed] [Google Scholar]
- Blankenstein T., Krawinkel U. Immunoglobulin VH region genes of the mouse are organized in overlapping clusters. Eur J Immunol. 1987 Sep;17(9):1351–1357. doi: 10.1002/eji.1830170920. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. RNA polymerase. Annu Rev Biochem. 1971;40:711–740. doi: 10.1146/annurev.bi.40.070171.003431. [DOI] [PubMed] [Google Scholar]
- Chamberlin M., McGrath J., Waskell L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature. 1970 Oct 17;228(5268):227–231. doi: 10.1038/228227a0. [DOI] [PubMed] [Google Scholar]
- D'Hoostelaere L. A., Klinman D. Characterization of new mouse V kappa groups. J Immunol. 1990 Oct 15;145(8):2706–2712. [PubMed] [Google Scholar]
- Duceman B. W., Rose K. M., Jacob S. T. Activation of purified hepatoma RNA polymerase I by homologous protein kinase NII. J Biol Chem. 1981 Nov 10;256(21):10755–10758. [PubMed] [Google Scholar]
- Green M. C., Sweet H. O., Bunker L. E. Tight-skin, a new mutation of the mouse causing excessive growth of connective tissue and skeleton. Am J Pathol. 1976 Mar;82(3):493–512. [PMC free article] [PubMed] [Google Scholar]
- Heinemann S., Bevan S., Kullberg R., Lindstrom J., Rice J. Modulation of acetylcholine receptor by antibody against the receptor. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3090–3094. doi: 10.1073/pnas.74.7.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Sentenac A., Fromageot P. Spot-immunodetection of conserved determinants in eukaryotic RNA polymerases. Study with antibodies to yeast RNA polymerases subunits. J Biol Chem. 1982 Mar 10;257(5):2613–2618. [PubMed] [Google Scholar]
- Jimenez S. A., Williams C. J., Myers J. C., Bashey R. I. Increased collagen biosynthesis and increased expression of type I and type III procollagen genes in tight skin (TSK) mouse fibroblasts. J Biol Chem. 1986 Jan 15;261(2):657–662. [PubMed] [Google Scholar]
- Karlsson F. A., Burman P., Löf L., Mårdh S. Major parietal cell antigen in autoimmune gastritis with pernicious anemia is the acid-producing H+,K+-adenosine triphosphatase of the stomach. J Clin Invest. 1988 Feb;81(2):475–479. doi: 10.1172/JCI113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik A., Schulze D. H., Bonilla F. A., Bona C., Kelsoe G. Stochastic pairing of heavy-chain and kappa light-chain variable gene families occurs in polyclonally activated B cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4932–4936. doi: 10.1073/pnas.87.13.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- Manheimer A. J., Bona C. A. Anti-immunoglobulin antibodies. VI. Age-dependent isotype and auto anti-immunoglobulin variation during secondary immune response in 129 mice. Mech Ageing Dev. 1985 May 13;30(2):187–199. doi: 10.1016/0047-6374(85)90007-7. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983 Jul 14;304(5922):177–179. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- Mosrin C., Thuriaux P. The genetics of RNA polymerases in yeasts. Curr Genet. 1990 May;17(5):367–373. doi: 10.1007/BF00334516. [DOI] [PubMed] [Google Scholar]
- Muryoi T., André-Schwartz J., Saitoh Y., Daian C., Hall B., Dimitriu-Bona A., Schwartz R. S., Bona C. A., Kasturi K. N. Self-reactive repertoire of tight skin (TSK/+) mouse: immunochemical and molecular characterization of anti-cellular autoantibodies. Cell Immunol. 1992 Oct 1;144(1):43–54. doi: 10.1016/0008-8749(92)90224-d. [DOI] [PubMed] [Google Scholar]
- Muryoi T., Kasturi K. N., Kafina M. J., Cram D. S., Harrison L. C., Sasaki T., Bona C. A. Antitopoisomerase I monoclonal autoantibodies from scleroderma patients and tight skin mouse interact with similar epitopes. J Exp Med. 1992 Apr 1;175(4):1103–1109. doi: 10.1084/jem.175.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muryoi T., Kasturi K. N., Kafina M. J., Saitoh Y., Usuba O., Perlish J. S., Fleischmajer R., Bona C. A. Self reactive repertoire of tight skin mouse: immunochemical and molecular characterization of anti-topoisomerase I autoantibodies. Autoimmunity. 1991;9(2):109–117. doi: 10.3109/08916939109006746. [DOI] [PubMed] [Google Scholar]
- Painter C. J., Monestier M., Chew A., Bona-Dimitriu A., Kasturi K., Bailey C., Scott V. E., Sidman C. L., Bona C. A. Specificities and V genes encoding monoclonal autoantibodies from viable motheaten mice. J Exp Med. 1988 Mar 1;167(3):1137–1153. doi: 10.1084/jem.167.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portmann L., Hamada N., Heinrich G., DeGroot L. J. Anti-thyroid peroxidase antibody in patients with autoimmune thyroid disease: possible identity with anti-microsomal antibody. J Clin Endocrinol Metab. 1985 Nov;61(5):1001–1003. doi: 10.1210/jcem-61-5-1001. [DOI] [PubMed] [Google Scholar]
- Reimer G., Rose K. M., Scheer U., Tan E. M. Autoantibody to RNA polymerase I in scleroderma sera. J Clin Invest. 1987 Jan;79(1):65–72. doi: 10.1172/JCI112809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. M., Stetler D. A., Jacob S. T. Protein kinase activity of RNA polymerase I purified from a rat hepatoma: probable function of Mr 42,000 and 24,600 polypeptides. Proc Natl Acad Sci U S A. 1981 May;78(5):2833–2837. doi: 10.1073/pnas.78.5.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Hügle B., Hazan R., Rose K. M. Drug-induced dispersal of transcribed rRNA genes and transcriptional products: immunolocalization and silver staining of different nucleolar components in rat cells treated with 5,6-dichloro-beta-D-ribofuranosylbenzimidazole. J Cell Biol. 1984 Aug;99(2):672–679. doi: 10.1083/jcb.99.2.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer U., Rose K. M. Localization of RNA polymerase I in interphase cells and mitotic chromosomes by light and electron microscopic immunocytochemistry. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1431–1435. doi: 10.1073/pnas.81.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac A. Eukaryotic RNA polymerases. CRC Crit Rev Biochem. 1985;18(1):31–90. doi: 10.3109/10409238509082539. [DOI] [PubMed] [Google Scholar]
- Stetler D. A., Jacob S. T. Immunization of rabbits with purified RNA polymerase I induces a distinct population of antibodies against nucleic acids as well as anti-RNA polymerase I antibodies, both characteristic of systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6797–6801. doi: 10.1073/pnas.82.20.6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler D. A., Jacob S. T. Phosphorylation of RNA polymerase I augments its interaction with autoantibodies of systemic lupus erythematosus patients. J Biol Chem. 1984 Nov 25;259(22):13629–13632. [PubMed] [Google Scholar]
- Stetler D. A., Sipes D. E., Jacob S. T. Anti-RNA polymerase I antibodies in sera of MRL lpr/lpr and MRL +/+ autoimmune mice. Correlation of antibody production with delayed onset of lupus-like disease in MRL +/+ mice. J Exp Med. 1985 Dec 1;162(6):1760–1770. doi: 10.1084/jem.162.6.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J., Sollner-Webb B. Transcription of mouse rDNA is regulated by an activated subform of RNA polymerase I. Cell. 1987 Sep 11;50(6):873–883. doi: 10.1016/0092-8674(87)90514-9. [DOI] [PubMed] [Google Scholar]
- Van de Water J., Gershwin M. E., Leung P., Ansari A., Coppel R. L. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988 Jun 1;167(6):1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]