Abstract
Rationale: Individuals with sleep-disordered breathing (SDB) are at increased cardiovascular risk, possibly due to SDB-related stresses contributing to atherosclerosis.
Objectives: We postulate that pathways associated with a prothrombotic potential are up-regulated in SDB.
Methods: Morning and evening plasminogen activator inhibitor-1 (PAI-1), morning fibrinogen, and morning D-dimer were measured in 537 Cleveland Family Study adults. Piecewise multivariable linear mixed models estimated relative mean change or mean change in the biomarker per 5-unit increase in apnea-hypopnea index (AHI) in two groups: AHI less than 15 and AHI greater than or equal to 15, and hypoxia defined as percentage of sleep time with SaO2 less than 90% (< 2%, ≥ 2%).
Measurements and Main Results: Nonlinear associations were demonstrated: morning and evening PAI-1 increased by 12% (95% confidence interval [CI], 5–20%; P < 0.001) and 11% (95% CI, 2–20%; P = 0.01), respectively per 5-unit AHI increase until an AHI of 15, when no further increase in PAI-1 was demonstrated. The association between AHI and morning PAI-1 remained significant after adjusting for evening PAI-1 level (10%; 95% CI, 3–17%; P < 0.01). Morning fibrinogen increased on average by 8.4 mg/dl (95% CI, 3.12–13.65; P = 0.002) per five-unit AHI increase until an AHI of 15. There was no association between AHI and morning D-dimer. Hypoxia severity was not associated with thrombotic marker levels.
Conclusions: PAI-1 and fibrinogen levels increase monotonically with AHI at degrees of SDB considered mildly to moderately abnormal, suggesting that even mild SDB levels may increase prothrombotic processes. There may be a plateau in this effect, occurring at levels considered to reflect only moderate SDB severity. These relationships with mild-to-moderate SDB were not observed with D-dimer.
Keywords: sleep apnea, thrombosis, cardiovascular disease
AT A GLANCE COMMENTARY.
Scientific Knowledge on the Subject
Sleep-disordered breathing (SDB) is associated with adverse cardiovascular outcomes and increased mortality. The mechanistic pathways for these associations may include increased atherothrombotic disease occurring secondary to SDB-related proinflammatory and prothrombotic stressors. Prior research has not consistently identified the extent to which biomarkers associated with cardiovascular disease are increased in association with SDB.
What This Study Adds to the Field
In the current study, we find that levels of the prothrombotic biomarkers plasminogen activator inhibitor-1 and fibrinogen increase with incremental increases in the apnea-hypopnea index until a plateau is reached at a moderate level of SDB. Associations persisted after consideration of confounders, including body mass index, medications, and smoking, suggesting that the changes in prothrombotic biomarkers were secondary to mild to moderate levels of SDB-related stressors and providing support for treating patients with even modest levels of SDB.
Sleep-disordered breathing (SDB) is a prevalent condition characterized by repetitive complete or partial upper airway collapse resulting in intermittent hypoxemia and sympathetic nervous system activation with attendant adverse cardiovascular outcomes. Studies have demonstrated an association between SDB and cardiovascular outcomes, including hypertension, stroke, and heart failure (1–4). Recent longitudinal data have brought to light the increased risk of cardiovascular mortality associated with SDB (5–7). Literature also supports an increased risk of death in those with SDB during the 12:00 a.m. to 6:00 a.m. hours compared with subjects without sleep apnea (8). The mechanisms of SDB-associated cardiovascular disease and mortality are uncertain; however, these may involve chronic SDB-induced atherothrombotic disease and potentially superimposed, more immediate processes, such as acute vascular thrombosis/infarction.
Atherothrombosis of the vasculature, including the coronary and cerebral vessels, is considered a disorder of hemostasis, inflammation, endothelial dysfunction, and lipid metabolism. We chose to focus on the role of thrombosis in SDB by examining the relation of SDB and plasminogen activator inhibitor-1 (PAI-1), a molecule that inhibits fibrinolysis by inactivation of tissue plasminogen activator given its described contribution to atherothrombotic events and associated risk of recurrent myocardial infarction (9–11). PAI-1 levels have been shown to vary with measures of hypoxemia (12, 13) as well as sympathetic nervous system activation (14), both known consequences of SDB. Although some data suggest increased PAI-1 levels in those with SDB (15, 16) and improvement with SDB treatment (17), these studies involved small sample sizes and individuals referred to sleep centers (15, 16), may have inadequately controlled for confounding factors (15), lacked a control group (17), and did not examine diurnal variability. We also investigated the association of SDB with fibrinogen given its role in clot formation and association with coronary artery disease (18). Several small studies have identified an association with fibrinogen and SDB; however, limitations of these studies include small sample sizes, lack of clarity regarding whether these relationships are independent of obesity, and lack of a comparison group (19, 20). D-dimer is a cross-linked fibrin degradation fragment that is highly sensitive in the detection of venous thromboembolic disease and may identify abnormalities of thrombosis in both the venous and arterial systems (21–23). The literature regarding an association between SDB and D-dimer is inconsistent (16, 17, 24).
In the current study, we report cross-sectional associations between SDB and thrombotic measures from the Cleveland Family Study, a cohort established to investigate the risk factors and consequences of SDB, which involves individuals with a wide spectrum of SDB and incorporates a collection of selective measures of evening and morning measures of thrombosis. The wide range of SDB severity in this sample provided an opportunity to explore the nature of the dose–response association between apnea-hypopnea index (AHI) and prothrombotic markers, in particular at the low levels of SDB common in the community, which have been associated with an increased risk of cardiovascular disease (3). Some of the results of this work have been previously reported in the form of an abstract (25).
METHODS
Study Population
The initial Cleveland Family Study cohort was assembled by identifying affected families with a proband with diagnosed sleep apnea, and neighborhood control subjects were also recruited. Recruitment and data collection methods have been previously described (26). The current report involves subjects participating in a clinical laboratory–based examination conducted between 2001 and 2006 designed to identify cardiovascular risk factors associated with SDB.
Data Collection
Testing was conducted in the Dahms Clinical Research Unit (Cleveland, OH) after obtaining Institutional Review Board approval and written informed consent from each participant. Height was measured using a rigid stadiometer, and weight with a calibrated digital scale using standardized methods, and these measures were used to calculate body mass index (BMI, kg/m2). Venous blood was sampled between 22:00 and 23:00 in the supine position before sleep onset. Blood was again drawn, supine, between 7:00 and 8:00 the next morning after overnight polysomnography and an overnight fast. After centrifuging and aliquoting using standardized protocols, samples were stored at −80°C until assayed at the University of Vermont Laboratory for Clinical Biochemistry Research. PAI-1 (ng/ml), fibrinogen (mg/dl), and D-dimer (ng/ml) were assayed. PAI-1 was measured by a two-site ELISA (27) using an assay that was sensitive to free PAI-1 (both latent and active), but not PAI-1 in complex with tissue plasminogen activator. Fibrinogen concentrations were quantified by the STa-R automated coagulation analyzer (Diagnostica Stago, Parsippany, NJ), which uses the clotting method developed by Clauss (28) in which the level of fibrinogen is directly correlated with the clotting time of a diluted plasma sample in the presence of excess thrombin (28). D-dimer was quantified with the STa-R analyzer using an immuno-turbidimetric assay (Liatest D-DI; Diagnostica Stago). Timing of the blood draws, the draw procedure, and sample processing were carefully monitored and protocols strictly followed to prevent contamination by platelets (29, 30). Analytical coefficients of variation for the assays were: 3.5% for PAI-1, 4.0% for fibrinogen, and 1% for D-dimer.
Overnight 14-channel polysomnography was performed using the Compumedics E-Series System (Abbotsford, Australia). Studies were scored using standard approaches (31, 32). Apneas and hypopneas were defined using Sleep Heart Health Study criteria, modified to include nasal pressure signal. Hypopneas were identified as a discernible decline in respiratory effort (from inductive respiratory bands) or airflow (from thermocouple or nasal pressure) for 10 seconds or more associated with a greater than or equal to 3% oxygen desaturation. The AHI was used to assess SDB severity, and was defined as the number of respiratory events (apneas and hypopneas) per hour of sleep, with the vast majority of apneas categorized as obstructive.
Statistical Analysis
Standard descriptive statistics were used to describe the study sample. Continuous and categorical variables were compared between subjects with AHI less than 15 and AHI greater than or equal to 15 using Wilcoxon and Pearson chi-square tests, respectively.
The primary outcomes examined were morning and evening PAI-1 (to capture known diurnal variability in PAI-1), morning fibrinogen, and morning D-dimer levels. To satisfy model assumptions, morning and evening PAI-1 and D-dimer levels were log transformed before model fitting.
To model the association between AHI and each thrombosis marker, linear mixed models with a compound symmetric covariance structure to account for within-family correlation were used. To assess the functional form of AHI relative to markers of thrombosis, a restricted cubic spline model was initially fit and showed an approximate linear threshold effect at an AHI of 15. Consequently, a piecewise linear mixed model with one knot at AHI = 15 was used. To facilitate interpretation, the parameter estimates from these models were used to estimate the relative (geometric) mean change in level of PAI-1 or D-dimer or the mean change in morning fibrinogen for each 5-unit AHI change.
To examine the effect of possible confounders on the association between AHI and each thrombosis marker, two multivariable models were also fit. Model 1 adjusted for age, sex, and race only; Model 2 additionally adjusted for BMI, self-reported cardiac/cerebrovascular disease (i.e., history of angina, coronary angioplasty, coronary artery bypass graft surgery, myocardial infarction, coronary heart disease, stroke, carotid endarterectomy, or heart failure), hypertension (blood pressure ≥ 140 mm Hg or diastolic blood pressure ≥ 90 mm Hg or use of antihypertensive medication), diabetes mellitus (fasting glucose ≥ 126 mg/dl or oral glucose tolerance test ≥ 200 mg/dl or use of hypoglycemic medication), menopausal status (pre- versus postmenopausal status), smoking status (current smoker versus noncurrent), aspirin use, and use of oral contraceptive/estrogen replacement therapy. To assess immediate SDB-related effects on morning PAI-1 levels, an additional model was fit adjusting for the evening PAI-1 level (Model 3).
The association between hypoxia (percentage of total sleep time < 90% oxygen saturation [dichotomized at 2% given its skewed distribution]) and arousal index (dichotomized at 15) with each thrombotic marker was also examined. To further examine any confounding influence of cardiac/cerebrovascular disease or hypertension, sensitivity analyses were performed excluding those participants with these respective disorders.
All tests were performed using SAS v 9.2 (SAS Institute Inc., Cary, NC) for analyses and R 2.9.2 for the graphs.
RESULTS
Primary Analyses
Of the 735 participants in the study, the analyses were restricted to participants 16 years of age or older (n = 644) and without severe chronic health conditions, such as systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, and liver or renal disease (n = 18) as well as subjects taking oral corticosteroids or anticoagulants (n = 36) (see flow diagram in the online supplement). Individuals who reported regular continuous positive airway pressure use for sleep apnea and who were studied when not using continuous positive airway pressure were excluded (n = 13). The final analytic sample included those individuals with complete PAI-1, fibrinogen, and D-dimer data (n = 537). Those individuals with missing (n = 39) versus nonmissing values were younger (37.0 ± 18.5 vs. 44.6 ± 16.8 yr) and had a lower BMI (30.3 ± 9.6 vs. 33.5 ± 8.9 kg/m2).
Table 1 shows subject characteristics according to level of AHI. Subjects were 44.6 ± 16.8 years of age, obese with BMI 33.5 ± 8.9 kg/m2, 57.0% women, and 54.9% African American. As expected, there was a higher percentage of men and subjects with obesity, diabetes mellitus, hypertension, and cardiac or cerebrovascular disease in those with AHI greater than or equal to 15 compared with those with AHI less than 15.
TABLE 1.
SUBJECT CHARACTERISTICS OF THE ANALYTIC SAMPLE DICHOTOMIZED BY APNEA-HYPOPNEA INDEX
| Demographics (n = 536) | Analytic Sample (n = 536) | AHI < 15 (n = 392) | AHI ≥ 15 (n = 144) | P Value |
|---|---|---|---|---|
| Subject characteristics | ||||
| Age, yr | 44.6 ± 16.8, 45.4 (29.7–55.2) | 42.2 ± 16.8, 43.0 (25.5–53.2) | 51.0 ± 15.0, 49.3 (42.6–62.0) | < 0.001 |
| Female sex | 56.9% | 62.8% | 41.0% | < 0.001 |
| Menopausal status* | 35.4% | 31.3% | 52.5% | 0.004 |
| African American race | 55.0% | 53.3% | 59.7% | 0.24 |
| BMI, kg/m2 | 33.5 ± 8.9, 32.2 (27.0–38.7) | 32.3 ± 8.9, 30.5 (25.9–36.7) | 36.9 ± 7.9, 35.9 (31.2–41.9) | < 0.001 |
| Waist circumference, cm | 100.2 ± 19.6, 98.4 (85.7–112.4) | 96.5 ± 19.4, 94.0 (81.9–108.8) | 110.2 ± 16.4, 109.5 (100.4–119.8) | < 0.001 |
| Current smoker | 27.6% | 27.3% | 28.5% | 0.77 |
| Medical conditions | ||||
| Hypertension | 36.2% | 30.6% | 51.4% | < 0.001 |
| Diabetes mellitus | 20.0% | 16.1% | 30.6% | < 0.001 |
| Cardiovascular disease | 11.4% | 9.2% | 17.4% | 0.003 |
| Medication use | ||||
| Aspirin use | 28.7% | 27.0% | 33.3% | 0.12 |
| Birth control or HRT use* | 12.4% | 14.2% | 5.1% | 0.04 |
| Statins | 11.0% | 7.1% | 21.5% | < 0.001 |
| Antihypertension | 23.0% | 18.6% | 34.7% | < 0.001 |
| Oral glucose | 9.7% | 8.4% | 13.2% | 0.09 |
| Insulin | 2.8% | 1.8% | 5.6% | 0.02 |
| Sleep measures | ||||
| Apnea-Hypopnea Index | 13.2 ± 19.6, 5.2 (1.6–16.1) | 4.3 ± 4.0, 2.6 (1.0–6.4) | 38.1 ± 23.4, 28.2 (20.2–50.2) | < 0.001 |
| ≥ 2% of sleep time with oxygen saturation < 90% | 22.7% | 8.0% | 62.5% | < 0.001 |
| Arousal index | 16.2 ± 9.3, 14.0 (9.7–20.1) | 13.5 ± 6.4, 12.5 (8.8–16.7) | 23.8 ± 11.5, 22.0 (14.9–31.0) | < 0.001 |
Definition of abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; HRT = hormone replacement therapy.
For continuous variables, mean ± standard deviation, median, and interquartile range are presented. For categorical variables, percentage is presented.
Among females (n = 305).
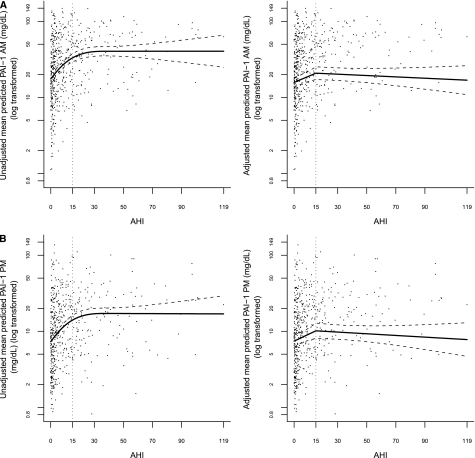
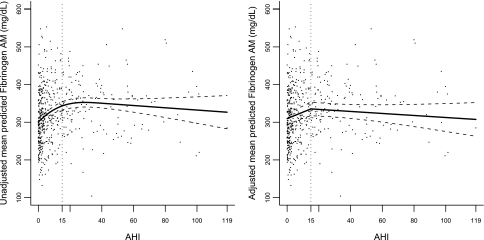
To initially examine the association between AHI and each of the markers, an unadjusted mixed model was fit using a restricted cubic smoothing spline to model AHI. An increase in AHI was associated with increases in the levels of morning and evening log PAI-1 as well as morning and evening fibrinogen until a threshold AHI of 15 was reached, at which point there appeared to be a plateau (Figures 1 and 2). In contrast, no association was observed between D-dimer levels and AHI.
Figure 1.
Points represent a scatterplot of (A) morning and (B) evening plasminogen activator inhibitor-1 (PAI-1) (on the log scale) and apnea-hypopneas index (AHI), and lines represent the model-based unadjusted and fully adjusted piecewise linear association between morning and evening PAI-1 (on the log scale) and AHI. To ensure normal distribution assumptions of the mixed model are satisfied, PAI-1 values were log transformed before fitting the piecewise linear association.
Figure 2.
Points represent a scatterplot of morning fibrinogen and apnea-hypopneas index (AHI), and lines represent the model-based unadjusted and fully adjusted piecewise linear association between morning fibrinogen and AHI.
Morning and evening PAI-1.
Table 2 presents the results from the piecewise linear mixed models that were developed to investigate the association between AHI and morning and evening PAI-1, and Figure 1 presents a scatterplot/graph of unadjusted and fully adjusted piecewise linear models between morning and evening PAI-1 (on the log scale) and AHI. For morning PAI-1, Model 1 demonstrated that at levels of AHI less than 15, the mean of morning PAI-1 increased by 26% (95% confidence interval [CI], 17–36%; P < 0.001) per 5-unit AHI increase. In contrast, at levels of AHI greater than or equal to 15, the mean of morning PAI-1 remained relatively the same per 5-unit AHI increase (P = 0.43). A test of the piecewise linear association between AHI and morning PAI-1 was highly significant (P < 0.001), indicating that for a 5-unit increase in AHI, the relative mean change in morning PAI-1 when AHI less than 15 was significantly different from the relative mean change in morning PAI-1 when AHI was greater than or equal to 15. After adjusting for potential confounders, including BMI, cardiovascular factors, and medications (Model 2), the association with lower levels of SDB (AHI < 15) and morning PAI-1 was attenuated, but persisted such that the mean of morning PAI-1 increased, on average, by 12% (95% CI, 5–20%; P < 0.001) per 5-unit AHI increase.
TABLE 2.
ESTIMATED RELATIVE MEAN CHANGE IN PLASMINOGEN ACTIVATOR INHIBITOR-1 WHEN APNEA-HYPOPNEA INDEX IS MODELED USING A PIECEWISE LINEAR ASSOCIATION (N = 536)
| Estimated Relative Mean Change in PAI-1 Given a 5-Unit Increase in AHI (95% CI) | Test if No Relative Mean Change in PAI-1 Given a 5-Unit Increase in AHI | Test if Relative Mean Change in PAI-1 is the Same for Both AHI Groups | |
|---|---|---|---|
| PAI-1 a.m. (ng/ml)* | |||
| Model 1 | |||
| AHI < 15 | 1.26 (1.17–1.36)† | < 0.001 | < 0.001 |
| AHI ≥ 15 | 1.01 (0.98–1.04) | 0.44 | |
| Model 2 | |||
| AHI < 15 | 1.12 (1.05–1.19) | 0.001 | 0.002 |
| AHI ≥ 15 | 0.99 (0.97–1.01) | 0.35 | |
| Model 3 | |||
| AHI < 15 | 1.10 (1.03–1.17) | 0.003 | 0.01 |
| AHI ≥ 15 | 0.99 (0.97–1.01) | 0.34 | |
| PAI-1 p.m. (ng/ml)* | |||
| Model 1 | |||
| AHI < 15 | 1.25 (1.14–1.36) | < 0.001 | < 0.001 |
| AHI ≥ 15 | 1.01 (0.98–1.04) | 0.47 | |
| Model 2 | |||
| AHI < 15 | 1.11 (1.02–1.20) | 0.01 | 0.02 |
| AHI ≥ 15 | 0.99 (0.96–1.01) | 0.34 | |
Definition of abbreviations: AHI = apnea-hypopnea index; CI = confidence interval; PAI-1 = plasminogen activator inhibitor-1.
Model 1: AHI + age, race, sex. Model 2: Model 1 + BMI, cardiac disease/cerebrovascular disease, hypertension, diabetes mellitus, aspirin use, oral contraceptive use, current smoker status, and menopausal status. Model 3: Model 2 + PAI-1 p.m.
PAI-1 a.m. and PAI-1 p.m. are log transformed to satisfy the assumption of normality in the model. Parameter estimates are then back transformed for ease of model interpretation.
For AHI < 15, when AHI increases by 5 units, the mean of PAI-1 a.m. increases by 26% (1.26; 95% CI, 1.17–1.36) adjusting for age, race, and sex.
With respect to evening PAI-1 levels and AHI, the results were similar to that of morning PAI-1. For evening PAI-1, Model 1 demonstrated that at levels of AHI less than 15, the mean of evening PAI-1 increased by 24% (95% CI, 14–36%; P < 0.001) per 5-unit AHI increase. Alternatively, at levels of AHI greater than or equal to 15, the relative mean change in morning PAI-1 remained relatively the same (P = 0.46) per 5-unit AHI increase. The test of the piecewise linear association between AHI and evening PAI-1 was significant (P < 0.001), indicating that for a 5-unit increase in AHI, the relative mean change in evening PAI-1 when AHI was less than 15 was significantly different from the relative mean change in evening PAI-1 when AHI was greater than or equal to 15. In Model 2, after adjusting for potential confounders, the association with lower levels of SDB (AHI < 15) and evening PAI-1 was attenuated (similar to associations noted with morning levels), but persisted, such that when AHI increased by 5 units, the mean in evening PAI-1 increased by 11% (95% CI, 2–20%; P = 0.02).
Given the biologic plausibility that morning levels of thrombotic markers may be increased due to overnight SDB-associated physiological stressors, and known diurnal variability in PAI-1, an analysis of PAI-1 morning levels was also performed after further adjusting for PAI-1 evening levels (Model 3). In this case, there remained a statistically significant association between morning PAI-1 and mild degrees of SDB (AHI < 15), such that when AHI increased by 5 units, the mean in morning PAI-1 increased by 10% (95% CI, 3–17%; P = 0.002).
Sensitivity analyses of morning and evening PAI-1 excluding those participants with cardiac/cerebrovascular disease (n = 475) or excluding those with hypertension (n = 342) revealed that unadjusted and fully adjusted results were consistent with those presented in the primary analyses. Tables are included in the online supplement.
There was no statistically significant association between percentage of sleep time at less than 90% oxygen saturation or arousal index with both morning and evening PAI-1 in the partially or fully adjusted models.
Morning and evening fibrinogen.
Although initially both morning and evening levels of fibrinogen were measured, an interim analysis showed these analyses to be highly correlated (r = 0.87; n = 397); therefore, primary analyses reported results for morning fibrinogen only. Table 3 presents the results from the piecewise linear mixed models that were developed to investigate the association between AHI and morning fibrinogen levels and Figure 2 presents a scatterplot/graph of unadjusted and fully adjusted piecewise linear models between morning fibrinogen and AHI. For morning fibrinogen, Model 1 demonstrated that at AHI levels less than 15, morning fibrinogen increased on average by 14.4 mg/dl (95% CI, 8.68–20.18; P < 0.001) per 5-unit AHI increase. Alternatively, no significant incremental change in morning fibrinogen was observed with increasing AHI when RDI greater than or equal to 15 (i.e., per 5-unit increment in AHI, morning fibrinogen increased only by 0.3 mg/dl [95% CI, −2.06 to 2.66; P = 0.80]). A test of the piecewise linear association between AHI and morning fibrinogen was highly significant (P < 0.001), indicating that for a 5-unit change in AHI, the mean change in morning fibrinogen when AHI was less than 15 was significantly different from the mean change in morning fibrinogen when AHI was greater than or equal to 15. After adjusting for potential confounders (Model 2), the association with lower levels of SDB (AHI < 15) and morning fibrinogen was attenuated, but persisted such that when AHI increased by 5 units, morning fibrinogen increased on average by 8.4 mg/dl (95% CI, 3.12–13.65; P = 0.002). Based on an analytic sample of 397 individuals who had evening levels assayed, results for evening fibrinogen were similar to morning fibrinogen. Specifically, after adjusting for covariates, evening fibrinogen increased on average by 8.7 mg/dl (95% CI, 2.72–14.62); P < 0.001) (results not shown).
TABLE 3.
ESTIMATED CHANGE IN MORNING FIBRINOGEN WHEN APNEA-HYPOPNEA INDEX IS MODELED USING A PIECEWISE LINEAR ASSOCIATION (N = 536)
| Estimated Mean (mg/dl) Change in Fibrinogen Given a 5-Unit Increase in AHI (95% CI) | Test if No Mean Change in Fibrinogen Given a 5-Unit Increase in AHI | Test if Mean Change in Fibrinogen is the Same for Both AHI Groups | |
|---|---|---|---|
| Model 1 | |||
| AHI < 15 | 14.55 (8.78 to 20.32)* | < 0.001 | < 0.001 |
| AHI ≥ 15 | 0.28 (−2.08 to 2.64) | 0.81 | |
| Model 2 | |||
| AHI < 15 | 8.58 (3.33 to 13.83) | 0.001 | 0.003 |
| AHI ≥ 15 | −1.34 (−3.56 to 0.88) | 0.24 |
Definition of abbreviations: AHI = apnea-hypopnea index; CI = confidence interval.
Model 1: AHI + age, race, sex. Model 2: Model 1 + BMI, cardiac disease/cerebrovascular disease, hypertension, diabetes mellitus, aspirin use, oral contraceptive use, current smoker status, and menopausal status.
For AHI < 15, when AHI increases by 5 units, mean fibrinogen a.m. increases by 14.55 mg/dl (95% CI, 8.78–20.32) adjusting for age, race, and sex.
Sensitivity analyses of morning fibrinogen excluding those participants with cardiac/cerebrovascular disease (n = 475) or excluding those with hypertension (n = 342) revealed that unadjusted and adjusted results were consistent with those presented in the primary analyses. Tables are included in the online supplement.
There was no statistically significant association observed between percentage of sleep time at less than 90% oxygen saturation or arousal index with the morning fibrinogen in the partially or fully adjusted models.
Morning D-dimer.
In both Models 1 and 2, there was no significant relationship between morning D-dimer and an AHI (for a 5-unit increase in AHI: 1.00; 95% CI, 0.99–1.02; P = 0.78). Additionally, there was no statistically significant association observed between total sleep time at less than 90% oxygen saturation and morning D-dimer in the partially and fully adjusted models. Although there was a significant 4% increase in the mean of D-dimer for a 5-unit increase in the arousal index in unadjusted analyses, when taking into account confounding factors (Model 2), this relationship was no longer statistically significant.
DISCUSSION
This study provides evidence for a positive linear relationship between mild to moderate levels of SDB and markers of thrombosis (both morning and evening PAI-1 levels and morning levels of fibrinogen), which appear to plateau at an AHI of approximately 15. These relationships persist even after extensive consideration of confounding factors, including BMI, cardiovascular disease, medications that have the potential to affect thrombotic processes, and smoking. In addition, PAI-1, which demonstrates large diurnal variability, also appeared to show morning variability in association with SDB, which was not explained by evening PAI-1 levels. This may indicate the sensitivity of PAI-1 levels to the influences of immediate overnight SDB-physiologic stress. In contrast, unlike PAI-1 and fibrinogen, D-dimer does not demonstrate the same linear relationships with mild to moderate SDB.
The relationship between morning and evening PAI-1 levels and increasing severity of SDB in those with mild to moderate SDB is of particular interest. Tissue plasminogen activator, an enzyme secreted by endothelial cells, initiates fibrinolysis and converts plasminogen to plasmin, which then proteolytically degrades the fibrin that holds the thrombus together. PAI-1 exerts its effects by inactivating tissue plasminogen activator. This results in a reduction in fibrinolytic activity, which may lead to increased atherothrombosis due to excess fibrin formation and to promotion of vascular disease progression. There is biologic plausibility to support a mechanistic link of hypoxia and sympathetic nervous system activation (12–14), both known SDB consequences, with increased PAI-1 activity.
Diurnal variation of PAI-1 activity has been reported such that higher PAI-1 levels occur in the morning (33–35). We observed both diurnal variation and variation of both evening and morning PAI-1 levels in association with increased AHI levels from 0 to 15. This suggests that overall daily levels of PAI-1 are elevated in association with mild to moderate SDB. Also, after adjusting for evening PAI-1 in addition to other covariates, the relative mean change in morning PAI-1 per 5-unit AHI increase was not substantively attenuated (10%) compared with the model that did not adjust for evening PAI-1 (11%). The persistence of an association between morning PAI-1 levels and incremental increases in AHI, even after adjusting for evening levels in conjunction with its relatively short half-life of 2 to 5 hours (36), suggests that morning PAI-1 may be a useful marker of overnight SDB-related stress. We have also observed similar relationships of SDB severity and morning soluble IL-6 receptor levels, although not with IL-6 levels (37).
Fibrinogen has been identified as a risk factor for cardiovascular disease likely due to multiple mechanisms, including: binding to activated platelets via glycoprotein IIb/IIIa, and contributing to platelet aggregation, promotion of fibrin formation, enhancing plasma viscosity, and serving as an acute-phase reactant. In the current study, morning fibrinogen levels were observed to increase relative to increases in AHI in those with mild to moderate SDB. Prior research has shown that individuals with ischemic stroke and SDB have higher levels of fibrinogen, which correlates with SDB and hypoxia severity (20).
Unlike PAI-1 and fibrinogen, D-dimer did not demonstrate a significant association with mild to moderate SDB, which is consistent with previously published findings. D-dimer is a breakdown product of a stabilized fibrin mesh and has been measured clinically as a highly sensitive marker of venous thromboembolism and disseminated intravascular coagulation (21, 23). Because elevations may occur generally with aging and in settings of venous disease (the latter of which has not been commonly reported in association with SDB), its use as a marker of SDB may be limited.
Perhaps the most interesting aspect of our findings was evidence for a plateau effect at an AHI of approximately 15. There is much uncertainty regarding thresholds and dose–response relationships between indices of SDB and various health outcomes. Understanding the levels of SDB that are associated with increased risk of cardiovascular disease is needed to most effectively target groups likely to benefit from SDB interventions. Most clinic-based samples have reported increased risk of adverse cardiometabolic outcomes at the severe levels of SDB common in referred samples (5, 38). However, our findings are consistent with the cross-sectional data from the Sleep Heart Health Study, which also showed an increase in the prevalence of cardiovascular disease at low levels of SDB, with further risk plateauing at an AHI of approximately 11 (3). It is possible that there is a physiologic threshold effect of hypoxic and sympathetic nervous system surges on the thrombotic milieu such that more severe levels of SDB may not be associated with progressive increases in thrombotic biomarkers. Counterregulatory mechanisms may potentially manifest after a certain level of SDB-physiologic burden is realized, such that thrombotic risk may plateau, and therefore higher levels of SDB may not be associated with a graded increase in thrombotic risk. Although clinical trials are needed to shape intervention guidelines, these data suggest that individuals with even modest levels of SDB (which describes a large proportion of the adult population) may have an enhanced prothrombotic biochemical profile, increasing their cardiovascular disease risk.
In addition to examining the AHI as the primary metric of SDB exposure, we also examined associations with levels of hypoxia, as ascertained by percentage of total sleep time at oxygen saturation less than 90% and the arousal index. Modeling percentage time in desaturation is challenging due to the marked skewness of this measure. When analyzing the dichotomized values, we did not observe associations with any thrombotic measure. This may reflect the more limited information and ability to fully model appropriate dose–response associations with use of this metric, or the limited power in the current sample to detect effects related to large degrees of hypoxia. Compared with time in desaturation, the AHI may more specifically measure stresses associated with intermittent hypoxia, which may be the relevant stimulus for adverse inflammatory/prothrombotic responses. Although arousals may be associated with surges in sympathetic nerve activity, the arousal index, which reflects sleep disruption occurring with or without airway obstruction, may not reflect SDB prothrombotic stresses as well as the AHI, which reflects disturbances specific to airway occlusion.
Several study limitations need to be acknowledged. Given the cross-sectional design, causality is uncertain. Inferences regarding dose–response relationships at the highest levels of the AHI distribution were based on a relatively modest sample size and it is possible that more significant associations with higher levels of AHI or levels of hypoxia with each biomarker may have been observed had the sample included larger numbers of individuals with severe SDB. Although we adjusted for many known confounders, residual confounding nonetheless may have influenced our results. Both PAI-1 and fibrinogen have been implicated in both incident and recurrent cardiovascular events (9, 39). Our results were primarily attributed to associations observed in individuals without established cardiac or cerebrovascular disease, and thus best address SDB as a putative antecedent risk factor for cardiovascular disease. Our findings may differ from those of other epidemiological studies demonstrating increased cardiovascular risk and mortality (6, 7) due to the relatively young age of our cohort members. Strengths of the current study include the moderately large sample size, rigorous collection of data, collection of evening and morning levels to assess diurnal variability, and analysis of data from a nonreferral sample. This is the largest study to date to examine the associations between fibrinogen and PAI-1 relative to SDB.
In summary, these data suggest that, at low-modest levels of SDB, incremental increases in AHI are associated with increases in levels of two prothrombotic biomarkers associated with cardiovascular disease. Future directions include exploring whether treatment of even mild to moderate levels of SDB improves biomarkers of thrombosis, and performing further work to understand the specific pathways and pathobiology of SDB-related increased risk of thrombosis.
Supplementary Material
Acknowledgments
The authors thank the dedicated staff of the Cleveland Family Study, including Joan Aylor, Kathryn Clark, Rawan Nawabit Salem, Jennifer Frame, and Heather Rogers, as well as the nurses of the University Hospitals of Cleveland Case Medical Center Dahms Clinical Research Unit. They also thank the members of the Cleveland Family Study, whose continuing enthusiasm has made this study possible.
Supported by NIH National Heart Lung Blood Institute grants 46380 and K23 HL079114, NIH M01 RR00080, American Heart Association National Scientist Development Award 0530188N, Central Society of Clinical Research, NHLBI K08 HL081385, and NCI 1 U54CA116867. The project described was also supported by UL1 RR024989 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201001-0020OC on June 10, 2010
Author Disclosure: R.M. received more than $100,001 from the National Institutes of Health (NIH), more than $100,001 from the American Heart Association, and $10,001–$50,000 from American College of Chest Physicians/ASP in industry-sponsored grants, and received continuous positive airway pressure machines from Philips Respironics for use in a clinical trial. F.X. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.C.B. received up to $1,000 from the NIH for serving on an advisory board and $10,001–$50,000 from the NIH in sponsored grants. R.P.T. received more than $100,001 from the Case School of Medicine and $10,001–$50,000 from Columbia University in sponsored grants. N.S.J. received $50,001–$100,000 from the NIH as salary support on grants. S.R.P. received $1,001–$5,000 from Health Right Products in industry-sponsored grants, and more than $100,001 from the NIH and $10,001–$50,000 from the ATS in sponsored grants. S.R. received more than $100,001 from Dymedix Inc. in industry-sponsored grants and more than $100,001 from the NIH in sponsored grants.
References
- 1.Javaheri S, Parker TJ, Liming JD, Corbett WS, Nishiyama H, Wexler L, Roselle GA. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation 1998;97:2154–2159. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 3.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19–25. [DOI] [PubMed] [Google Scholar]
- 4.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041. [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046–1053. [DOI] [PubMed] [Google Scholar]
- 6.Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep 2008;31:1079–1085. [PMC free article] [PubMed] [Google Scholar]
- 7.Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin Sleep Cohort. Sleep 2008;31:1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 8.Gami AS, Howard DE, Olson EJ, Somers VK. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 2005;352:1206–1214. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK. Prospective study of fibrinolytic factors and incident coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol 2001;21:611–617. [DOI] [PubMed] [Google Scholar]
- 10.Hamsten A, de Faire U, Walldius G, Dahlen G, Szamosi A, Landou C, Blomback M, Wiman B. Plasminogen activator inhibitor in plasma: risk factor for recurrent myocardial infarction. Lancet 1987;2:3–9. [DOI] [PubMed] [Google Scholar]
- 11.Kohler HP, Grant PJ. Plasminogen-activator inhibitor type 1 and coronary artery disease. N Engl J Med 2000;342:1792–1801. [DOI] [PubMed] [Google Scholar]
- 12.Pinsky DJ, Liao H, Lawson CA, Yan SF, Chen J, Carmeliet P, Loskutoff DJ, Stern DM. Coordinated induction of plasminogen activator inhibitor-1 (PAI-1) and inhibition of plasminogen activator gene expression by hypoxia promotes pulmonary vascular fibrin deposition. J Clin Invest 1998;102:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan SF, Mackman N, Kisiel W, Stern DM, Pinsky DJ. Hypoxia/hypoxemia-induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis. Arterioscler Thromb Vasc Biol 1999;19:2029–2035. [DOI] [PubMed] [Google Scholar]
- 14.Venugopal B, Sharon R, Abramovitz R, Khasin A, Miskin R. Plasminogen activator inhibitor-1 in cardiovascular cells: rapid induction after injecting mice with kainate or adrenergic agents. Cardiovasc Res 2001;49:476–483. [DOI] [PubMed] [Google Scholar]
- 15.Rangemark C, Hedner JA, Carlson JT, Gleerup G, Winther K. Platelet function and fibrinolytic activity in hypertensive and normotensive sleep apnea patients. Sleep 1995;18:188–194. [DOI] [PubMed] [Google Scholar]
- 16.von Kanel R, Loredo JS, Ancoli-Israel S, Mills PJ, Natarajan L, Dimsdale JE. Association between polysomnographic measures of disrupted sleep and prothrombotic factors. Chest 2007;131:733–739. [DOI] [PubMed] [Google Scholar]
- 17.von Kanel R, Loredo JS, Ancoli-Israel S, Dimsdale JE. Association between sleep apnea severity and blood coagulability: Treatment effects of nasal continuous positive airway pressure. Sleep Breath 2006;10:139–146. [DOI] [PubMed] [Google Scholar]
- 18.Woodward M, Lowe GD, Rumley A, Tunstall-Pedoe H. Fibrinogen as a risk factor for coronary heart disease and mortality in middle-aged men and women. The Scottish Heart Health Study. Eur Heart J 1998;19:55–62. [DOI] [PubMed] [Google Scholar]
- 19.Nobili L, Schiavi G, Bozano E, De Carli F, Ferrillo F, Nobili F. Morning increase of whole blood viscosity in obstructive sleep apnea syndrome. Clin Hemorheol Microcirc 2000;22:21–27. [PubMed] [Google Scholar]
- 20.Wessendorf TE, Thilmann AF, Wang YM, Schreiber A, Konietzko N, Teschler H. Fibrinogen levels and obstructive sleep apnea in ischemic stroke. Am J Respir Crit Care Med 2000;162:2039–2042. [DOI] [PubMed] [Google Scholar]
- 21.Bockenstedt P. D-dimer in venous thromboembolism. N Engl J Med 2003;349:1203–1204. [DOI] [PubMed] [Google Scholar]
- 22.Reganon E, Vila V, Martinez-Sales V, Vaya A, Lago A, Alonso P, Aznar J. Association between inflammation and hemostatic markers in atherothrombotic stroke. Thromb Res 2003;112:217–221. [DOI] [PubMed] [Google Scholar]
- 23.Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, Kovacs G, Mitchell M, Lewandowski B, Kovacs MJ. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003;349:1227–1235. [DOI] [PubMed] [Google Scholar]
- 24.Shitrit D, Peled N, Shitrit AB, Meidan S, Bendayan D, Sahar G, Kramer MR. An association between oxygen desaturation and D-dimer in patients with obstructive sleep apnea syndrome. Thromb Haemost 2005;94:544–547. [PubMed] [Google Scholar]
- 25.Mehra R, Zhu X., Tracy RP, Jenny NS, Storfer-Isser A, Patel SR, Redline S. Association of sleep-disordered breathing with measures of thrombosis and endothelial function. American Thoracic Society abstracts; Toronto, Ontario, Canada: 2008.
- 26.Redline S, Tishler PV, Tosteson TD, Williamson J, Kump K, Browner I, Ferrette V, Krejci P. The familial aggregation of obstructive sleep apnea. Am J Respir Crit Care Med 1995;151:682–687. [DOI] [PubMed] [Google Scholar]
- 27.Declerck PJ, Alessi MC, Verstreken M, Kruithof EK, Juhan-Vague I, Collen D. Measurement of plasminogen activator inhibitor 1 in biologic fluids with a murine monoclonal antibody-based enzyme-linked immunosorbent assay. Blood 1988;71:220–225. [PubMed] [Google Scholar]
- 28.Clauss A. Rapid physiological coagulation method in determination of fibrinogen [in German]. Acta Haematol 1957;17:237–246. [DOI] [PubMed] [Google Scholar]
- 29.Macy EM, Meilahn EN, Declerck PJ, Tracy RP. Sample preparation for plasma measurement of plasminogen activator inhibitor-1 antigen in large population studies. Arch Pathol Lab Med 1993;117:67–70. [PubMed] [Google Scholar]
- 30.Tracy R, Bovill E. Plasminogen activator inhibitor-1. In: Beutler E, Lichtman M, Coller B, Kipps T, editors. Williams hematology. New York: McGraw Hill; 2005. pp. L110-L111.
- 31.Rechtschaffen A, Kales A, editors. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Washington: U.S. Department of Health, Education, and Welfare Public Health Service - NIH/NIND. 1968.
- 32.Redline S, Sanders MH, Lind BK, Quan SF, Iber C, Gottlieb DJ, Bonekat WH, Rapoport DM, Smith PL, Kiley JP. Methods for obtaining and analyzing unattended polysomnography data for a multicenter study. Sleep Heart Health Research Group. Sleep 1998;21:759–767. [PubMed] [Google Scholar]
- 33.Angleton P, Chandler WL, Schmer G. Diurnal variation of tissue-type plasminogen activator and its rapid inhibitor (PAI-1). Circulation 1989;79:101–106. [DOI] [PubMed] [Google Scholar]
- 34.Ohkura N, Oishi K, Fukushima N, Kasamatsu M, Atsumi GI, Ishida N, Horie S, Matsuda J. Circadian clock molecules CLOCK and CRYs modulate fibrinolytic activity by regulating the PAI-1 gene expression. J Thromb Haemost 2006;4:2478–2485. [DOI] [PubMed] [Google Scholar]
- 35.Oishi K, Shirai H, Ishida N. Identification of the circadian clock-regulated E-box element in the mouse plasminogen activator inhibitor-1 gene. J Thromb Haemost 2007;5:428–431. [DOI] [PubMed] [Google Scholar]
- 36.Gombau L, Schleef RR. Processing of type 1 plasminogen activator inhibitor (PAI-1) into the regulated secretory pathway. J Biol Chem 1994;269:3875–3880. [PubMed] [Google Scholar]
- 37.Mehra R, Storfer-Isser A, Kirchner HL, Johnson N, Jenny N, Tracy RP, Redline S. Soluble interleukin 6 receptor: a novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med 2006;166:1725–1731. [DOI] [PubMed] [Google Scholar]
- 38.Harsch IA, Schahin SP, Radespiel-Troger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, et al. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 2004;169:156–162. [DOI] [PubMed] [Google Scholar]
- 39.van der Krabben MD, Rosendaal FR, van der Bom JG, Doggen CJ. Polymorphisms in coagulation factors and the risk of recurrent cardiovascular events in men after a first myocardial infarction. J Thromb Haemost 2008;6:720–725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.