Abstract
Rationale: Despite the importance of bronchiolitis obliterans syndrome (BOS) in lung transplantation, little is known regarding the factors that influence survival after the onset of this condition, particularly among bilateral transplant recipients.
Objectives: To identify factors that influence survival after the onset of BOS among bilateral lung transplant recipients.
Methods: The effect of demographic or clinical factors, occurring before BOS, upon survival after the onset of BOS was studied in 95 bilateral lung transplant recipient using Cox proportional hazards models.
Measurements and Main Results: Although many factors, including prior acute rejection or rejection treatments, were not associated with survival after BOS, BOS onset within 2 years of transplantation (early-onset BOS), or BOS onset grade of 2 or 3 (high-grade onset) were predictive of significantly worse survival (early onset P = 0.04; hazard ratio, 1.84; 95% confidence interval, 1.03–3.29; high-grade onset P = 0.003; hazard ratio, 2.40; 95% confidence interval, 1.34–4.32). The effects of both early onset and high-grade onset on survival persisted in multivariable analysis and after adjustment for concurrent treatments. Results suggested an interaction might exist between early onset and high-grade onset. In particular, high-grade onset of BOS, regardless of its timing after transplant, is associated with a very poor prognosis.
Conclusions: The course of BOS after bilateral lung transplantation is variable. Distinct patterns of survival after BOS are evident and related to timing or severity of onset. Further characterization of these subgroups should provide a more rational basis from which to design, stratify, and assess response in future BOS treatment trials.
Keywords: lung transplantation, prognosis, bronchiolitis obliterans syndrome
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Despite the adverse impact of bronchiolitis obliterans syndrome (BOS) in lung transplantation, little is known regarding factors that influence survival after the onset of this condition, particularly among bilateral transplant recipients.
What This Study Adds to the Field
BOS within 2 years of transplant or with onset grades of 2 or 3 predict worse survival after this condition develops, providing prognostic information in the care of these patients.
Shortly after the first human lung transplants were performed, a condition of progressive airflow obstruction was recognized and found to correlate histologically with the development of small airway fibrosis known as obliterative bronchiolitis (OB) (1). Because of poor sensitivity of chest radiography or transbronchial biopsies for OB, bronchiolitis obliterans syndrome (BOS) was developed as a means to identify patients with presumed OB based on a sustained decline from a patient's best post-transplant lung function (2). Within 5 years of transplant, almost half of all lung recipients meet criteria for BOS (3, 4). Once BOS ensues, quality of life is diminished and the risk for death is substantially increased (5). In fact, BOS represents the leading cause of death among patients who survive longer than 1 year after lung transplantation (6).
Although BOS uniformly has a significant impact on survival, studies have suggested heterogeneity exists in its clinical course. In particular, the time to onset, rate of lung function decline, type of transplant operation, native disease, or prior acute rejection have been postulated to influence the clinical course of BOS (4, 7–12). Unfortunately, previous studies in this area have generally included small numbers of patients with BOS composed predominately of single-lung or heart–lung recipients transplanted in a much earlier era. The relevance of these studies to lung recipients transplanted in the recent era is uncertain, particularly because bilateral transplant now accounts for a majority of all lung transplant operations.
The purpose of this study was therefore (1) to validate the adverse impact of BOS on survival in a cohort of 222 bilateral lung recipients transplanted in the recent era, (2) to define overall survival after the development of BOS, and (3) to identify demographic or clinical factors that influence survival after the onset of BOS. In particular, we sought to assess the importance of factors implicated in the development of BOS, such as acute rejection or cytomegalovirus (CMV) pneumonitis; factors related to the onset of BOS, such as its timing or severity; or concurrent treatments administered before or after the onset of BOS. Some of the results of this study have been previously reported in the form of an abstract (13).
METHODS
Patient Population
The BOS study cohort consisted of 95 bilateral lung transplant recipients with established BOS grade 1 or greater who received a first transplant at Duke University Medical Center between January 1, 2000 and December 31, 2004. During this time, a total of 284 transplants were performed and our program preferentially offered bilateral transplant to all recipients regardless of native disease. Excluded from analysis were retransplants (n = 8), pediatric patients (n = 6), single-lung recipients (n = 9), heart–lung recipients (n = 6), subjects who died within 90 days of transplant (n = 17), and subjects who died within 180 days either without any pulmonary function tests (n = 9) or with inadequate number of pulmonary function tests for BOS evaluation (n = 7). The remaining 222 BOS eligible bilateral transplant recipients were followed through October 2008 (mean follow up of 5.8 yr), at which time 43% (95/222) met criteria for BOS.
Clinical Management Protocols
Immunosuppression, assessment of rejection by biopsy, and assessment of allograft dysfunction by pulmonary function tests were consistently applied to all subjects as previously described elsewhere (14). Briefly, all recipients received a CD25 antagonist induction agent followed by triple immunosuppression with cyclosporine (before May 2002) or tacrolimus (after May 2002), azathioprine, and corticosteroids. Ganciclovir prophylaxis was given based on CMV antibody serology status of donor and recipient. Patients were prospectively screened before transplantation for gastric reflux using 24-hour pH probes and, if present, underwent Nissen fundoplication after transplant. Bronchoscopies were performed at defined intervals, 1, 3, 6, 9, and 12 months after transplant, annually thereafter, and as clinically indicated. All recipients underwent serial pulmonary function testing at least quarterly from transplant onward. Acute rejection (minimal or greater) was first treated with methylprednisone followed by a prednisone taper. Recurrent or refractory acute rejection was treated with antithymocyte globulin (ATG) or alemtuzumab. Patients with BOS were treated similar to those with recurrent or refractory rejection. In addition, azithromycin was used at the discretion of the treating physician.
Clinical Outcomes
Acute cellular rejection was defined according to the International Society for Heart and Lung Transplantation criteria as perivascular or peribronchial mononuclear inflammation (15). Acute rejection score was calculated by summing the acute rejection grades before BOS. Cytomegalovirus pneumonitis (CMV-P) was determined by immunohistochemistry staining of all biopsies obtained before BOS, regardless of clinical status. BOS was defined according to International Society for Heart and Lung Transplantation guidelines as a persistent drop in the current FEV1 relative to the average of the two highest FEV1 values measured at least 3 weeks apart, and after other causes of lung function deterioration were excluded (2, 16). High-grade onset BOS was defined by a BOS grade of 2 or 3 (current FEV1 ≤ 66% of baseline) at the initial time of BOS diagnosis. Survival analysis considered death or retransplant as the primary endpoint. Cause of death determination was made by study authors, blinded to BOS status, after review of clinical history before death and autopsy information, if available.
Statistical Analysis
Descriptive statistics were used for cohort demographics with standard deviation or interquartile range (IQR) given to describe the variance around the mean or median, respectively. Continuous variables were analyzed with independent two-tailed Student t tests or Wilcoxon rank sum tests according to distribution. Fisher exact tests or chi-square tests were used to analyze binary variables as appropriate. Estimation of the impact of BOS (considered as a time-dependent covariate) on survival among all 222 BOS-eligible subjects was performed with a Cox proportional hazards model. To assess the relationship between clinical predictors (occurring before the onset of BOS) and survival after BOS (time to death), Cox proportional hazards and Kaplan-Meier survival analysis models were used. Proportional hazard assumptions were assessed and satisfied. Effect sizes were estimated with Cox regression models and presented as hazard ratios (HRs) and corresponding 95% confidence interval (CI). Because survival time was first measured at the onset of BOS, demographic and clinical predictors occurring before BOS onset were time independent. Treatments occurring after the onset of BOS were considered as time-dependent predictors in the analysis (see online supplement) (17). Statistical significance was defined as a two-tailed P value less than or equal to 0.05. All analyses were conducted with SAS statistical software version 9.1.3 (Cary, NC).
RESULTS
Characteristics of Patients with BOS
The demographic and clinical characteristics of the 95 patients with BOS are shown in Table 1. The majority of subjects with BOS were white and male, with a median age at transplant of 53 years. Obstructive lung disease was the most common indication for transplant. A majority of patients (76%) experienced at least one acute rejection episode, with a median acute rejection score of 2 before the onset of BOS (range 0–15). Treatments before BOS included Nissen fundoplication (41%, 39/95), alemtuzumab (7%, 7/95), and ATG (35%, 33/95). Median time to the onset of BOS was 3.0 years (IQR, 1.6–4.1 yr), with 34% (32/95) of patients experiencing early-onset BOS (occurring within 2 yr of transplant). On entering BOS, 74% (70/95) were grade 1 and 26% (25/95) were grades 2 or 3 (high-grade BOS).
TABLE 1.
DEMOGRAPHIC AND CLINICAL CHARACTERISTICS OF 95 BILATERAL LUNG TRANSPLANT RECIPIENTS WITH BOS
| Female sex | 40% (40) |
|---|---|
| Mean age at transplant (range), yr | 49 (18–66) |
| Race | |
| White | 91% (86) |
| African American | 9% (9) |
| Native disease | |
| Obstructive | 47% (45) |
| Pulmonary hypertension | 2% (2) |
| Cystic | 21% (20) |
| Restrictive | 30% (28) |
| Transplant era* | 49% (47) |
| Acute rejection history† | |
| Any acute rejection | 73% (69) |
| Acute rejection score‡§ | 3 (2–5) |
| Acute rejection score range§ | 1–15 |
| CMV mismatch (D+/R−) | 23% (22) |
| CMV-pneumonia† | 26% (25) |
| Treatments before BOS | |
| Nissen fundoplication | 41% (39) |
| Antithymocyte globulin | 35% (33) |
| Alemtuzumab | 7% (7) |
| Time to BOS, yr‡ | 3.0 (1.6–4.1) |
| Early-onset BOS‖ | 34% (32) |
| High-grade onset** | 26% (25) |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; CMV = cytomegalovirus; D = donor; R = recipient.
Data are presented as % (n) unless otherwise noted.
Transplanted after March 2002.
Before the onset of BOS.
Median (IQR)
Among patients with acute rejection.
BOS onset within 2 years of lung transplant.
Initial BOS grade of 2 or 3.
Impact of BOS on Post-Transplant Survival
To demonstrate the deleterious effect of BOS (considered as a time-dependent covariate), we compared survival outcomes among the 222 BOS-eligible, bilateral lung transplant recipients. A shorter time to the onset of BOS in years was significantly associated with an increased hazard for death (P < 0.0001; unadjusted HR, 10.15 per yr; 95% CI, 6.15–16.75). The adverse impact of earlier time to BOS remained significant when the model was adjusted for age, native disease, race, sex, and transplant era (P < 0.0001; adjusted HR, 10.07 per yr; 95% CI, 6.06–16.73). At a mean follow-up of 5.8 years, 51% (48/95) of the patients with BOS had died, in contrast with 22% (28/127) of patients without BOS.
Natural History of BOS
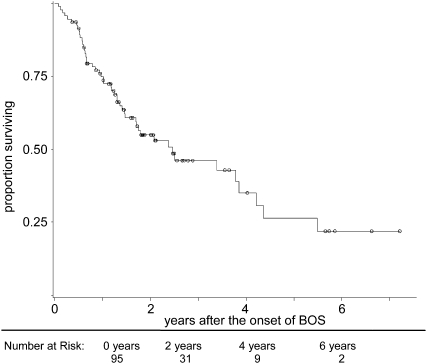
Given the heterogeneity of BOS, we wished to characterize its progression, as well as describe patient survival and cause of death after its onset. None of the patients meeting criteria for BOS improved to BOS grade 0. Sixty-one percent (43/70) of those who first entered BOS in grade 1 progressed to grades 2/3 in a median time of 208 days (IQR, 42–451). The remaining 39% (27/70) of patients stabilized in BOS grade 1. Among the 25 patients with high-grade BOS, 8% (2/25) experienced improvement in lung function and stabilized in BOS grade 1. The rest of the patients with high-grade BOS evidenced no improvement in lung function. Median survival after the onset of BOS was limited to 2.5 years (IQR, 0.8–5.5 yr; Figure 1). Kaplan-Meier post-BOS survival estimates at 1, 3, and 5 years were 74%, 46%, and 26%, respectively. Respiratory failure accounted for the majority of deaths among all patients with BOS (58%, 28/48), followed by infection (23%, 11/48), malignancy (8%, 4/48), and cardiac causes (4%, 2/48).
Figure 1.
Survival after the onset of bronchiolitis obliterans syndrome (BOS) among 95 bilateral lung transplant recipients. Kaplan-Meier survival estimates at 1, 3, and 5 years after the onset of BOS were 74%, 46%, and 26%, respectively. Median survival was limited to 2.5 years (IQR, 0.8–5.5 yr).
Effect of Pre-BOS Demographic and Clinical Factors on Survival after BOS
We next sought to determine whether patient factors occurring before the onset of BOS, including demographic characteristics, prior rejection, rejection treatments, or CMV-P, affect survival after BOS. Recipient sex, race, native disease, transplant era, incidence of or severity of prior acute rejection, CMV mismatch status, CMV-P, or rejection treatments before BOS were not associated with survival after its onset (Table 2). In contrast, as shown in Table 2 and described in detail in the following sections, an earlier time to BOS and initial severity of BOS (onset grade) were significantly associated with worse survival after BOS.
TABLE 2.
IMPACT OF CLINICAL AND DEMOGRAPHIC FACTORS, OCCURRING BEFORE BRONCHIOLITIS OBLITERANS SYNDROME, ON MORTALITY AFTER BRONCHIOLITIS OBLITERANS SYNDROME
| Covariates | HR | 95% CI | P Value |
|---|---|---|---|
| Female sex | 1.28 | 0.72–2.27 | 0.41 |
| Age per yr | 0.99 | 0.97–1.01 | 0.32 |
| Age ≥ 65 yr | 0.75 | 0.18–3.11 | 0.69 |
| White race | 0.98 | 0.41–2.31 | 0.96 |
| Native disease | |||
| Obstructive | 0.72 | 0.40–1.30 | 0.27 |
| Pulmonary hypertension | 2.09 | 0.50–8.76 | 0.31 |
| Cystic | 0.96 | 0.47–2.07 | 0.97 |
| Restrictive | 1.34 | 0.72–2.47 | 0.35 |
| Transplant era* | 1.15 | 0.62–2.11 | 0.71 |
| Acute rejection | |||
| Any acute rejection | 0.86 | 0.47–1.56 | 0.61 |
| Acute rejection score | 0.98 | 0.88–1.09 | 0.77 |
| CMV mismatch (D+/R−) | 1.63 | 0.87–3.06 | 0.13 |
| CMV pneumonitis | 1.30 | 0.66–2.53 | 0.44 |
| Treatments | |||
| Nissen fundoplication | 0.98 | 0.55–1.77 | 0.96 |
| Antithymocyte globulin | 1.09 | 0.56–1.85 | 0.96 |
| Alemtuzumab | 2.28 | 0.89–5.82 | 0.09 |
| Early-onset BOS† | 1.84 | 1.03–3.29 | 0.04 |
| High-grade onset‡ | 2.40 | 1.34–4.32 | 0.003 |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; CI = confidence interval; CMV = cytomegalovirus; D = donor; HR = hazard ratio; R = recipient.
Transplanted after March 2002.
BOS onset within 2 years of lung transplant.
Initial BOS grade of 2 or 3.
Effect of Timing of BOS on Survival after BOS
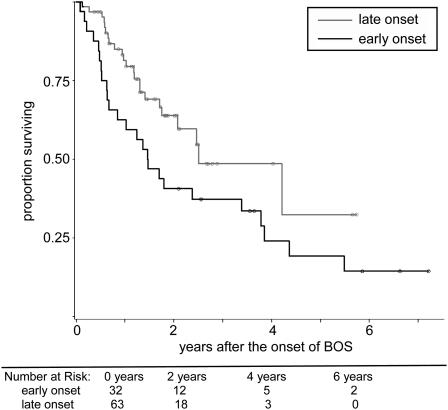
Early onset of BOS (within 2 yr of transplant), occurring in 34% (32/95) of patients, was associated with significantly increased mortality as compared with late onset (P = 0.04; HR, 1.84; 95% CI, 1.03–3.29), as shown in Figure 2. One-, 3-, and 5-year Kaplan-Meier survival estimates after BOS for the early-onset group were 59%, 37%, and 19% in contrast with 80%, 49%, and 32% in the late-onset group (P = 0.04). Median survival from the onset of BOS for patients with early versus late onset was 1.47 years and. 2.51 years, respectively. Similar results were also obtained when time to BOS onset was considered as continuous, rather than dichotomous, variable (see online supplement).
Figure 2.
The impact of time to bronchiolitis obliterans syndrome (BOS) onset on survival after the onset of BOS among 95 bilateral lung transplant recipients. Early-onset BOS (onset within 2 yr of lung transplant), occurring in 34% (32/95) of patients, was associated with significantly increased mortality as compared with late onset (P = 0.04; hazard ratio, 1.84; 95% CI, 1.03–3.29). Four-year Kaplan-Meier survival estimates after BOS for the early-onset group were 24% in contrast with 32% in the late-onset group; P = 0.04.
Effect of BOS Severity on Survival after BOS
High-grade onset BOS (grades 2 or 3), occurring in 26% (25/95) of patients, was associated with significantly increased mortality as compared with grade 1 onset (P = 0.0034; HR, 2.40; 95% CI, 1.34–4.32). One-, 3-, and 5-year Kaplan-Meier post-BOS survival estimates for the high-grade onset group were 48%, 22%, and 11% in contrast with 81%, 55%, and 32% for the grade 1 onset group, P = 0.003 (Figure 3). Median survival from the onset of BOS for the high-grade onset and grade 1 onset groups was 1.03 and 3.79 years, respectively.
Figure 3.
The impact of bronchiolitis obliterans syndrome (BOS) onset grade on survival after the onset of BOS among 95 bilateral lung transplant recipients. High-grade onset BOS (initial BOS grades 2 or 3), occurring in 26% (25/95) of patients, was associated with significantly increased mortality as compared with grade 1 onset (P = 0.0034; HR, 2.40; 95% CI, 1.34–4.32). Four-year Kaplan-Meier survival estimates after BOS for high-grade onset versus grade 1 onset were 11% and 45% respectively; P = 0.003.
Potential Confounding Effects of Variation in Pulmonary Function Test Measurements on BOS Phenotypes
To evaluate whether sampling bias, related to variation in the pulmonary function test (PFT) measurements over time, could have affected the apparent onset timing or onset grade of BOS, we examined the median number of PFTs per patient by post-transplant year. Among the early- versus late-onset groups and the high-grade versus grade 1 onset groups, there were no significant differences in the median number of PFTs obtained in year 1, years 2 and 3, or years 4 and 5 post transplant (see Table E1 in online supplement). These results demonstrate that all patients underwent similar serial PFT measurements over time after lung transplantation providing equal opportunity for every patient to develop early-onset or high onset grade BOS.
To further examine this point, we considered the median time interval between BOS onset and the preceding PFT measurement among patients with either of these BOS phenotypes. No significant difference was observed in the time interval between BOS onset and the preceding PFT among the patients with high-grade and grade 1 onset (high grade: 81 median days [IQR, 41–112] vs grade 1: 70 median days [IQR, 41–99]; P = 0.79). These results reinforce the idea that the assignment of high–onset grade BOS was not influenced by variation in the timing of PFT measurements.
In contrast, there was a statistically significant difference in the median time interval between BOS onset and the preceding PFT among the early- and late-onset groups (47 d [IQR, 33–98] vs 91 d [IQR, 56–112], respectively; P = 0.03), consistent with the increased overall frequency of PFT measurements that were performed in Year 1 as compared with subsequent years (Table E1). Because sampling over time was similar across all patients with BOS, however, each patient had an equal opportunity to develop early onset of BOS. Thus, those patients who developed late-onset BOS were sampled just as frequently at early time points as those who actually did develop early-onset BOS.
Finally, to further ensure this difference in PFT intervals among early- versus late-onset patients did not affect our survival results, we performed a sensitivity analysis, in which we reassigned every late-onset patient an onset time 44 days before the actual BOS onset time (the median interval difference between the groups). This analysis resulted in the reclassification of three patients into the early-onset group, but did not alter the significantly adverse impact of early-onset BOS on survival (HR, 1.93; 95% CI, 1.07–3.48; P = 0.03).
Relationship of Early Onset to High-Grade Onset
We then considered the effects of BOS onset timing, high-grade onset, and their interaction in a multivariable model. The effects of early onset and high-grade onset on survival persisted independent of each other (P = 0.05 and P = 0.007, respectively), whereas the interaction term was not significant (P = 0.21). To more fully explore the relationship between BOS onset timing and onset grade on survival after BOS we performed additional analyses examining survival by each subgroup.
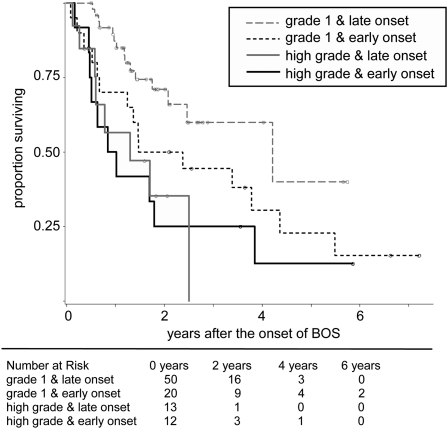
Among all patients, 13% (12/95) presented in early onset and high-grade onset, 21% (20/95) presented in early onset and grade 1 onset, 14% (13/95) presented in late onset and high-grade onset, and 52% (50/95) presented in late onset and grade 1 onset. Overall survival was significantly different among these four groups (Figure 4; P = 0.007). Interestingly, although survival appeared equally poor among patients with high-grade onset regardless of early versus late time to onset, survival among patients with grade 1 onset appeared to differ depending on early versus late onset, suggesting an interaction might exist between timing of onset and onset grade.
Figure 4.
Distinct patterns of survival after bronchiolitis obliterans syndrome (BOS) among 95 bilateral lung transplant recipients: survival by high-grade BOS onset, survival by grade 1 and early onset, and survival by grade 1 and late onset. Among the patients with BOS, 13% (12/95) presented in high-grade and early onset, 14% (13/95) presented in high-grade and late onset, 21% (20/95) presented in grade 1 and early onset, and 52% (50/95) presented in grade 1 and late onset. Overall survival was significantly different among these four groups (P = 0.007).
Potential Confounding Effects of Treatments on Survival after BOS
After the onset of BOS, 11% (10/95) of patients underwent Nissen fundoplication, 13% (12/95) received alemtuzumab, 37% (33/95) received ATG, and 58% (55/95) received azithromycin. To ensure that differences in survival were not related to effects of treatment, we first considered the impact of Nissen fundoplication, ATG, alemtuzumab, or azithromycin as time-dependent variables occurring after the onset of BOS on survival after BOS. None was associated with survival after BOS (Table 3). We additionally examined the distribution of treatments by onset grade or timing of BOS groups. The use of Nissen fundoplication, azithromycin, alemtuzumab, or ATG was similar among the groups and did not vary by early versus late onset or high-grade onset versus grade 1 onset groups (Table E2). Finally, we performed an analysis that considered either early onset or high-grade onset in conjunction with each of these potential confounding treatments (entered into the model as time-dependent predictors) and found the HRs associated with early onset or high-grade onset remained essentially unchanged (Table E3).
TABLE 3.
MORTALITY AFTER BRONCHIOLITIS OBLITERANS SYNDROME IS UNAFFECTED BY POST–BRONCHIOLITIS OBLITERANS SYNDROME TREATMENTS
| Treatment | % N | HR | 95% CI | P Value |
|---|---|---|---|---|
| Nissen fundoplication | 11 (10) | 1.52 | 0.63–3.67 | 0.35 |
| Alemtuzumab | 13 (12) | 1.37 | 0.58–3.24 | 0.47 |
| Antithymocyte globulin | 37 (35) | 1.25 | 0.69–2.29 | 0.46 |
| Azithromycin | 58 (55) | 1.44 | 0.78–2.65 | 0.24 |
Definition of abbreviations: CI = confidence interval; HR = hazard ratio.
DISCUSSION
We demonstrate the deleterious effect of BOS on survival in a large cohort of bilateral lung transplant recipients. Patients with BOS experienced an annual hazard for death that was 10 times that of their BOS-free counterparts, and once BOS ensued, 5-year survival was estimated at only 26%. Despite the profound adverse consequences of BOS, very little is known regarding the factors influencing survival after its onset, particularly among bilateral lung transplant recipients. We therefore sought to identify factors, occurring before or at the onset of BOS, which predict survival after BOS. Our analysis demonstrated that an early onset of BOS (occurring within 2 years of transplant) or an initial high grade at BOS onset (2 or 3) were associated with significantly worse survival. In contrast, many other clinical or demographic factors, including those implicated as BOS risk factors, such as acute rejection or CMV-P, were not predictive of survival after the development of BOS. These results might imply that the mechanisms that contribute to the progression of BOS differ from those that contribute to its onset.
Our results are consistent with several previous studies that suggested heterogeneity in the clinical course after BOS was related to the rapidity of lung function decline before the onset of BOS. Nathan and colleagues first described several patterns of BOS progression in a case series of 15 single-lung transplant recipients, including a rapidly progressive variant (12). More recently, Jackson and colleagues performed an analysis in which changes in lung function over time after transplant were assumed to follow one of two patterns: an acute onset, defined by a drop into BOS greater than or equal to 15% from FEV1 baseline, or a linear decline from baseline (8). Median survival after the onset of BOS was 29 months in the acute-onset group versus 58 months in the chronic-onset group. Our results validate the idea that the rapidity of progression into BOS influences survival. Our work further extends this concept to bilateral transplant recipients and is the first study to demonstrate that high initial BOS grade best identifies those at greatest risk for death after BOS onset. Our analysis thus provides a useful approach to assess prognosis at the time of BOS onset, because clinicians can rely on the established grading system to identify patients with onset grades of 2 or 3 who are at higher risk for death after BOS.
Other studies have examined the impact of the timing of BOS onset on the clinical course of BOS, although results in this area have been inconsistent. Brugière and colleagues reported that an early onset of BOS (occurring within 3 yr of transplant) was associated with significantly worse survival in 29 single-lung transplant recipients (11). In contrast, the poor prognosis of early-onset BOS (when dichotomized at 18 mo) was not confirmed in a study by Burton and colleagues (10). The analysis, however, only included patients who survived at least 3 years post transplant, potentially excluding early BOS-related deaths leading to an underestimation of the effect of early-onset BOS on survival. Our analysis dichotomized early onset of BOS at 2 years (based on the first quartile of the distribution of BOS onset in our sample) and found an increased risk for death with earlier onset of BOS, consistent with the finding of Brugière and colleagues. When time to BOS onset was analyzed as a continuous variable in years, it also was associated with an increased risk of death for each year of earlier BOS onset.
More recently, Lama and colleagues identified factors that influence the trajectory of lung function decline after the onset of BOS in a cohort of 111 predominately single-lung transplant recipients (7). Rapid-onset BOS (defined by Lama as a > 20% change from baseline within 6 mo of BOS onset) or early-onset BOS (defined as occurring within 2 years of transplant) were both associated with a more rapid loss of pulmonary function. Although this analysis used an elegant mixed model to estimate FEV1 trajectories, the study did not evaluate the relationship of BOS onset grade to lung function decline, nor did it consider the impact of any of these factors on survival after BOS. Nonetheless, our results complement this previous study and demonstrate that many of the factors that were associated with a steeper decline in lung function after BOS also predict worse survival after BOS. In contrast with our results, Lama and colleagues found that female sex and interstitial lung disease were associated with a worse lung function trajectory, factors not significant in our survival analysis. These differences might relate to the large proportion of single-lung recipients in the Lama study or, alternatively, are simply variables that correlate with lung function trajectory but not survival.
Another important feature of our analysis was the application of modern management protocols, including immunosuppression, azithromycin, and fundoplication, to this cohort of patients. Somewhat disappointingly, our results would suggest that median survival after the onset of BOS remains unchanged as compared with the previous era. Similar to our results, Valentine and colleagues reported median survival was just under 3 years after the onset of BOS in heart–lung patients transplanted from 1981 to 1995, and Heng and colleagues reported a median survival of just over 3 years in a cohort of primarily heart–lung and single-lung recipients transplanted from 1984 to 1995 (4, 9). Consistent with this idea, we did not observe a benefit to modern treatments, including azithromycin, lympholytic therapy, or fundoplication, on survival after BOS. We would caution, however, against using these results to conclude that treatments are not useful in the setting of BOS; instead, only appropriately designed and powered prospective randomized trials can determine the impact of any of these interventions on the natural history of BOS.
Finally, our results have several important implications regarding the design of future studies of patients with BOS. We demonstrate that the course of BOS is variable, with different patterns of survival related to the timing or initial severity of BOS onset. Our analysis considered potential differences in PFT sampling and demonstrated that assignment of these phenotypes and our survival results are not biased by variation in the frequency of PFT sampling. Collectively our results imply that several distinct phenotypes of BOS exist. In particular, high-grade onset of BOS, regardless of its timing after transplant, is associated with a very poor prognosis, whereas patients who enter BOS late with grade 1 at onset have the best prognosis. Further characterization and validation of these phenotypes may offer new insights into the mechanisms that lead to BOS. In addition, enhanced understanding of BOS subgroups should provide a more rational basis from which to design, stratify, and assess response in clinical treatment trials of bilateral lung transplant recipients with BOS.
Supplementary Material
Supported by National Institutes of Health grant KL2RR024127 and American Society of Transplantation Clinical Faculty Development Award (L.D.S.), National Heart, Lung, and Blood Institute SCCOR 1P50-HL084917–01 and K24–091140–01 (S.M.P.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201002-0211OC on May 27, 2010
Author Disclosure: C.A.F.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.D.S. received fees from Pfizer for a lecture on lung transplant (up to $1,000) and received a career development research grant from the NIH ($50,001–$100,000). D.W.Z. served as a consultant for MPEX pharmaceuticals and APT pharmaceuticals ($1,001–$5,000). He served on the Board or Advisory Board for Schering Plough, CSL Behring, and Ortho McNeil ($1,001–$5,000). His spouse/life partner received lecture fees from Astellas, grant support from Enzon ($10,001–$50,000), and receives royalties from Elsevier Sciences ($1,001–$5,000) and Up to Date (up to $1,000). He also receives royalties from Up to Date (up to $1,000). W.J.T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.A.D. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.M.P. served on the Data Safety Monitoring Board for Clinical Trial for Watermark and received lecture fees from Robert Michael Educational for CME presentations ($1,001–$5,000). He received grant support from Roche, he is the PI of Investigator Initiated Multicenter Trial through DCRI, and he received grant support from the NIH (more than $100,001).
References
- 1.Burke CM, Theodore J, Dawkins KD, Yousem SA, Blank N, Billingham ME, Van Kessel A, Jamieson SW, Oyer PE, Baldwin JC, et al. Post-transplant obliterative bronchiolitis and other late lung sequelae in human heart-lung transplantation. Chest 1984;86:824–829. [DOI] [PubMed] [Google Scholar]
- 2.Estenne M, Maurer JR, Boehler A, Egan JJ, Frost A, Hertz M, Mallory GB, Snell BI, Yousem SA. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant 2002;21:297–310. [DOI] [PubMed] [Google Scholar]
- 3.Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J 2003;22:1007–1018. [DOI] [PubMed] [Google Scholar]
- 4.Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, Theodore J. Actuarial survival of heart-lung and bilteral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant 1996;15:371–383. [PubMed] [Google Scholar]
- 5.Jan W, Van den Berg K, Geertsma A, Van der Bij W, Koeter GH, De Boer WJ, Postma DS, Ten Vergert EM. Bronchiolitis obliterans syndrome after lung transplantation and health-related quality of life. Am J Respir Crit Care Med 2000;161:1937–1941. [DOI] [PubMed] [Google Scholar]
- 6.Reichenspurner H, Girgis RE, Robbins RC, Yun M, Nitschke M, Berry GJ, Morris RE, Theodore M, Reitz BA. Stanford experience with obliterative bronchiolitis after lung and heart-lung transplantation. Ann Thorac Surg 1996;62:1467–1472. [DOI] [PubMed] [Google Scholar]
- 7.Lama VN, Murray S, Lonigro RJ, Toews GB, Chang A, Lau C, Flont A, Chan KM, Martinez FJ. Course of FEV1 after onset of bronchiolotis obliterans syndrome in lung transplant recipients. Am J Respir Crit Care Med 2007;175:1192–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson CH, Sharples LD, McNeil K, Stewart S, Wallwork J. Acute and chronic onset of bronchiolitis obliterans syndrome (BOS): are they different entities? J Heart Lung Transplant 2002;21:658–666. [DOI] [PubMed] [Google Scholar]
- 9.Heng D, Sharples LD, McNeil K, Steward S, Wreghitt T, Wallwork J. Bronchiolitis obliterans syndrome: incidence, natural history, prognosis, and risk factors. J Heart Lung Transplant 1998;17:1255–1263. [PubMed] [Google Scholar]
- 10.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant 2007;26:681–686. [DOI] [PubMed] [Google Scholar]
- 11.Brugière O, Pessione F, Thabut G, Mal H, Jebrak G, Lesèche G, Fournier M. Bronchiolitis obliterans syndrome after single-lung transplantation. Chest 2002;121:1883–1889. [DOI] [PubMed] [Google Scholar]
- 12.Nathan SD, Ross DJ, Belman MJ, Shain S, Elashoff JD, Kass RM, Koerner SK. Bronchiolitis obliterans in single lung transplant recipients. Chest 1995;104:467–472. [DOI] [PubMed] [Google Scholar]
- 13.Finlen Copeland CA, Turbyfill WJ, Willner D, Zaas D, Snyder LD, Palmer SM. 2009. Predictors of survival after onset of BOS in lung transplant recipients [abstract]. American Thoracic Society Annual Meeting, San Diego, California (May 15–20, 2009).
- 14.Snyder LD, Finlen Copeland CA, Hartwig MG, Lin SS, Davis RD, Palmer SM. Lung transplantation at Duke University Medical Center. Clin Transpl 2007;99–111. [PubMed]
- 15.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant 1996;15:1–15. [PubMed] [Google Scholar]
- 16.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. J Heart Lung Transplant 1993;12:713–716. [PubMed] [Google Scholar]
- 17.Allison P. Survival analysis using SAS: a practical guide. Cary, NC: SAS Institute Inc.; 1995.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.