Abstract
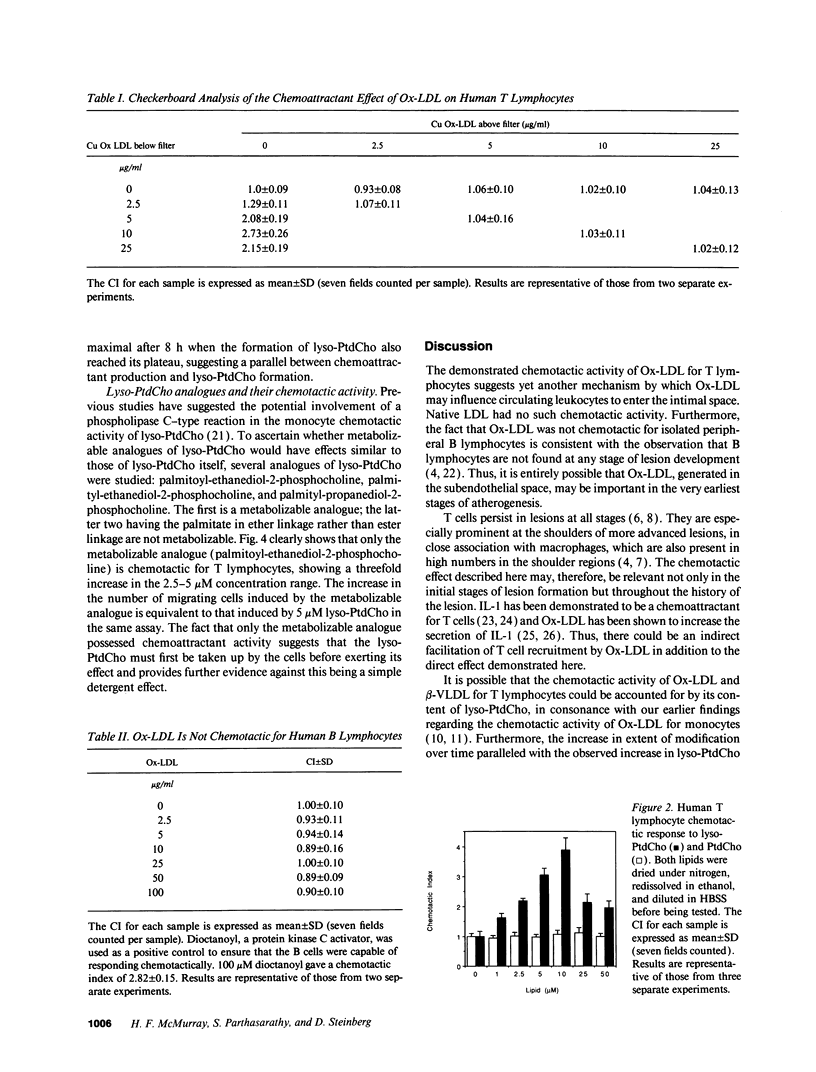
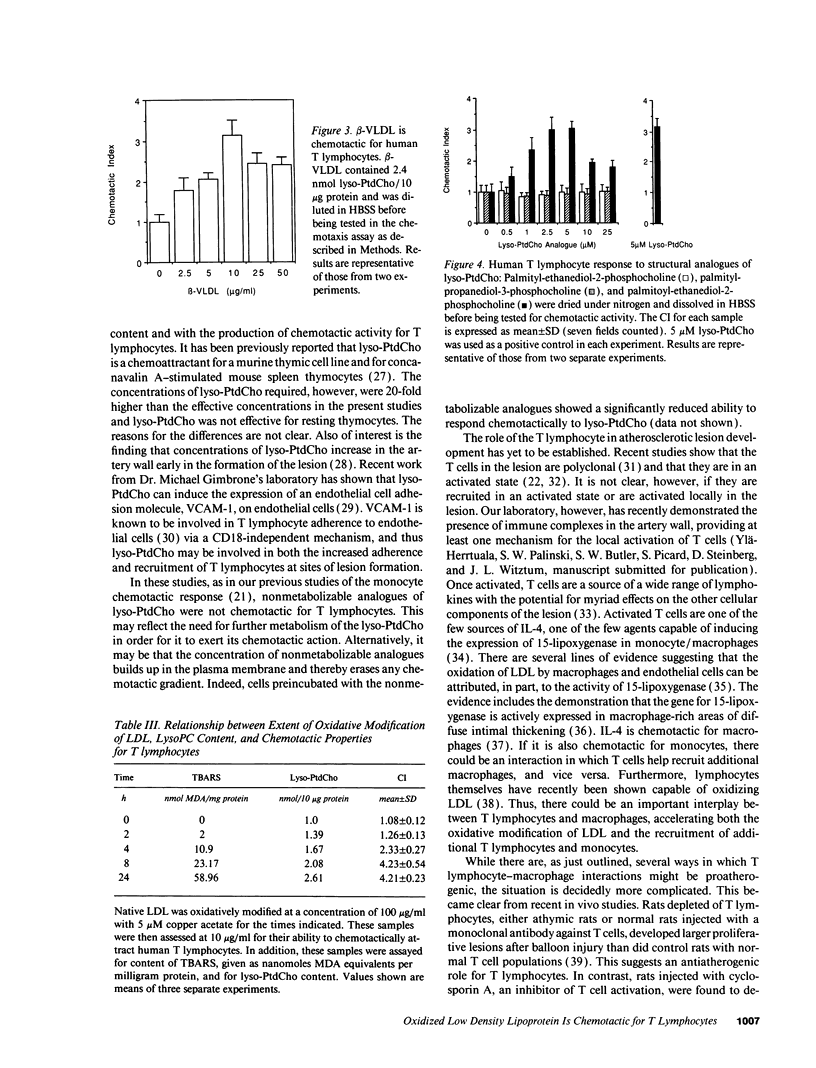
Oxidatively modified low density lipoprotein (Ox-LDL) is a known chemoattractant for monocytes. Here we demonstrate, using a modified Boyden chamber assay, that human peripheral blood T lymphocytes, but not B lymphocytes, also respond chemotactically to Ox-LDL, showing a threefold increase over control and an optimum response at 10 micrograms/ml. Copper and endothelial cell-oxidized LDL and beta-VLDL were used and gave similar results. The activity was not chemokinetic and native LDL possessed no chemoattractant activity. The chemoattractant activity was found to reside in the lipid fraction of Ox-LDL. Lysophosphatidylcholine is a major phospholipid component of Ox-LDL and is known to be chemotactic for monocytes. We show that lysophosphatidylcholine is also chemotactic for T lymphocytes with a maximal fourfold increase at 10 microM. Nonmetabolizable analogues of lysophosphatidylcholine had no such chemotactic effect. Thus, Ox-LDL, by virtue of its lysophosphatidylcholine content, may contribute to the recruitment of both T lymphocytes and monocytes into developing atherosclerotic lesions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aqel N. M., Ball R. Y., Waldmann H., Mitchinson M. J. Monocytic origin of foam cells in human atherosclerotic plaques. Atherosclerosis. 1984 Dec;53(3):265–271. doi: 10.1016/0021-9150(84)90127-8. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Buonassisi V., Venter J. C. Hormone and neurotransmitter receptors in an established vascular endothelial cell line. Proc Natl Acad Sci U S A. 1976 May;73(5):1612–1616. doi: 10.1073/pnas.73.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Conrad D. J., Kuhn H., Mulkins M., Highland E., Sigal E. Specific inflammatory cytokines regulate the expression of human monocyte 15-lipoxygenase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):217–221. doi: 10.1073/pnas.89.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Frostegård J., Wu R., Giscombe R., Holm G., Lefvert A. K., Nilsson J. Induction of T-cell activation by oxidized low density lipoprotein. Arterioscler Thromb. 1992 Apr;12(4):461–467. doi: 10.1161/01.atv.12.4.461. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Holm S., Fotev Z., Hedrich H. J., Fingerle J. T lymphocytes inhibit the vascular response to injury. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10530–10534. doi: 10.1073/pnas.88.23.10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Lojsthed B., Stemme S., Kocher O., Gabbiani G. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis. 1988 Aug;72(2-3):135–141. doi: 10.1016/0021-9150(88)90074-3. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Seifert P. S., Olsson G., Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- Hiester A. A., Metcalf D. R., Campbell P. A. Interleukin-4 is chemotactic for mouse macrophages. Cell Immunol. 1992 Jan;139(1):72–80. doi: 10.1016/0008-8749(92)90100-4. [DOI] [PubMed] [Google Scholar]
- Hoffman R. D., Kligerman M., Sundt T. M., Anderson N. D., Shin H. S. Stereospecific chemoattraction of lymphoblastic cells by gradients of lysophosphatidylcholine. Proc Natl Acad Sci U S A. 1982 May;79(10):3285–3289. doi: 10.1073/pnas.79.10.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Htin-Aung T lymphocyte motility toward IL-1 in patients with interstitial lung diseases. Bull Chest Dis Res Inst Kyoto Univ. 1990 Mar;23(1-2):38–47. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Ku G., Doherty N. S., Wolos J. A., Jackson R. L. Inhibition by probucol of interleukin 1 secretion and its implication in atherosclerosis. Am J Cardiol. 1988 Jul 25;62(3):77B–81B. doi: 10.1016/s0002-9149(88)80057-2. [DOI] [PubMed] [Google Scholar]
- Kume N., Cybulsky M. I., Gimbrone M. A., Jr Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest. 1992 Sep;90(3):1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb D. J., Wilkins G. M., Leake D. S. The oxidative modification of low density lipoprotein by human lymphocytes. Atherosclerosis. 1992 Feb;92(2-3):187–192. doi: 10.1016/0021-9150(92)90277-n. [DOI] [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Miossec P., Cavender D., Ziff M. Interleukin 1 derived from human endothelial cells enhances the binding and chemotactic step of T lymphocyte emigration. Clin Exp Immunol. 1988 Aug;73(2):250–254. [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., van der Walt J. D., Munro C. S., Chalmers J. A., Cox E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987 Apr;18(4):375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., FLOREY H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958 Apr;75(2):245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Quinn M. T., Schwenke D. C., Carew T. E., Steinberg D. Oxidative modification of beta-very low density lipoprotein. Potential role in monocyte recruitment and foam cell formation. Arteriosclerosis. 1989 May-Jun;9(3):398–404. doi: 10.1161/01.atv.9.3.398. [DOI] [PubMed] [Google Scholar]
- Portman O. W., Alexander M. Lysophosphatidylcholine concentrations and metabolism in aortic intima plus inner media: effect of nutritionally induced atherosclerosis. J Lipid Res. 1969 Mar;10(2):158–165. [PubMed] [Google Scholar]
- Quinn M. T., Kondratenko N., Parthasarathy S. Analysis of the monocyte chemotactic response to lysophosphatidylcholine: role of lysophospholipase C. Biochim Biophys Acta. 1991 Apr 3;1082(3):293–302. doi: 10.1016/0005-2760(91)90205-v. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Endothelial cell-derived chemotactic activity for mouse peritoneal macrophages and the effects of modified forms of low density lipoprotein. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5949–5953. doi: 10.1073/pnas.82.17.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Steinberg D. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S. M., Parthasarathy S., Steinberg D. Evidence for a dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J Lipid Res. 1991 Mar;32(3):449–456. [PubMed] [Google Scholar]
- Stemme S., Holm J., Hansson G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992 Feb;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Stemme S., Jonasson L., Holm J., Hansson G. K. Immunologic control of vascular cell growth in arterial response to injury and atherosclerosis. Transplant Proc. 1989 Aug;21(4):3697–3699. [PubMed] [Google Scholar]
- Stemme S., Rymo L., Hansson G. K. Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest. 1991 Dec;65(6):654–660. [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]



