Abstract
Viable human CD56+CD16− peripheral blood Natural Killer (NK) cells show specific in vitro binding under shear forces to ligands expressed by endothelial cells in cryostat sections of gestation day (gd)7 mouse decidua basalis. In serial assays, numbers of cells adhering to gd7 tissue are constant for men but have cyclical variation for fertile women, suggesting a brief gain in functional decidual homing potential of this NK cell subset during the menstrual cycle. Regardless of gender, numbers of adhering cells from an individual donor, increase dramatically when the substrate is decidua basalis from a later gestational timepoint. Here, we report that human blood CD56+CD16− NK cells which adhere as single cells over gd7 decidua basalis, adhere as large clusters over gd8 and gd9 tissues, suggestive of antigen recognition and lymphocyte activation. We asked which cells within mouse decidua basalis trigger this response in CD56+CD16− cells. Using decidua from mice transgenic for myeloid dendritic cell (mDC) expression of enhanced yellow fluorescent protein (eYFP), we found cluster formation was independent of mDC contact. Use of decidua from alymphoid mice showed clustering behavior required substrate lymphocytes. By use of decidua containing NK cells but lacking T and B cells, decidual T and/or B lymphocytes were identified as the cells altered after gd7 in a manner that activates CD56+CD16− cell clustering. This time point is just prior to mouse spiral arterial modification and its detection by these indicator cells implicates adaptive, decidual immune responses in the regulation of NK cell function.
Keywords: Decidua basalis, Lymphocyte adhesion, Lymphocyte activation, Natural Killer cell, Shear force
Introduction
Natural Killer lymphocytes (NK cells), identified by expression of CD56 (neural cell adhesion molecule, NCAM), are ~7–12% of human blood lymphocytes. CD56+ NK cells can be phenotyped into two subsets based on the expression on CD16 (FcγRIII). The first subset, CD56+CD16+ NK cells, comprise ~90% of blood NK cells. The second low abundance subset, CD56+CD16− NK cells, express much more CD56. Because of this difference in the level of CD56 expression, these two subsets are termed CD56Dim and CD56Bright cells, respectively. Another lymphocyte, the NKT cell, co-expresses CD56, CD3 and a T cell receptor and is considered a T cell. While most mammalian NK cells are found in the circulation or spleen, CD56+CD16− NK cells are transiently the predominant lymphocytes in post-ovulatory uterus and persist in decidua during the first half of pregnancy [1, 2]. These cells, called uterine (u) Natural Killer cells, appear with endometrial decidualization in mice and persist to mid gestation and decline numerically once they have established the essential environment for physiological modification of spiral arteries [3]. While the origins of uNK cells are not firmly established, it is hypothesized that at least some traffic from blood to the uterus [4]. They may additionally develop from hematopoietic stem cells that reside in the uterus [5, 6].
The trafficking potential of lymphocytes from circulation into different tissues can be compared using an assay of leukocyte adhesion to cytostat sections of the test tissues under shear forces (Stamper-Woodruff adhesion assay) [7]. This in vitro assay takes advantage of the fact that indicator cells demonstrate specific binding to ligands expressed by endothelial cells of frozen sections of murine substrate tissue. Although this assay does not measure mechanisms of tethering and diapedesis, enumeration of adherent cells has been shown to be indicative of their potential to extravasate. For example, numbers of cells adhering to mouse lymph node high endothelial venules in vitro increase with fever range hyperthermia and have been directly correlated with fever-induced changes in in vivo homing [8–10]. Similarly, T and NK cells from autoimmune diabetic patients show preferential functional adhesion to pancreatic islets in mouse tissue sections, consistent with the pathologic lymphoid cell infiltrates that lead to type 1 diabetes [11]. We previously reported, using gd6-7 mouse implantation sites as substrates, that the adhesion patterns of blood NK cells at ovulation is predictive of successful human embryo implantation [12]. Serial studies of women successfully undergoing fertility treatment showed a rapid loss of CD56+CD16− blood NK cell adhesion to mouse decidua after ovulation that was followed by a second period of adhesive gain up to week 6 of pregnancy, then declined over the next 26 weeks [13]. This prediction of high recruitment of uNK cells between 2–6 weeks of gestation followed by less recruitment potential is consistent with the known time course for CD56+CD16− (u)NK cell appearance in human decidua. In all the above reports, gains in adhesion were linked to gains in functional SELL (L-selectin, CD62L) and ITGA4 (α4-integrin, CD49D) by lymphocytes and gains in their counter-receptors in the substrate tissue [12–15].
Throughout our studies employing mouse decidua as an adhesion substrate, we noted that use of sections from more advanced pregnancies (gd 8–12) led to huge gains in numbers of adherent NK cells from a common blood sample, even when analysed on a single slide [16]. Further, NK cells from the same blood adhered to gd6-7 tissue as single cells but adhered in progressively larger clusters to sections from tissues of more advanced gestational ages [16]. These clusters did not adhere over the fetus or placenta but were found broadly across the decidua basalis, suggesting that a major functional change within the decidua basalis was being detected by the blood indicator lymphocytes. Lymphocyte clustering has been described in intact, antigen activated lymph nodes in organ bath cultures using two-photon confocal imaging or intravital microscopy [17–19]. Clustering of the viable indicator blood lymphocytes on sections of gd8-9 decidua could represent immune cell recognition of DC activated by conceptus antigens since co-localization of these cell types has been reported in early human decidua [20]. Alternately, it could represent onset of a specific change in murine uNK cells as they initiate the process of spiral arterial modification, in endothelia within the decidua, in invasive trophoblast or in other cell types found within decidua. Using genetically altered mice, we undertook this study to attempt to identify the cell type within gd9 mouse decidua basalis responsible for transforming decidua into a substrate competent for induction of clustering behavior in human blood NK cells and to define the subclasses of human NK cells participating in the clustering phenomenon.
Materials and Methods
Mice
Four strains of mice were homozygously mated to provide timed implantation sites; C57BL6, the wild type control; Rag2−/− on the BALB/c background as mice lacking T and B cells; alymphoid Rag2−/−/Il2rg−/− on the BALB/c background as mice lacking NK, T and B cells and a transgenic mouse strain whose mDC express eYFP under control of CD11c [21] on a C57BL6 background. The morning of copulation plug detection was designated gd0. Uteri were collected the mornings of gd6 to 9. All use of mice was approved by Queen’s University Animal Care Committee.
Isolation and staining of human blood NK cells
Fifteen male and female (not selected for menstrual cycle day) healthy adult subjects were recruited to participate in the study and provided informed consents approved by the Queen’s University Health Sciences Human Subjects Research Ethics Board. Participants donated 20 mL of blood that was collected into evacuated vials containing the anti-coagulant acid citrate dextrose (ACD). Mononuclear cells were obtained after density centrifugation over Histopaque 1077 (Sigma, Oakville, ON, Canada). Cells were then washed and labeled with anti-CD56 antibodies conjugated with magnetic beads (CD56 Multisort kit, Miltenyi Biotec, Auburn, CA, USA). Labeled cells were isolated using AutoMACS (Miltenyi Biotec, Auburn, CA, USA) using the “Posselds” program setting. To identify the three CD56+ subsets, a tri-color stain was performed. The NK cells collected from magnetic isolation were labeled with CellTracker Blue CMAC (7-amino-4-chloromethylcoumarin) (Invitrogen, Burlington, ON, Canada), anti-CD16-PE (Beckman Coulter, Mississauga, ON, Canada) and anti-CD3-FITC (Miltenyi Biotec, Auburn, CA, USA) and incubated at 37°C for 30 min. Blocking experiments were also performed by treating cells with function blocking antibodies to SELL (10 μg/mL anti-CD62L; BD Pharmingen, Mississauga, ON, Canada) and ITGA4 (10 μg/mL anti-CD49D; BD Pharmingen, Mississauga, ON, Canada). Cells were then washed and resuspended at 5×105 cells per 100 μL of unsupplemented RPMI media (Sigma, Oakville, ON, Canada) and used for the adhesion assay. For experiments using wild type control tissue, tissue from n=3 mice for each gd (gd6, 7, 8, 9) were used with each gd paired with blood from n=3 individuals (a total of 12 individual blood donors for pairing with wild type mouse tissue). For experiments using tissue from Rag2−/− mice, Rag2−/−Il2rg−/− mice and eYFP mice, gd9 tissue from n=3 mice for each type of mouse were used. The same blood from additional blood donor individuals (n=3) were used for adhesion assays using tissue from Rag2−/− mice, Rag2−/−Il2rg−/− mice and eYFP mice.
Adhesion assay
A modified Stamper-Woodruff Assay [7] was performed using gd6 to 9 mouse uterine tissue [11–15]. Tissue was embedded and frozen in Shandon Cryomatrix (Thermo Scientific, Ottawa, ON, Canada) then cut at 12 μm using a cryostat and melted onto charged glass slides (Fisher, Ottawa, ON, Canada). Tissue sections were encircled using a hydrophobic Dako Pen (DAKO, Mississauga, ON, Canada) and 5×105 lymphocytes in a 100 μL suspension of unsupplemented RPMI medium were applied onto the tissue while rotating on an orbital shaker (60 rpm at 4°C). Once the lymphocytes had been applied, rotation was increased to 112 rpm for 30 min at 4°C. The slides were then rinsed in PBS to remove non-adherent cells, fixed for 30 min in 4% paraformaldehyde, washed, mounted, and coverslipped before being visualized under ultraviolet illumination. Numbers of adherent cells were independently counted in 25 high power fields (HPF) by two people and the counts were averaged.
Histology
Standard hematoxylin and eosin (HE) staining was done on a cryosection from each of the implantation site tissue blocks used for the adhesion assays. These sections were photographed for reference alignment with the fluorescent images using Zeiss Axiomat and image analysis software.
Statistics
One way ANOVA on ranks was used to analyze data from adhesion experiments. Tukey’s test was used to further identify which groups differed from others. A value of p<0.05 was considered significant.
Results
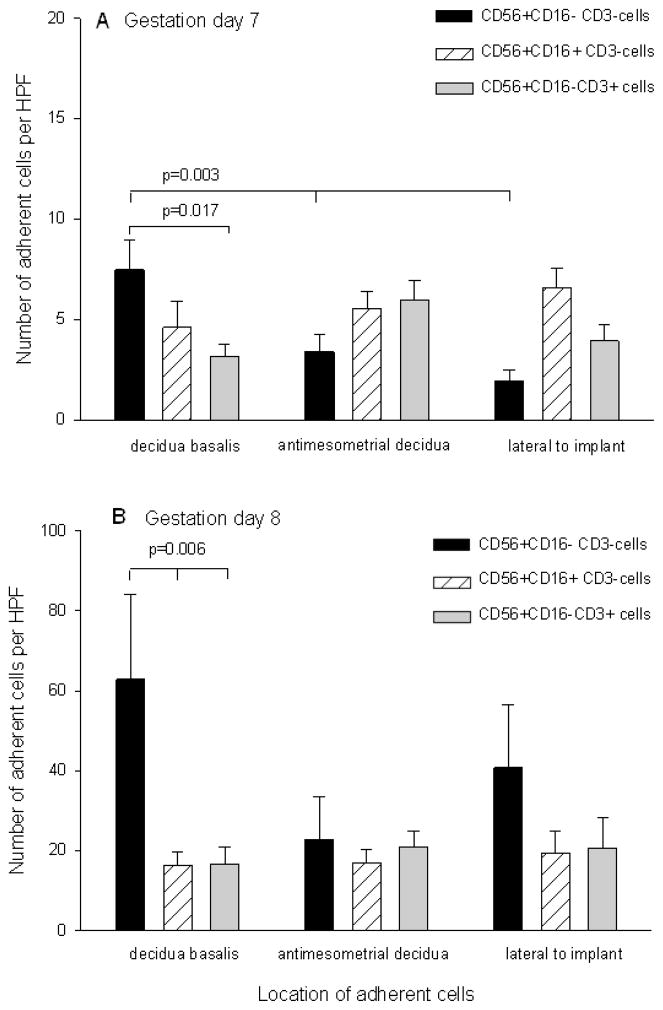
We first sought to determine which subsets of NK cells were adherent to mouse implantation sites on gd7, 8 and 9. Figure 1 shows the number of adherent cells as subdivided by cell subset to each area of the implantation site. Black bars represent adherent CD56+CD16−CD3− cells, hatched bars show CD56+CD16+CD3− cells and gray bars depict NKT cells. Due to the large clusters at gd9, enumeration became inaccurate (not shown). Thus, we focused on differences within and between gd7 (Figure 1A) and gd8 (Figure 1B). At gd7, the number of CD56+CD16−CD3− cells adhering to decidua basalis exceeded those cells adhering to other regions of the implantation site (p=0.003). There were no differences in the numbers of CD56+CD16+CD3− cells or NKT cells adhering to any area of the implantation site. Similarly, there were no differences found amongst subsets in the anti-mesometrial or lateral areas, but there was a significant increase in the number of adherent CD56+CD16−CD3− cells in the decidua basalis (p=0.017) relative to the other two subsets. At gd8 (Figure 1B), there were no differences in the numbers of adherent cells in any of the three subsets in anti-mesometrial or lateral areas, but there was a significant increase in the number of CD56+CD16−CD3− cells adhering to decidua basalis as compared to the other two subsets (p=0.006). The number of adherent cells increased significantly between gd7 and gd8 (p<0.001). Specifically, there was increased adhesion of the CD56+CD16−CD3− subset to decidua basalis at gd8 as compared to the same cells at gd7 or of CD56+CD16+CD3− and NKT cells to decidua basalis, anti-mesometrial and lateral areas of the implant site at gd7 (p<0.05).
Figure 1.
Location of adherent NK cells on cryosections of mouse implantation sites. Panel A shows number of adherent cells per high power field (HPF) on gd7 implantation sites, panel B shows number of adherent cells HPF on gd8 implantation sites. Black bars represent CD56+CD16−CD3− cells, hatched bars represent CD56+CD16+CD3− cells and gray bars represent CD56+CD16−CD3+ cells. A p value <0.05 was considered significant.
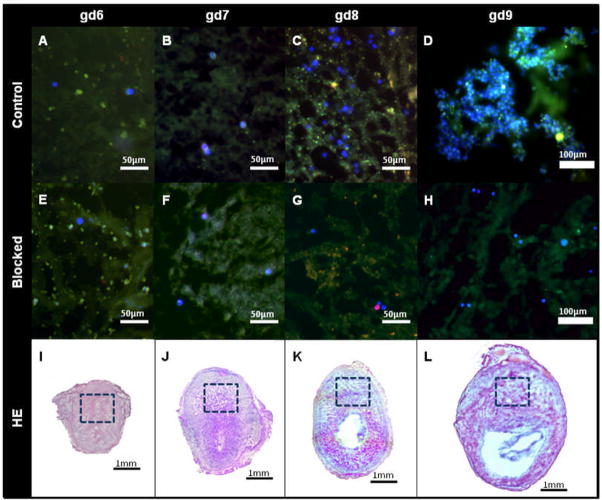
Next, we asked if clumping adhesion of human blood NK cell to mouse decidua was dependant upon the gestational age of the substrate tissue. When gd6 and gd7 tissues were used, NK cells were seen as individual adherent cells across maternal tissue, but with preferential attachment to the decidua basalis. Preferential attachment to decidua basalis was also seen for gd8 and gd9 substrates. However, when gd8-9 tissue was used there were many more adherent CD56+CD16−CD3− NK cells. These cells occasionally formed large clusters when gd8 tissue was used, and when gd9 tissue was used clusters appeared more often and were larger and individual cells within them became impossible to enumerate. Figure 2(A–D) shows representative images of cells adhering to decidua basalis for each gd. The clusters of cells consisted of groups of closely associated cells that densely packed together in an aggregate fashion. Figure 2D shows such groups of densely aggregated cells. Figure 2(E–H) demonstrates that the formation of clusters is prevented when the applied cells are pretreated with function blocking antibodies to SELL and ITGA4. Figure 2(I–L) shows HE stained matched tissue sections. The boxed areas represent the regions of preferential human blood NK cell adhesion shown in Figure 2(A–D).
Figure 2.
Adhesion of human blood NK cells to decidua basalis of mouse implantation sites. Panels A to H show CD56+CD16−CD3− cells stained blue, CD56+CD16+CD3− cells stained red and CD56+CD16−CD3+ cells stained green. Variable tissue autofluorescence is present. Panels A to H illustrate the decidua basalis, the preferred site of human CD56+CD16+CD3− cell adherence. Panels E–H; pre-treatment of cells with anti-ITGA4 and anti-SELL prevent cluster formation. Panels I–L; decidua basalis is boxed in hematoxylin and eosin stained sections.
To determine whether human blood NK cells were forming clusters over uterine mDC, substrate decidua from CD11c-eYFP transgenic mice were used in assays. As shown in Figure 3A, CD56+CD16−CD3− cells form clusters over eYFP gd9 decidua. However, no clusters were associated with eYFP expressing mDC. On rare occasions, an association between individual CD56+CD16−CD3− NK cells and gd9 uterine mDC could be observed as shown in Figure 3B.
Figure 3.
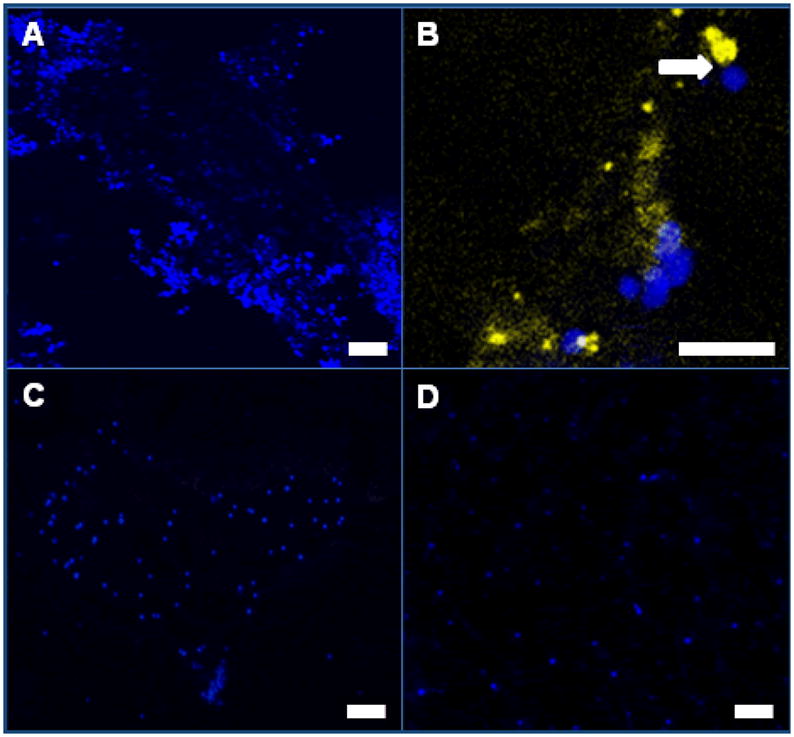
Clustering of human CD56+CD16−CD3− NK cells in adhesion assays occurs independently of DC cell contact in gd9 decidua but requires the presence of resident T and/or B cells. Panel A; formation of clusters of CD56+CD16−CD3− NK cells (stained blue) over decidua of mice whose DC express eYFP. Panel B; CD56+CD16−CD3− NK clusters were not associated with DC. Panel C; human NK cells do not display clustering function over gd9 implantation sites from Rag2−/−/Il2rg−/− mice (NK−, T−, B−). Panel D; human NK cells do not display clustering function over gd9 implantation sites from Rag2−/− mice (NK+, T−, B−). As shown in panel D, the decidua has areas of heightened autofluorescence.
We next asked if clustering adhesion depended upon changes to decidual lymphocytes. Assays were conducted using substrate from the alymphoid Rag2−/−/Il2rg−/− mouse to address this question. As shown in Figure 3C, human blood NK cells did not form clusters over uterine sections from gd9 alymphoid mice, suggesting that activation of lymphocytes found in mouse decidua was essential for promotion of clustering in the indicator cells. We next asked if changes in mouse uNK cells in the substrate were necessary to trigger indicator NK cell clustering by using implantation sites from Rag2−/− mice (NK+, T−, B−). Normal activation of uNK cells and spiral arterial modification occur in mice spontaneously mutant at this locus [22, 23, 23]. When gd9 Rag2−/− tissue was used as substrate, no indicator cell clustering was seen (Figure 3D). This suggests that gestationally-induced changes in T and/or B cells underlie indicator cell clustering and may have importance in activation of uNK cells in vivo.
Discussion
In this investigation we observed that human CD56+CD16−CD3− blood NK indicator cells form clusters over mature mouse decidua basalis that contains T and B cells, and that this functional behavior is independent of mDC and uNK cells. Clustering behavior depends on upon both the day of gestation of the substrate decidual tissue and either SELL and/or ITGA4 expression by the CD56+CD16−CD3− blood NK cells. The CD56+CD16+CD3− subset gained in cell adhesion but did not respond to the changes in the substrate by cluster formation. The changed behavior was most notable in cells overlying decidua basalis. These findings reveal that a significant immunological change occurs in mouse decidua basalis after gd7 that is detected functionally by human NK cells.
Murine uNK cells promote angiogenesis via secretion of the vascular endothelial growth factor (Vegf) family of cytokines, they relax the decidual stroma by secretion of matrix metalloproteases and synthesis of inducible nitric oxide synthase [24] which allows deeper invasion by trophoblast and vascular relaxation, and they secrete interferon gamma (Ifng) which triggers modification of spiral arteries [22]. Because uNK cells dynamically change the architectural structure and milieu of the uterus during pregnancy [25], it was to our surprise that clustering was not observed over Rag2−/− implantation site sections in which uNK cell function remains (Figure 3D).
One possible explanation of the clustering we observed is that it detects an antigen presentation phase. Studies by Chtanova et al. have shown that neutrophils form clusters in the subcapsular sinus of the mouse draining lymph node after infection with Toxoplasma gondii [26]. There have been numerous reports that T cells also form clusters, stable for up to 24 hr, with DC in the lymph node during initiation of immune responses leading to activation or to tolerance [21, 27–29]. We sought to determine if the clusters of NK cells we observed formed over DC in a similar fashion to the manner in which T cells form clusters in vivo around DC. Uterine DC have the potential to present fetal antigen to local leukocytes, and in early human decidua, some DC-SIGN (dendriticcell-specific ICAM-grabbing nonintegrin, CD209) expressing proliferating antigen-presenting cells are found to have intimate contact with uNK cells [30]. Locksley et al. have shown in mice that DC activation by Listeria seeded rapid clustering of Ifng producing NK cells in the spleen [31]. Clustering was necessary for Ifng production in these NK cells, which were responsive to pertussis toxin. However, we did not see changes in fluorescence emission indicating overlay of blood NK cell clusters on mDC. This suggests direct mDC-NK cell contact was not responsible for the shift in function of the viable NK cells.
In previous studies this adhesion assay was used with gd6-7 substrate to study trafficking potential of human blood NK cells and mouse splenocytes to the uterus [12–14, 32, 33]. Specific attachment of cells was achieved via elevated SELL and ITGA4 expression [12–14, 33]. In the current investigation we demonstrated that both adhesion and clustering of CD56+CD16−CD3− NK cells depended on either SELL and/or ITGA4, suggesting the alternative or supplementary explanation that clustered attachment of CD56+CD16−CD3− NK cells is a response to elevated expression of trafficking ligands in the substrate. This explanation is consistent with reports that peak uNK cell numbers are achieved in mouse uterus between gd8-14 depending on the strain [34–36] with gd10-12 usually reported as their numerically most abundant days in C56BL6 [34].
A third explanation is that there are other factors that contribute to this phenomenon. Interleukin 15 (Il15), a progesterone-regulated, essential growth factor for NK cells, might play a role in CD56+CD16−CD3− NK cell clustering. Since Il15 is presented via cell to cell contact in trans, this mechanism may have the potential to restrict clustering to specific Il15-producing locations in the decidua basalis as it is involved in regulating the differentiation of uNK cells [37]. While only a minority of mouse uNK cells associate in vivo, with progesterone receptor positive (Pgr+) stromal cells [38], Pgr+ mouse decidual cells still might be capable of triggering clustering through synthesis of molecules other than Il15. How NK cell clusters were sustained was not investigated, however in the case of T cell clustering it has been reported that microtubule organizing centers become polarized and IL2 is secreted to adjacent cells in a cell-synapse delivery mechanism [39]. CD56+CD16−CD3− NK cells may be participating in similar paracrine communication, or by homophilic interactions [40–42] such as CD56 dimerization to form the large aggregates we observed. It should also be noted that adhesion and clustering occurred at 4°C, therefore a mechanism that allows for immediate hyperactivation of SELL or ITGA4 is more likely to be responsible for this response than transcriptional or translational mechanisms.
We have demonstrated specific CD56+CD16−CD3− human NK cell clustering over mouse decidua basalis that is dependent on day of gestation and occurs only in the presence of endometrial T and B lymphocytes. This novel observation further highlights the immunological gestational changes that occur to the mouse uterus. This work provides evidence of an early function of adoptive immune responses across the mouse decidua basalis. It is unknown if during human pregnancy endometrial T and B lymphocytes also induce similar functional changes in NK cells. The timing of this increased adhesion and NK cell clumping provides further support for the hypothesis that blood NK cells have the capacity to home to the uterus during pregnancy and that they are regulated to undergo functional changes by the decidual environment.
Acknowledgments
We thank Dr. Michael L. Dustin for his kind provision of eYFP breeding pairs. This study was funded through the NSERC/CIHR Collaborative Health Research Program.
References
- 1.King A, Burrows T, Loke YW. Human uterine natural killer cells. Nat Immun. 1996;15(1):41–52. [PubMed] [Google Scholar]
- 2.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 3.Anne CB, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol Rev. 2006;214:161–185. doi: 10.1111/j.1600-065X.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 4.Carlino C, Stabile H, Morrone S, Bulla R, Soriani A, Agostinis C, et al. Recruitment of circulating NK cells through decidual tissues: a possible mechanism controlling NK cell accumulation in the uterus during early pregnancy. Blood. 2008;111(6):3108–3115. doi: 10.1182/blood-2007-08-105965. [DOI] [PubMed] [Google Scholar]
- 5.Lynch L, Golden-Mason L, Eogan M, O’Herlihy C, O’Farrelly C. Cells with haematopoietic stem cell phenotype in adult human endometrium: relevance to infertility? Hum Reprod. 2007;22(4):919–926. doi: 10.1093/humrep/del456. [DOI] [PubMed] [Google Scholar]
- 6.Kitaya K, Yamaguchi T, Honjo H. Central role of interleukin-15 in postovulatory recruitment of peripheral blood CD16(−) natural killer cells into human endometrium. J Clin Endocrinol Metab. 2005;90(5):2932–2940. doi: 10.1210/jc.2004-2447. [DOI] [PubMed] [Google Scholar]
- 7.Stamper HB, Jr, Woodruff JJ. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans SS, Wang WC, Bain MD, Burd R, Ostberg JR, Repasky EA. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97(9):2727–2733. doi: 10.1182/blood.v97.9.2727. [DOI] [PubMed] [Google Scholar]
- 9.Evans SS, Bain MD, Wang WC. Fever-range hyperthermia stimulates alpha4beta7 integrin-dependent lymphocyte-endothelial adhesion. Int J Hyperthermia. 2000;16(1):45–59. doi: 10.1080/026567300285411. [DOI] [PubMed] [Google Scholar]
- 10.Wang WC, Goldman LM, Schleider DM, Appenheimer MM, Subjeck JR, Repasky EA, et al. Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J Immunol. 1998;160(2):961–969. [PubMed] [Google Scholar]
- 11.Burke SD, Dong H, Hazan AD, Croy BA. Aberrant endometrial features of pregnancy in diabetic NOD mice. Diabetes. 2007;56(12):2919–2926. doi: 10.2337/db07-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Heuvel MJ, Horrocks J, Bashar S, Hatta K, Burke S, Evans SS, et al. Periovulatory increases in tissue homing potential of circulating CD56(bright) cells are associated with fertile menstrual cycles. J Clin Endocrinol Metab. 2005;90(6):3606–3613. doi: 10.1210/jc.2004-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van den Heuvel MJ, Hatta K, Peralta CG, Han VK, Clark DA. CD56+ cells are recruited to the uterus in two waves: at ovulation and during the first 2 weeks after missed menses. Am J Reprod Immunol. 2008;59(2):90–98. doi: 10.1111/j.1600-0897.2007.00546.x. [DOI] [PubMed] [Google Scholar]
- 14.van den Heuvel MJ, Horrocks J, Bashar S, Taylor S, Burke S, Hatta K, et al. Menstrual cycle hormones induce changes in functional interactions between lymphocytes and decidual vascular endothelial cells. J Clin Endocrinol Metab. 2005;90(5):2835–2842. doi: 10.1210/jc.2004-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peralta CG, Han VK, Horrocks J, Croy BA, van den Heuvel MJ. CD56 bright cells increase expression of {alpha}4 integrin at ovulation in fertile cycles. J Leukoc Biol. 2008;84(4):1065–1074. doi: 10.1189/jlb.0308164. [DOI] [PubMed] [Google Scholar]
- 16.Chantakru S, Wang WC, van den Heuvel M, Bashar S, Simpson A, Chen Q, et al. Coordinate regulation of lymphocyte-endothelial interactions by pregnancy-associated hormones. J Immunol. 2003;171(8):4011–4019. doi: 10.4049/jimmunol.171.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296(5574):1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- 18.Cahalan MD, Parker I, Wei SH, Miller MJ. Two-photon tissue imaging: seeing the immune system in a fresh light. Nat Rev Immunol. 2002;2(11):872–880. doi: 10.1038/nri935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci U S A. 2003;100(5):2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kammerer U, Kruse A, Barrientos G, Arck PC, Blois SM. Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol Invest. 2008;37(5):499–533. doi: 10.1080/08820130802191334. [DOI] [PubMed] [Google Scholar]
- 21.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, et al. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6(7):707–714. doi: 10.1038/ni1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61(2):493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- 23.Croy BA, Chapeau C, Reed N, Stewart IJ, Peel S. Is there an essential requirement for bone-marrow-derived cells at the fetomaternal interface during successful pregnancy? A study of pregnancies in immunodeficient mice. In: Wegmann TG, Gill TJ, Nisbet-Brown E, editors. Molecular and cellular immunology of the maternal fetal interface. New York: Oxford University Press; 1991. pp. 168–188. [Google Scholar]
- 24.Hunt JS, Miller L, Vassmer D, Croy BA. Expression of the inducible nitric oxide synthase gene in mouse uterine leukocytes and potential relationships with uterine function during pregnancy. Biol Reprod. 1997;57(4):827–836. doi: 10.1095/biolreprod57.4.827. [DOI] [PubMed] [Google Scholar]
- 25.Guimond MJ, Wang B, Croy BA. Engraftment of bone marrow from severe combined immunodeficient (SCID) mice reverses the reproductive deficits in natural killer cell-deficient tg epsilon 26 mice. J Exp Med. 1998;187(2):217–223. doi: 10.1084/jem.187.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chtanova T, Schaeffer M, Han SJ, van Dooren GG, Nollmann M, Herzmark P, et al. Dynamics of neutrophil migration in lymph nodes during infection. Immunity. 2008;29(3):487–496. doi: 10.1016/j.immuni.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cahalan MD, Parker I. Close encounters of the first and second kind: T-DC and T-B interactions in the lymph node. Semin Immunol. 2005;17(6):442–451. doi: 10.1016/j.smim.2005.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bousso P, Robey E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat Immunol. 2003;4(6):579–585. doi: 10.1038/ni928. [DOI] [PubMed] [Google Scholar]
- 29.Hommel M, Kyewski B. Dynamic changes during the immune response in T cell-antigen-presenting cell clusters isolated from lymph nodes. J Exp Med. 2003;197(3):269–280. doi: 10.1084/jem.20021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, et al. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162(3):887–896. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang SJ, Liang HE, Reizis B, Locksley RM. Regulation of hierarchical clustering and activation of innate immune cells by dendritic cells. Immunity. 2008;29(5):819–833. doi: 10.1016/j.immuni.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croy BA, di Santo JP, Greenwood JD, Chantakru S, Ashkar AA. Transplantation into genetically alymphoid mice as an approach to dissect the roles of uterine natural killer cells during pregnancy--a review. Placenta. 2000;21 (Suppl A):S77–S80. doi: 10.1053/plac.1999.0518. [DOI] [PubMed] [Google Scholar]
- 33.van den Heuvel MJ, Peralta CG, Hatta K, Han VK, Clark DA. Decline in number of elevated blood CD3(+) CD56(+) NKT cells in response to intravenous immunoglobulin treatment correlates with successful pregnancy. Am J Reprod Immunol. 2007;58(5):447–459. doi: 10.1111/j.1600-0897.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 34.Xie X, He H, Colonna M, Seya T, Takai T, Croy BA. Pathways participating in activation of mouse uterine natural killer cells during pregnancy. Biol Reprod. 2005;73(3):510–518. doi: 10.1095/biolreprod.104.033951. [DOI] [PubMed] [Google Scholar]
- 35.Paffaro VA, Jr, Bizinotto MC, Joazeiro PP, Yamada AT. Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta. 2003;24(5):479–488. doi: 10.1053/plac.2002.0919. [DOI] [PubMed] [Google Scholar]
- 36.Kusakabe K, Okada T, Sasaki F, Kiso Y. Cell death of uterine natural killer cells in murine placenta during placentation and preterm periods. J Vet Med Sci. 1999;61(10):1093–1100. doi: 10.1292/jvms.61.1093. [DOI] [PubMed] [Google Scholar]
- 37.Ye W, Zheng LM, Young JD, Liu CC. The involvement of interleukin (IL)-15 in regulating the differentiation of granulated metrial gland cells in mouse pregnant uterus. J Exp Med. 1996;184(6):2405–2410. doi: 10.1084/jem.184.6.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh MJ, Croy BA. A map of relationships between uterine natural killer cells and progesterone receptor expressing cells during mouse pregnancy. Placenta. 2008;29(4):317–323. doi: 10.1016/j.placenta.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Sabatos CA, Doh J, Chakravarti S, Friedman RS, Pandurangi PG, Tooley AJ, et al. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity. 2008;29(2):238–248. doi: 10.1016/j.immuni.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236(4803):799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- 41.Hoffman S, Edelman GM. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci U S A. 1983;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutishauser U, Hoffman S, Edelman GM. Binding properties of a cell adhesion molecule from neural tissue. Proc Natl Acad Sci U S A. 1982;79(2):685–689. doi: 10.1073/pnas.79.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]