Abstract
Maternal cardiovascular adaptations occur in normal pregnancy, systemically and within the uterus. In humans, gestational control of blood pressure is clinically important. Transient structural remodeling of endometrial spiral arteries normally occurs in human and mouse pregnancies. In mice, this is dependent on uterine Natural Killer cell function. Using normal and immune deficient mice, we asked whether spiral artery remodeling critically regulates gestational mean arterial pressure and/or placental growth. Radiotelemetric transmitters were implanted in females and hemodynamic profiles to a dietary salt challenge and to pregnancy were assessed. Implantation sites from non-instrumented females were use for histological morphometry. Both normal and immune deficient mice had normal sensitivity to salt and showed similar five-phase gestational patterns of mean arterial pressure correlating with stages of placental development, regardless of spiral artery modification. After implantation, mean arterial pressure declined during the pre-placental phase to reach a mid-gestation nadir. With gestation day 9 opening of placental circulation, pressure rose, reaching baseline before parturition while heart rate dropped. Heart rate stabilized before parturition. Placental sizes deviated during late gestation when growth stopped in normal mice but continued in immune deficient mice. As an indication of the potential for abnormal hemodynamics, two pregnant females delivering dead offspring developed late gestational hypertension. This study characterizes a dynamic pattern of blood pressure over mouse pregnancy that parallels human gestation. Unexpectedly, these data reveal that spiral artery remodeling is not required for normal gestational control of blood pressure or for normal placental growth.
Keywords: Blood pressure monitoring, Hemodynamics, Lymphocytes, Mice, Pregnancy, Remodeling
Background
Well defined, systemic cardiovascular adaptations occur during normal pregnancy. These include significant increases in heart rate, cardiac output and blood volume. These changes often accompany physiological cardiac hypertrophy which, in non-pregnant individuals, would lead to hypertension. In pregnancy, however, despite blood volume gain, mean arterial pressure (MAP) is maintained or declines. This gestational anti-hypertensive mechanism is postulated to involve systemic vasodilation as well as transient insensitivity of the vasculature to vasoactive substances (such as products of the renin-angiotensin system; RAS). Hemodynamic alterations occur during the 1st trimester of human pregnancy, prior to completion of placental development and are thought to be critical for the support of the high metabolic demands of a growing fetus.(1)
Regional and local structural changes that ensure adequate perfusion of the early maternal-fetal interface are also required for pregnancy success. These are less well defined. In humans, rats and mice, uterine arteries and veins undergo substantial outward circumferential remodeling. Coincidently, the endometrial vascular bed enlarges via vasculogenesis and angiogenesis, supporting endometrial decidualization and uterine enlargement. Spiral arteries (SA), the major vessels participating in endometrial remodeling at implantation sites, arise from the uterine arteries. SA begin as high-resistance, low-capacity arteries and remodel to low-resistance, high-capacity venous-like structures.(2;3) In women, SA remodeling is initiated by invasive trophoblast and uterine Natural Killer (uNK) cells and is completed by mid-gestation.(3;4) In mice, uNK cells are solely responsible for SA modification.(5) UNK cells are a specialized, terminally-differentiated NK cell subset that are poorly cytotoxic but secrete large amounts of angiogenic, chemotactic and inflammatory cytokines.(6)
Important human pregnancy complications are linked with inadequate local vascular adaptations including fetal loss,(7) intrauterine growth restriction, gestational hypertension and preeclampsia (PE).(8;9) We sought to determine the relative importance of physiological remodeling of SA on systemic hemodynamics of pregnant mice, using continuous assessment by radiotelemetry and on growth of the placenta subsequent to SA remodeling. We compared hemodynamic and placental morphometric data from normal mice to NK cell- T cell- B cell-(Rag2−/−γc−/−; immune deficient) mice that lack SA remodeling. Neither gestational hypertension nor deficient placental growth was an outcome of impaired SA remodeling.
Methods
Animals
C57BL/6J mice (B6) were purchased from the Jackson Laboratory (Bar Harbor, ME) and conventionally housed. BALB/cAnNCrl mice (BALB/c) were purchased from Charles River Canada (St. Constant, QU) and housed under barrier husbandry. BALB/c-Rag2−/−γc−/− were bred under barrier husbandry at Queen’s University from pairs generously donated by Dr. Mamuro Ito, Central Institute for Experimental Animals, Kawasaki, Japan. All mice had free access to food and water and entered studies at 10 weeks of age. Animal usage complied with protocols approved by Queen’s University’s Animal Care Committee.
Telemetry Implant Surgery and Data Acquisition
TA11PA-C10 radiotransmitters (Data Sciences International; DSI, St. Paul, MN) were implanted via the common carotid, as reported by others.(10) Briefly, weighed virgin females were anesthetized with isoflurane. The cervical ventral midline was incised (2cm) and the submandibular glands were separated with sterile cotton swabs. The left common carotid artery was visualized, retracted and temporarily occluded with a microvessel clamp. The artery was punctured with a 26-gauge needle, the catheter tip of the transmitter was advanced to the aortic arch using cannulation forceps, and the catheter was sutured in place. A subcutaneous pocket was excavated over the right flank, the transmitter body was inserted into this and the incision was closed (4–0 Vicryl). Mice were individually housed and received buprenorphine (0.1mg/kg, bid s.c. as required). At day 10 after surgery, continuous, 24-hr data collection began using the Dataquest A.R.T.™ Acquisition System (DSI, version 4.1). Collected parameters were MAP, systolic arterial pressure (SAP), diastolic arterial pressure (DAP), heart rate (HR), pulse pressure (PP) and activity.
Study Protocol
Hemodynamic data were collected for 30 seconds every four minutes. For the salt challenges, immune competent controls (allogenic B6, n=5; congenic BALB/c, n=4) and Rag2−/−γc−/− (n=4) were fed four days each with diets containing normal salt (0.67%), low salt (0.43%), high salt (8.5%), then normal salt (Purina, Richmond, IN). Food consumption, water intake and body weight were measured daily. Then, females were selected for estrus and paired with genetically-matched males for timed matings. No data were collected during mating. At copulation plug detection, males were removed and pregnancy dated as gestation day (gd) 0.5. Mated females (B6, n=4; BALB/c, n=5; and Rag2−/−γc−/−, n=9) were recorded until birth when neonates were removed.
Histology
For placental morphometry, non-transmitter implanted Rag2−/−γc−/− (n=3/timepoint) and immune competent BALB/c (n=3/timepoint) females were deeply anesthetized at gd10, gd12, gd14, gd16 and gd18 using tribromoethanol (250mg/kg) prior to euthanasia (cervical dislocation) and dissection. For spiral artery morphometry, other gd12 females were perfused with 30mL 4% neutral buffered paraformaldehyde (PFA; Sigma-Aldrich, Oakville, ON, Canada) with 0.1M sucrose (Sigma) pH7.4. Uteri were removed after 30 minutes. Tissues were fixed (non-perfused samples) or post-fixed (perfused samples) 6 hr in PFA, rinsed in 70% ethanol, processed automatically into paraffin and embedded using routine methods.(11) Implantation sites were serial sectioned at 7μm, stained with H&E. To estimate placental size, three placentas per dam were scored, 15 sections from each placenta; selecting the centre (largest) section and moving outward. SA were scored as previously described.(12) Lumen diameters (LD) were derived from circumferential (C) determinations for each vessel cross-section (LD=C/π). Wall thicknesses were calculated by (wall diameter – LD)/2. Calculations were made using Zeiss AxioVision Software (V4.6, Oberkochen, Germany).
Statistics
Data were analyzed using Prism 4.03 Statistical Software (GraphPad, San Diego, CA), and are presented as means ± SEM. All hemodynamic data were analyzed using 24-hr means. Salt challenge data were analyzed using the lowest day MAP for each animal on low salt and the highest day MAP on high salt. Because MAP from gd0-3 was stable in preliminary studies, this MAP was averaged to define baseline and each subsequent gd was compared to obtain the delta value. Data were analyzed by paired 1-way repeated measures ANOVA with Dunnett’s post hoc test within strain and 1-way ANOVA with Bonferroni’s post hoc test between strains. When sample sizes were unequal, Bartlett’s correction was used. Morphometric measurements were compared between groups using 2-tailed t-tests. P<0.05 was considered significant.
Results
Dietary Salt Challenge
Baseline MAP was not different between B6 (109.1±1.26 mmHg), BALB/c (113.0±1.41 mmHg) or Rag2−/−γc−/− mice (116.4±1.28 mmHg). Dietary salt challenge indicated that arterial pressures of unmated females of each genotype were similarly sensitive to changes in dietary salt. All mice showed a decrease in MAP with low salt that did not differ between genotypes: B6 −4.8%±0.88 mmHg; BALB/c −4.8%±1.31 mmHg; Rag2−/−γc−/− −5.7%±1.74 mmHg. Similarly, the three strains exhibited increases in MAP with salt loading; B6 8.3%±1.30 mmHg; BALB/c 7.1%±1.95 mmHg; Rag2−/−γc−/− 4.5%±0.75 mmHg, P>0.05 between strains. The amplitude of change in MAP between low and high salt diets was not different between B6 and BALB/c or between the genotypes of BALB/c, indicating similar degrees of salt sensitivity: B6 10.2±1.58 mmHg; BALB/c 13.3±1.70 mmHg; Rag2−/−γc−/− 11.5±2.18 mmHg. Further, no hemodynamic differences were observed between virgin females of these genotypes in any other parameters (SAP, DAP, HR, PP or activity; not shown).
Gestational Studies-Spiral Arterial and Placental Morphometry
We previously reported that pregnant B6 and BALB/c experience mid-gestational SA remodeling whereas B6-Rag2−/−γc−/− do not.(13) To confirm that pregnant BALB/c-Rag2−/−γc−/− lack remodeled SA, gd12 histological studies were undertaken. For remodeled SA in BALB/c females, the wall:lumen ratio was nearly half that observed in Rag2−/−γc−/− (0.21±0.009 vs 0.39±0.020, P<0.0001, Figure 1A). In BALB/c mice, SA lumens were dilated to 90.3±3.57μm (Figure 1B) compared to 79.1±2.72μm (P<0.05) in Rag2−/−γc−/− mice, (12.4% narrower). Vessel walls in BALB/c were thin with minimal smooth muscle (wall thickness, 10.1±0.40μm; Figure 1C) compared with Rag2−/−γc−/−, with thicker muscle walls (16.2±0.55μm; P<0.0001) that contained extracellular matrix deposits. Based on Poiseuille’s Law (R=1/r4), the impact of the lack of SA remodeling on vascular resistance (R) was calculated to be ~1.7 fold greater in Rag2−/−γc−/− SA than in gd-matched immune competent BALB/c.
Figure 1.
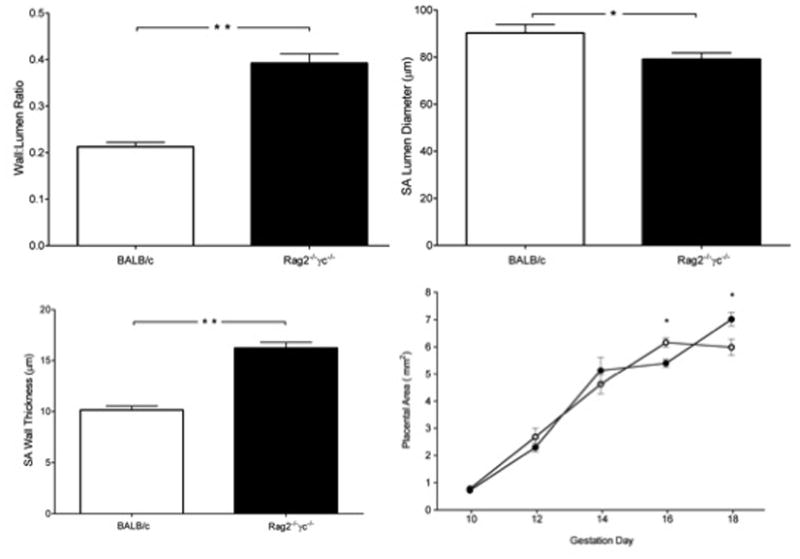
Spiral artery modification in immune competent (white bar; n=3) and Rag2−/−γc−/− mice (black bar; n=3) at gd12. A) Spiral artery wall thickness to arterial lumen ratio, B) spiral artery lumen diameters and C) spiral artery wall media thickness. D) Placental area over gestation in immune competent (open symbols; n=3 per gestation day) and Rag2−/−γc−/− mice (closed symbols; n=3 per gestation day). Data is presented as mean ± SEM. *P<0.05, **P<0.001.
To determine if differences in SA modification might alter placental growth and thus confound our planned hemodynamic study, a time course study of placental cross-sectional areas was conducted comparing Rag2−/−γc−/− and BALB/c mice. At gd9-9.5, placental development is completed by fusion of the allantois to the chorion (trophoblast component), establishing fetal circulation within the placenta. Thus, mid-sagittal placental surface areas were measured from gd10-18 (Figure 1D). In both strains, placental growth was equivalent and linear from gd10-14. At gd16, placental surface area was greater in BALB/c than in Rag2−/−γc−/− mice (6.16mm2±0.17 vs. 5.40mm2±0.14; P<0.001). Between gd16-18 BALB/c placentas showed no growth while Rag2−/−γc−/− placentas exhibited further growth. At gd18, BALB/c placentas were smaller than those of Rag2−/−γc−/− (5.99mm2±0.29 vs. 7.02mm2±0.26; P<0.01).
MAP Profile of Pregnant Immune Competent Mice with Gestational SA Modification
We employed two strains of immune competent mice; B6 to enable comparisons with data published by others and BALB/c, congenic to the experimental immune deficient mice. Continuous data collection enabled detection of subtle hemodynamic changes. Following mating, no difference was detected between pre-pregnancy baseline MAP and that of gd0-3 (pre-implantation) for B6 or BALB/c mice. Thus, a gd0-3 MAP was calculated for each group and used as its baseline value for later gestational comparisons. For both strains, MAP remained constant until gd5 then declined, reaching a nadir at gd9 (Figure 2A, B). MAP returned to baseline by gd12 and remained stable until peri-partum (gd18+). Continuous data are only presented to gd18 due to variation in the timing of parturition between females. The final day of study that included parturition and maternal care until 7AM are presented as (P) in the figures. Statistical comparisons are not reported for the 24hr preceding parturition. MAP values were not statistically different between B6 and BALB/c at any gd. Further, there were no statistically significant differences in SAP, DAP, PP, HR and activity between these strains (data not shown). Thus, B6 and BALB/c data were pooled as the immune competent control for comparisons with gestational data from the immune deficient mice.
Figure 2.
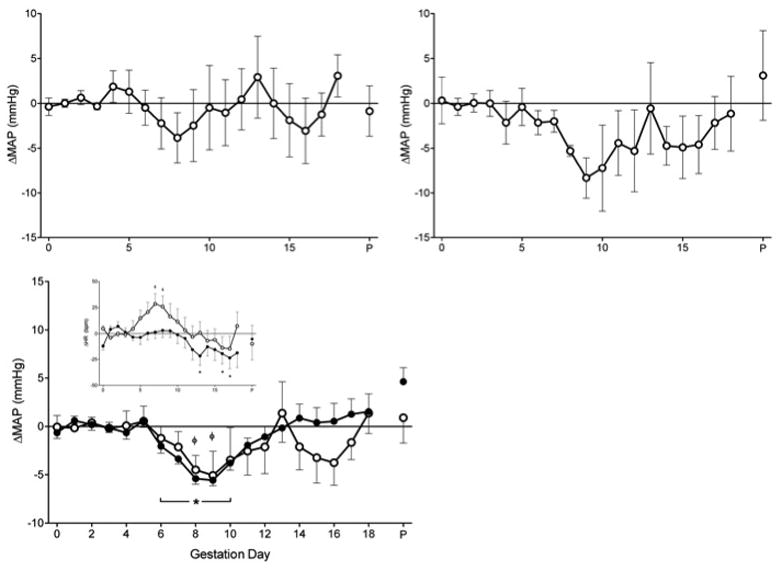
ΔMAP and HR of mice across gestation. A) ΔMAP of B6, n=4 and B) BALB/c, n=5. C) ΔMAP of immune competent mice (n=9; open circles) and Rag2−/−γc−/− mice (n=9; closed circles); D) ΔHR across gestation. No difference observed between groups for gd0-3 baseline MAP for BALB/c (113.3±1.19 mmHg), B6 (114.0±3.78 mmHg) and Rag2−/−γc−/−(112.6±0.90 mmHg) mice or HR for BALB/c (595.1bpm±10.13), B6 (596.5bpm±17.05) or Rag2−/−γc−/− (613.4bpm±10.96) mice. Values are represented as mean ± SEM of 24hr continuous recordings. ΦP<0.05 vs. baseline within immune competent group, *P<0.05 vs. baseline within Rag2−/−γc−/− group.
MAP Profile in Pregnant Immune Deficient Mice Lacking SA Modification
Pregnant Rag2−/−γc−/−mice exhibited a nearly identical MAP profile to that of immune competent mice (Figure 2C). Specifically, in pregnant Rag2−/−γc−/−, MAP was stable until gd5, after which a small but statistically significant decline in MAP occurred between gd6 and 9 (gd6; −2.04±0.72, gd9; −5.57±0.57, P<0.01). MAP then increased back towards baseline by gd12 and was relatively stable until just before parturition. No statistically significant differences were found in MAP profiles between Rag2−/−γc−/− andimmune competent mice.
Heart Rate during Mouse Pregnancy
Figure 2D presents gestational HR profiles for immune competent strains and Rag2−/−γc−/− mice. The HR pattern of pregnant immune competent mice was relatively constant to gd4, rose (+25-30bpm) to peak values between gd7-9, then declined until parturition. HR was slightly lower in Rag2−/−γc−/− than in immune competent mice (significantly different only at gd7, P<0.05). Despite this, HR declines similarly in both groups beginning at mid-gestation. This decrease in HR from is −25bpm in both groups during late gestation. The decline in Rag2−/−γc−/− HR was significant compared to baseline at gd13, 16 and 17 (P<0.05).
Systolic, Diastolic and Pulse Pressures during Mouse Pregnancy
The general trend of gestational profiles of SAP and DAP in immune competent and Rag2−/−γc−/− were similar to MAP (Figure 3A, B). For immune competent mice, SAP declined significantly at gd8-9 (−6-7mmHg; P<0.01), but returned to baseline by gd12. Changes in DAP were of less magnitude at these times. For Rag2−/−γc−/−, SAP declined significantly. At gd9 SAP had dropped 6–7 mmHg (P<0.001) while DAP had fallen 4.50±0.51 mmHg (P<0.001). As in immune competent mice, both values returned to baseline at gd12. There were no differences in SAP or DAP between immune competent and Rag2−/−γc−/− mice at any time point.
Figure 3.
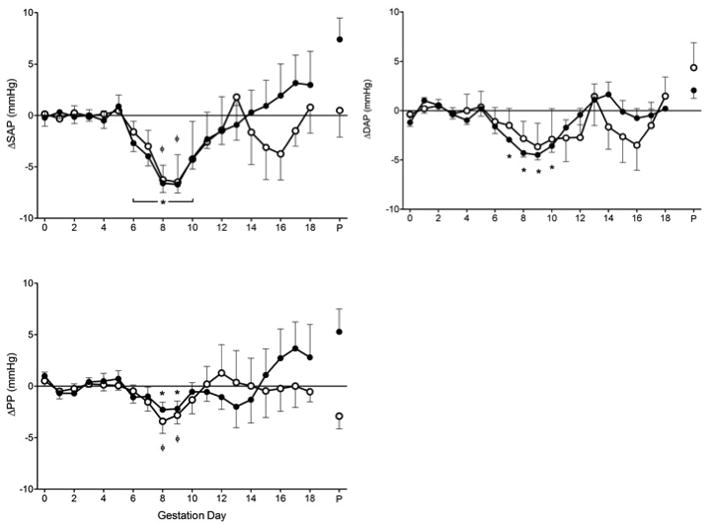
A) ΔSAP, B) ΔDAP and (C) Δ pulse pressure (PP) of immune competent (n=9; open circles) and Rag2−/−γc−/− mice (n=9; closed circles) across gestation. No differences observed for gd0-3 baseline for SAP, DAP or PP between immune competent (126.7±2.60 mmHg; 100.0±2.33 mmHg; 26.6±3.24 mmHg) and Rag2−/−γc−/− (127.1±1.28 mmHg; 97.0±0.98 mmHg; 30.1±1.34 mmHg) mice. Values are represented as mean ± SEM of 24hr continuous recordings. ΦP<0.05 vs. baseline within immune competent group, *P<0.05 vs. baseline within Rag2−/−γc−/− group.
PP, a more recent predictor of cardiovascular risk, had a similar pattern in immune competent and Rag2−/−γc−/− mice (Figure 3C). In general, early gestational PP was stable; there was a small, significant decline (2–3 mmHg) between gd8-9, and a return to baseline by gd12. No differences between groups were detected at any time point.
Activity during Mouse Gestation
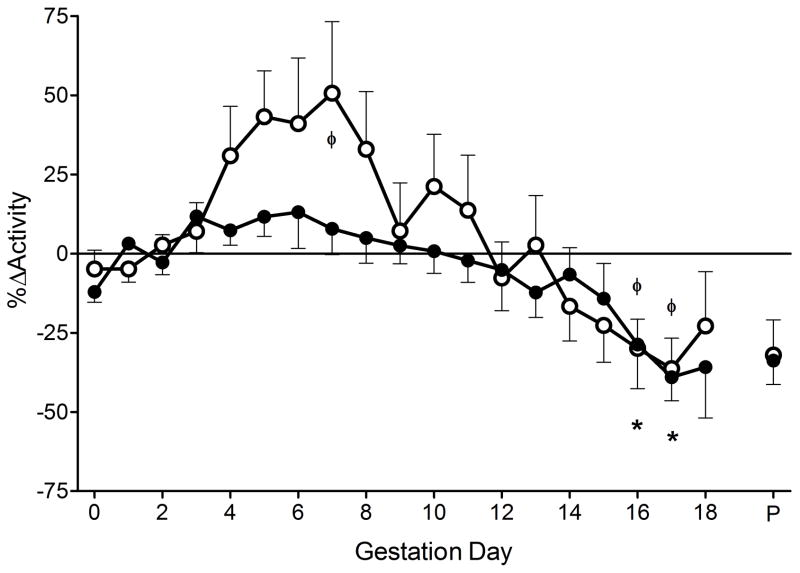
Others have demonstrated that mutations in the mouse Rag2 gene result in fatigue and lower activity than in control animals. (14) In our immune competent mice, activity was relatively constant during the gd0-3 baseline then rose to a peak between gd5-7 (Figure 4). Following this, activity declined slowly, passing baseline at gd12, and reaching a statistically significant lower level at gd16-17 (−30%; P<0.05). This pattern is identical to that of HR in immune competent mice, coincident with the timing of increased HR and activity. Rag2−/−γc−/− mice showed less activity early in pregnancy then it declined at a similar rate from gd8 as in immune competent mice to reach −40% at gd16-17 (P<0.001).
Figure 4.
Percent change in activity of immune competent (n=9; open circles) and Rag2−/−γc−/−mice (n=9; closed circles) across gestation. No gd0-3 baseline activity differences observed between immune competent (9.5±1.38) and Rag2−/−γc−/− (11.3±1.22) mice. Values are represented as mean ± SEM of 24hr continuous recordings. ΦP<0.05 vs. baseline within immune competent group, *P<0.05 vs. baseline within Rag2−/−γc−/− group.
Reproductive Outcomes and Complications
Birth data for the animals in this study, including pups found dead, were 4.9±0.7 Rag2−/−γc−/− pups (15 litters), and 4.6±0.8 BALB/c pups (5 litters), P>0.05. Rag2−/−γc−/− pup weights averaged 1.48g, which is within normal range for congenic pups (currently, no comparative syngeneic data exist). Previous studies report BALB/c pup weights as 1.39g for 9 litters (15) and 1.53g for 11 litters.(16)
In addition to surgical and equipment failures, reproductive complications occurred in radiotransmitter implanted females. As in other mouse populations, some females failed to copulate while others failed to conceive after mating. The latter are endocrinologically pseudopregnant until day 7 after mating, when estrus returns. The pseudopregnancy MAP profile was compared to pregnant Rag2−/−γc−/− mice to gd11, when pseudopregnancy was confirmed by absence of weight gain or palpable fetuses (Figure 5A). In pseudopregnancy, MAP was stable, suggesting that post-implantation conceptuses rather than hormonal changes (i.e. ovarian effects without a conceptus) account for the early gestational drop in MAP of pregnant mice (P<0.05).
Figure 5.
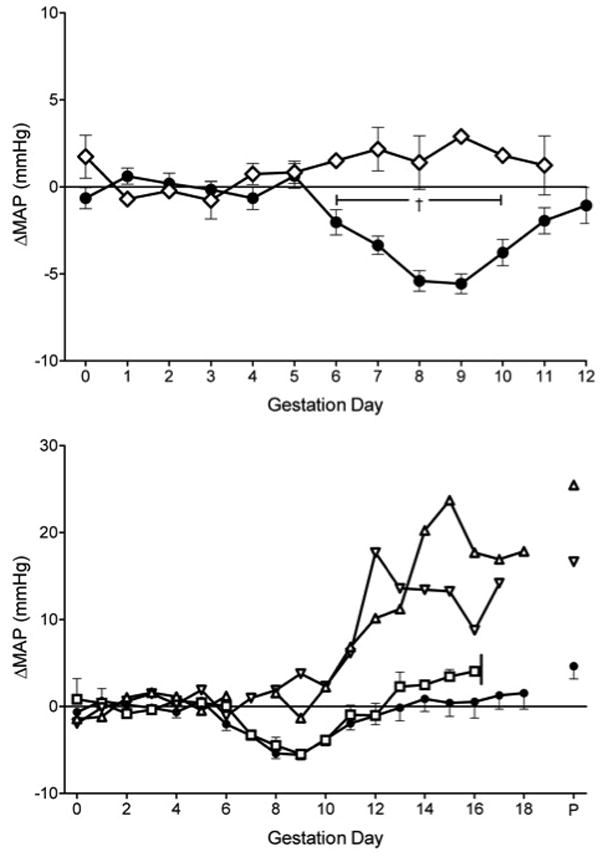
Effect of gestational complications on MAP in radiotransmitter-implanted mice compared to Rag2−/−γc−/− pregnant mice with normal outcomes, (n=9; closed circles). A) ΔMAP profile of pseudopregnant mice (n=3; open diamonds), B) ΔMAP of mice that experienced dystocia beginning at gd16 (n=2; open squares) and mice who gave birth to dead pups (open triangles). Mean ± SEM of 24-hour continuous recordings. †P<0.05 between groups.
Dystocia, the inability to vaginally deliver newborns at term, was seen in two radiotransmitter implanted Rag2−/−γc−/− females. Loss of condition and mobility onset acutely at gd16-17. These distressed females were euthanized and examined. The litters were 5 and 10 and all fetuses, including the ones entered into the pelvic inlet, were of normal size and viable. These females had identical hemodynamic profiles to Rag2−/−γc−/− mice with normal pregnancy (Figure 5B), suggesting there is no prodromal hemodynamic event to dystocia.
Two additional Rag2−/−γc−/− gave birth to small litters of dead pups. One female gave birth to a single stillborn while the other gave birth to one viable and two dead pups. MAP profiles for these animals were distinctly different and showed hypertension in later gestation (Figure 5B). No etiology for these late gestation deaths was found by histological or bacterial analyses (Laboratory Animal Pathology, University of Guelph, Guelph, ON).
Discussion
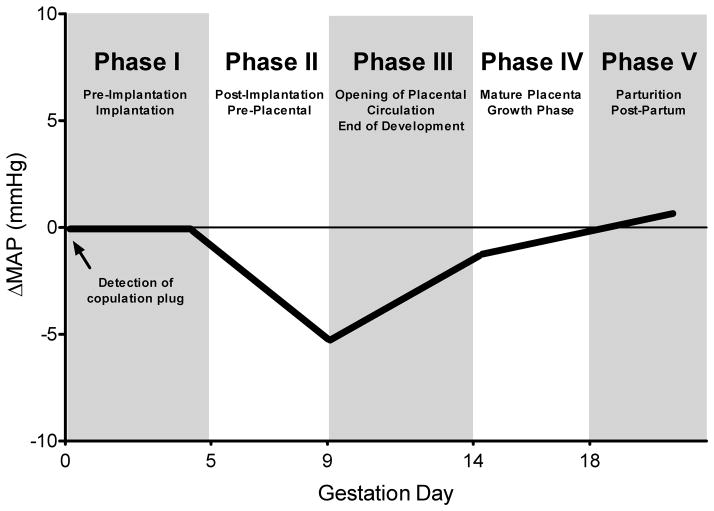
This study identified a distinct, five-phase pattern of arterial pressure in successful pregnancies of two distinct strains of normal, immune competent mice that have SA remodeling (Figure 6). Phase I of the pattern had stable arterial pressures with pre-pregnancy baseline hemodynamic values. This gd0-5 interval is the stage of pre-implantation development that ends with gd3.75 blastocyst hatching and implantation at ~gd4.0. During phase II (gd5-9), maternal MAP declined, corresponding to the interval of pre-placental conceptus development. By this stage, blastocyst trophoectoderm has differentiated into the placental primordium called ectoplacental cone and endometrial decidualization has occurred on the anti-mesometrial side of the uterus. Decidualization then spreads to the mesometrial side where, at gd6, uNK cells suddenly appear in abundance as rapidly dividing cells.(17) A decline in MAP during this early post-implantation/pre-placental stage has not been previously delineated in rodents but is known in women.(1) Phase III onset at gd9, extended to gd14, and coincided with histologically-recognized remodeling of SA in immune competent mice. This is also an interval of placental growth.(18–20) During Phase III, MAP rose gradually back towards the pre-pregnancy baseline while HR declined. In Phase IV (gd14-18), a time of rapid fetal growth after placental maturation, these patterns of change continued at a more modest rate.(18–20) Phase V covered the peri-partum interval, which was not remarkably distinct.(21) Similar profiles were found during radiotelemetry monitored 2nd pregnancies in study animals (data not shown).
Figure 6.
Representative profile for mean arterial pressure (MAP) of normal mice across gestation showing a 5-phase, placental development related pattern.
Unexpectedly, the pattern of gestational MAP in Rag2−/−γc−/− mice, which do not undergo SA remodeling, was indistinguishable from normal mice. Although we estimated that the lack of remodeling of individual spiral arteries increased vascular resistance in Rag2−/−γc−/− implantation sites by 60% relative to those in normal females, pregnant Rag2−/−γc−/− females had no predisposition towards hypertension, placenta growth restriction or fetal loss. This suggests that under normal conditions, the upstream maternal vasculature can compensate for inadequate SA remodeling without causing placental insufficiency. This outcome likely results from the absence of lymphocyte responses, which, in human placental insufficiency, are thought to promote strong Type 1 inflammatory responses and cell killing. While inadequately remodeled SA may be a predisposing factor to placental insufficiency, it does not itself trigger hypertension. Our data support a novel distinction between SA remodeling versus placental signals as the initiating factor for gestational hypertension. Placental insufficiency and/or conceptus stress signals initiate downstream effectors. Placental tissues release microparticles, angiogenic and vasoactive compounds, chemokines and cytokines (vascular endothelial growth factor; VEGF, placental growth factor; PlGF, soluble fms-like tyrosine kinase-1; sFlt-1), which all act upon the maternal vasculature to restrict the volume of SA flow and further exacerbate placental hypoxic and/or reperfusion injury. This continuing cycle is amplified by the maternal immune system until a severe systemic inflammation manifests as clinical hypertension and proteinuria. The importance of circulating molecules in PE has been found clinically and experimentally; sFlt-1 is higher in PE women prior to clinical signs and it induces PE-like symptoms in rodent models.(22;23) Many uterine cell types express angiogenic and hemodynamic molecules, including human and murine uNK cells. Thus, we expect differences the angiogenic environment of implantation sites in pregnant Rag2−/−γc−/− compared with controls (decreased PlGF and VEGF due to lack of uNK cells and a possible relative elevation in sFlt-1 due to decreased ligand availability).(24)(25) These factors were not assayed in this study, as no differences in primary outcomes (hypertension or placental insufficiency) were detected between strains.
Pregnant Rag2−/−γc−/− females must utilize as yet unknown adaptive responses including alterations in circulatory control pathways or vasculogenesis to provide adequate placental perfusion and conceptus growth. These adaptations may involve systemic changes including increased vasodilatory capacity, particularly of the vasculature upstream of the uterine vascular bed. This would permit decreased arterial resistance upstream of non-remodeled SA. We postulate that due to immune deficiency, Rag2−/−γc−/− mice do not have damaging responses to hypoxia or oxidative stress and thus retain greater organ and endothelial cell function during pregnancy. Studies are in progress to validate these concepts using micro-ultrasonography and immunohistochemical detection of tissue oxygenation. The as yet undefined, lymphocyte-independent adaptive responses of pregnancy must be highly successful because the sizes of the placentae of Rag2−/−γc−/− fetuses just prior to birth exceeded those of gd-matched congenic fetuses and Rag2−/−γc−/− pups had no evidence of growth restriction.
Chapman et al. monitored hemodynamic parameters of healthy cycling women who became pregnant and reported several 1st trimester time points. They found a decline in gestational MAP earlier than had been previously appreciated.(1) A nadir in MAP occurred at 8 weeks, prior to opening of the placental circulation.(26) Arterial pressure in women remained low at 12 weeks, when the uteroplacental circulation opens, then increased but remained below or near baseline. This pattern is similar to what we describe here in mice and supports the hypothesis that in both species, the hemodynamic changes in pregnancy are integrated temporally with specific events in placental development.(27) We postulate that in both women and mice, systemic blood pressure drops prior to opening of the uteroplacental circulation to enhance local hypotension and protect fragile new developing decidual capillaries, vascular structures and nascent fetal villi. When the placental circulation opens, the maternal vessels are mature and the placental tissue has matured to withstand higher pressures and makes greater metabolic demand. During late gestation, when placental blood flow is high, minor variation is seen in MAP between groups (~gd14-term). This variation, which is not marked, may be due to differences in placental growth and metabolic demand. The concept that placental development regulates gestational MAP is further supported by the stable profiles obtained from mated females that did not conceive. This observation shows hormones do not trigger the early drop in gestational MAP but does not assess the role of endometrial decidualization.
Others have examined the patterns of MAP during rodent pregnancy. Gestational hemodynamic data from radiotelemetric studies have been reported for normal rats(28;29) and normal B6 mice(30;31) but none of these publications provide fully characterized hemodynamic profiles for normal animals, particularly using continuous radiotelemetry. Nishizawa et al. compared SAP and DAP using indirect tail-cuff measures between syngenically (B6 × B6) and allogeneically mated (B6 x BALB/c) mice from gd7-17. Their control groups that received twice daily placebo injections from gd6 showed drops in pressures between gd7 and gd10 followed by rebound to baseline for both groups and continued to decline for DAP in the syngeneically-mated group. However, these changes were not indicated as significantly different.(32) Zenclussen et al. employed tail-cuff recording on alternate days in a study of BALB/c mice receiving transfers of either unstimulated or type 1 cytokine-activated T cells.(33) In non-pregnant females, neither treatment altered MAP. In pregnant females receiving non-activated cells, MAP was reported as stable between mating and gd14. In contrast, females receiving cytokine activated (i.e. non-antigen specific) T cells at gd9 and gd12 increased MAP by 93 mmHg at gd13 and almost half of the fetuses were resorbing. Our studies reveal that the fetal loss induced in this model complicates the resolution of immune from non-immune mediated effects on MAP. Our finding that two Rag2−/−γc−/− females became hypertensive prior to delivering small litters of dead pups implies that resorbing/dead fetuses may have a dramatic effect on MAP as early as mid-gestation. This finding again implies that conceptus tissue affects maternal hemodynamic control during pregnancy.
Because there is limited pathology subsequent to the deficit in SA modification and normal blood pressure regulation in Rag2−/−γc−/− pregnancy, a central role for the maternal immune system in the pathogenesis of PE can be postulated. Lymphocytes, through expression of a functional RAS, have been linked with generation of hypertension in non-pregnant rodents and humans. Human T and NK cells proliferate in vitro and modulate their cytokine production response to Ang II. In contrast, animal studies have addressed effects from T and B cell but not NK cell deficiencies.(34) For example, in radiotelemetric studies of non-pregnant Rag-1−/− mice (T−, B−, NK+; sex not stated), Guzik et al. found absence of T cells blunted the ability to induce hypertension by challenge with Ang II or dietary salt. Adoptive transfer of purified T cells but not B cells restored the Ang II response.(35) The T cells’ role was attributed to production of Tnfa. The recent development of a genetically-defined T+, B+, NK- mouse will now permit similar studies addressing the functional roles of RAS in NK cells.(36) However, it is predicted from our experiments that immune responses to placental signals are critical amplifiers of pathogenic processes leading to pregnancy complications.
Perspectives
Our overall definition of the gestational pattern of MAP in normal mice and its linkage to stages of placental development is the major contribution of this study. By focusing on changes in human gestational MAP at times of specific developmental events (such as presence or absence of change in MAP at the time of opening of the placental circulation, or days or rate of gain to achieve pre-pregnancy baseline MAP), it may be possible to more specifically identify the time at which a developmental programming errors onset that then trigger clinically significant complications. This temporal characterization should also aid in identification of causation. We also provide clear evidence that normal SA remodeling is not essential for normal pregnancy outcomes in mice. Further, we have provided the pregnant Rag2−/−γc−/− mouse as a hemodynamically, well-defined animal platform lacking SA remodeling but having normal placental growth. This makes it an ideal animal for subsequent reconstruction studies in which specific molecules, such as Tnfa, sFlt-1, PlGF or the AT1 agonist antibodies, molecules that are postulated to have major roles in pregnancy complications, can be independently and incrementally assessed for induction of hypertension, placental pathology and fetal loss.(29) This platform also potentially permits assessment of highly targeted therapeutic approaches.
Acknowledgments
We thank Ms. Corry Smallegange for her support of our mouse surgery and Drs. Colin D. Funk and John T. Fisher for their generous access to equipment necessary for completion of these studies.
Funding Sources
This research was supported by the Natural Sciences and Engineering Council, Canada (NSERC), Canadian Institute of Health Research (CIHR), the Canadian Foundation for Innovation and the Heart and Stroke Foundation of Ontario (T-6140). S.D.B. is supported by a Canada Doctoral Award from CIHR. B.A.C. is supported by the Canada Research Chairs Program.
Footnotes
Disclosures
The authors disclose no conflicts of interest.
Reference List
- 1.Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D, Johnson A, Osorio F, Goldberg C, Moore LG, Dahms T, Schrier RW. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney Int. 1998;54:2056–2063. doi: 10.1046/j.1523-1755.1998.00217.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Dong H, Wang B, Zhu S, Croy BA. Dynamic changes occur in patterns of endometrial EFNB2/EPHB4 expression during the period of spiral arterial modification in mice. Biol Reprod. 2008;79:450–458. doi: 10.1095/biolreprod.108.067975. [DOI] [PubMed] [Google Scholar]
- 3.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Smith SD, Dunk CE, Aplin JD, Harris LK, Jones RL. Evidence for Immune Cell Involvement in Decidual Spiral Arteriole Remodeling in Early Human Pregnancy. Am J Pathol. 2009;174:1959–1971. doi: 10.2353/ajpath.2009.080995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev. 2006;214:161–185. doi: 10.1111/j.1600-065X.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 7.Tayade C, Fang Y, Hilchie D, Croy BA. Lymphocyte contributions to altered endometrial angiogenesis during early and midgestation fetal loss. J Leukoc Biol. 2007;82:877–886. doi: 10.1189/jlb.0507330. [DOI] [PubMed] [Google Scholar]
- 8.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–1249. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 10.Butz GM, Davisson RL. Long-term telemetric measurement of cardiovascular parameters in awake mice: a physiological genomics tool. Physiol Genomics. 2001;5:89–97. doi: 10.1152/physiolgenomics.2001.5.2.89. [DOI] [PubMed] [Google Scholar]
- 11.Prophet EB, Mills B, Arrington JB, Sobin LH. Armed Forces Institute of Pathology Laboratory Methods in Histotechnology. Washington DC: American Registry of Pathology; 1992. [Google Scholar]
- 12.Burke SD, Dong H, Hazan AD, Croy BA. Aberrant endometrial features of pregnancy in diabetic NOD mice. Diabetes. 2007;56:2919–2926. doi: 10.2337/db07-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashkar AA, di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192:259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golumbek PT, Keeling RM, Connolly AM. RAG2 gene knockout in mice causes fatigue. Muscle Nerve. 2007;36:471–476. doi: 10.1002/mus.20834. [DOI] [PubMed] [Google Scholar]
- 15.Pal S, Peterson EM, de la Maza LM. A murine model for the study of Chlamydia trachomatis genital infections during pregnancy. Infect Immun. 1999;67:2607–2610. doi: 10.1128/iai.67.5.2607-2610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzas EI, Hollo K, Rubliczky L, Garzo M, Nyirkos P, Glant TT. Effect of pregnancy on proteoglycan-induced progressive polyarthritis in BALB/c mice: remission of disease activity. Clin Exp Immunol. 1993;94:252–260. doi: 10.1111/j.1365-2249.1993.tb03440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peel S. Granulated Metrial Gland Cells. Springer-Verlag; 1989. [DOI] [PubMed] [Google Scholar]
- 18.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187:217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borzychowski AM, Chantakru S, Minhas K, Paffaro VA, Yamada AT, He H, Korach KS, Croy BA. Functional analysis of murine uterine natural killer cells genetically devoid of oestrogen receptors. Placenta. 2003;24:403–411. doi: 10.1053/plac.2002.0924. [DOI] [PubMed] [Google Scholar]
- 20.Zhou YQ, Foster FS, Qu DW, Zhang M, Harasiewicz KA, Adamson SL. Applications for multifrequency ultrasound biomicroscopy in mice from implantation to adulthood. Physiol Genomics. 2002;10:113–126. doi: 10.1152/physiolgenomics.00119.2001. [DOI] [PubMed] [Google Scholar]
- 21.Wong AY, Kulandavelu S, Whiteley KJ, Qu D, Langille BL, Adamson SL. Maternal cardiovascular changes during pregnancy and postpartum in mice. Am J Physiol Heart Circ Physiol. 2002;282:H918–H925. doi: 10.1152/ajpheart.00641.2001. [DOI] [PubMed] [Google Scholar]
- 22.Karumanchi SA, Stillman IE. In vivo rat model of preeclampsia. Methods Mol Med. 2006;122:393–399. doi: 10.1385/1-59259-989-3:393. [DOI] [PubMed] [Google Scholar]
- 23.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 24.Tayade C, Hilchie D, He H, Fang Y, Moons L, Carmeliet P, Foster RA, Croy BA. Genetic deletion of placenta growth factor in mice alters uterine NK cells. J Immunol. 2007;178:4267–4275. doi: 10.4049/jimmunol.178.7.4267. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Tanaka T, Nakamura H, Umesaki N, Hirai K, Ishiko O, Ogita S, Kaneda K. Granulated metrial gland cells in the murine uterus: localization, kinetics, and the functional role in angiogenesis during pregnancy. Microsc Res Tech. 2003;60:420–429. doi: 10.1002/jemt.10280. [DOI] [PubMed] [Google Scholar]
- 26.Burton GJ, Jauniaux E, Watson AL. Maternal arterial connections to the placental intervillous space during the first trimester of human pregnancy: the Boyd collection revisited. Am J Obstet Gynecol. 1999;181:718–724. doi: 10.1016/s0002-9378(99)70518-1. [DOI] [PubMed] [Google Scholar]
- 27.Rossant J, Tam PPL. Mouse Development: Patterning, Morphogenesis, and Organogenesis. Academic Press; 2002. [Google Scholar]
- 28.Buhimschi CS, Gokdeniz R, Saade GR, Buhimschi IA, Garfield RE. The effect of chronic nitric oxide synthase inhibition on blood pressure and heart rate in unrestrained pregnant rats as recorded by radiotelemetry. Am J Obstet Gynecol. 1999;181:159–164. doi: 10.1016/s0002-9378(99)70453-9. [DOI] [PubMed] [Google Scholar]
- 29.Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–746. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- 30.Davisson RL, Hoffmann DS, Butz GM, Aldape G, Schlager G, Merrill DC, Sethi S, Weiss RM, Bates JN. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39:337–342. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann DS, Weydert CJ, Lazartigues E, Kutschke WJ, Kienzle MF, Leach JE, Sharma JA, Sharma RV, Davisson RL. Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension. 2008;51:1058–1065. doi: 10.1161/HYPERTENSIONAHA.107.107219. [DOI] [PubMed] [Google Scholar]
- 32.Nishizawa H, Hasegawa K, Suzuki M, Achiwa Y, Kato T, Saito K, Kurahashi H, Udagawa Y. Mouse model for allogeneic immune reaction against fetus recapitulates human pre-eclampsia. J Obstet Gynaecol Res. 2008;34:1–6. doi: 10.1111/j.1447-0756.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 33.Zenclussen AC, Fest S, Joachim R, Klapp BF, Arck PC. Introducing a mouse model for pre-eclampsia: adoptive transfer of activated Th1 cells leads to pre-eclampsia-like symptoms exclusively in pregnant mice. Eur J Immunol. 2004;34:377–387. doi: 10.1002/eji.200324469. [DOI] [PubMed] [Google Scholar]
- 34.Jurewicz M, McDermott DH, Sechler JM, Tinckam K, Takakura A, Carpenter CB, Milford E, Abdi R. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol. 2007;18:1093–1102. doi: 10.1681/ASN.2006070707. [DOI] [PubMed] [Google Scholar]
- 35.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gascoyne DM, Long E, Veiga-Fernandes H, de BJ, Williams O, Seddon B, Coles M, Kioussis D, Brady HJ. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat Immunol. 2009;10:1118–1124. doi: 10.1038/ni.1787. [DOI] [PubMed] [Google Scholar]