Abstract
Fur mite outbreaks remain a persistent problem in laboratory mouse colonies. All currently published treatment methods are labor-intensive, expensive, or unreliable. During a recent outbreak with Myobia musculi and Myocoptes musculinus in a large colony (approximately 30,000 cages), we developed a feed-based treatment regime in which ivermectin was the active ingredient. Rodent feed was compounded with 3 different concentrations of ivermectin (12, 24, and 48 ppm) and γ-irradiated. Postcompounding analysis revealed loss of ivermectin during manufacturing, but the remaining drug was stable for at least 6 mo. In an 8-wk toxicity study in a C57BL/6NTac mouse breeding colony, ad-libitum feeding of the 3 diets yielded estimated doses of 1.3, 2.7, and 5.4 mg/kg. Adult mice lacked adverse clinical effects, except that 1 of the 144 mice in the 48-ppm group developed tremors and ataxia and was euthanized. No significant differences between doses were revealed by CBC, serum chemistry, body weight, or gross necropsy. Plasma drug concentrations plateaued at a dose-dependent level 7 to 10 d after initiation of treatment and decreased to undetectable levels 6 to 9 d after its discontinuation. Fertility of the P0 generation was unaffected. Pup mortality was higher in the 24- and 48-ppm groups, reaching 100% at the higher dose. Animals exposed to ivermectin as neonates had normal weaning weights, but mice receiving 24-ppm feed had lower adult weights. Our results indicate that using feed containing 12 ppm ivermectin (estimated ingested dose, 1.3 mg/kg) was safe in a C57BL/6NTac breeding colony.
Fur mites are a persistent problem in laboratory mouse colonies.8,12 Fur mite infestations can affect animals’ health and compromise experimental results.16 Clinical signs in infested mice include localized pruritus, alopecia, ulcerative dermatitis, lymphadenopathy, and weight loss.39,40 Immunocompetent mice mount a robust humoral response characterized by hypergammaglobulinemia, markedly elevated IgE levels, lymphocytopenia, eosinophilia, and alterations in inflammatory cytokines.18,35 Therefore, effective treatment is imperative in the interest of both the animals and the research in which they are used.
More than a dozen reports have been published in laboratory animal science journals over the last 25 y describing various fur mite treatments. 3,4,7,9,13,14,23,26,28,29,36,39,41 Despite the large number of regimens and acaricides described, fur mites remain a concern for laboratory mouse colonies. The last 2 comprehensive surveys, which were conducted almost 10 y apart (1996 and 2006) and assessed prevalence of adventitious agents in major United States research institutions, indicated that fur mites were present in 30% to 40% of the institutions responding.8,17 Despite great advancements in laboratory animal science and technologies, the prevalence of rodent fur mite infestation in laboratory mouse colonies apparently has remained relatively stable, as compared with that of other adventitious infective agents.
We recently experienced a fur mite outbreak with both Myobia musculi and Myocoptes musculinus in all 3 of our vivaria, affecting 30,000 cages (described elsewhere in this issue).30 Briefly, the infestation was identified simultaneously in 3 distinct but interconnected vivaria. The fur mites most likely were introduced on imported animals, whose infestation evaded detection during quarantine. Subsequently, the infestation was spread inadvertently and rapidly by transferring large numbers of cages between vivaria. Ultimately, about 40% of the animal rooms became infested. Because of the fulminant nature of this outbreak, the entire rodent population in all 3 facilities required treatment. A treatment regimen that could be deployed facility-wide in an efficient and cost-effective manner was needed.
Most protocols describing effective fur mite eradication require individual treatment of animals or cages or the preparation of medicated water bottles for each cage.3,4,7,9,14,36,39 These methods, when applied to very large colonies, are cumbersome, labor-intensive, expensive, and (most importantly) lack proven long-term efficacy. In our program, we routinely and successfully use rodent feed compounded with anthelminthics, antibiotics, and supplemental vitamins to treat mice.25 In our hands, medicated feed has served as an efficient and effective way of delivering therapeutics to animals requiring treatment for days to weeks.
Since the advent of avermectin class drugs in 1981, most fur mite treatment protocols have incorporated their use. These agents have been administered through various routes, doses, and schedules, as a monotherapy or in combination with other agents.3,7,14,23,28,36,41 Avermectins have powerful broad-spectrum antiparasitic activity against most endo- and ectoparasites and act by binding GABA-gated chloride and invertebrate-specific glutamate-gated anion channels in peripheral neuromuscular synapses, suppressing nerve impulse conduction.37 Ivermectin was the first avermectin developed specifically for and introduced to the veterinary market.21 Since its introduction, ivermectin at doses as low as 0.2 mg/kg has proven effective in killing rodent fur mites in small, well-defined outbreaks and controlled experiments.4,9,14,23,32,41 Today, ivermectin is readily available in bulk quantities at very low cost. By delivering ivermectin in feed, we could avoid the difficult labor and expense associated with individual treatment of more than 120,000 mice or administering the drug weekly in more than 30,000 water bottles.
Although ivermectin has a wide safety margin in most mammalian species, some animals are particularly sensitive to ivermectin toxicity. Neonatal mice, which have an immature blood brain barrier, and adult mice and dogs with defective P-glycoprotein are most at risk to ivermectin toxicity, even when given at doses as low as 0.4 mg/kg.20,21,31 In addition, typical therapeutic doses have been shown to cause adverse effects in various mouse strains or stocks and dog breeds with normal levels of P-glycoprotein.5,21 In addition, ivermectin has been shown to alter aspects of behavior and immune function in mice.6,10,18
Reported ivermectin doses for mice vary from 0.2 to 4 mg/kg and appear to have been chosen arbitrarily.3,9-11,14,19,23,24,28,33 Moreover, most dose recommendations are much higher than the no-effect-dose reported in the literature.21 Therefore, we sought to identify the highest-possible ivermectin concentration that could be provided to mice in feed for an extended period of time without causing adverse effects on neonates and adults or during pregnancy and lactation.
Materials and Methods
Animals.
Male (n = 288) and female (n = 288) C57BL/6NTac mice (age, 6 to 8 wk) were obtained from Taconic Farms (Germantown, NY). On arrival, animals were free of antibodies to mouse hepatitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse parvovirus, minute virus of mice, murine norovirus, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, mouse adenovirus, K virus, polyoma virus, mouse cytomegalovirus, mouse thymic virus, Haantan virus, lactic dehydrogenase elevating virus, cilia-associated respiratory bacillus, and Mycoplasma pulmonis. Animals were also free of Helicobacter spp., Salmonella spp., Clostridium piliforme, Corynebacterium kutscheri, Citrobacter rodentium and endoparasites and ectoparasites. All mice were housed in solid-bottom polysulfone shoebox cages maintained in individually ventilated cages (Thoren Systems, Hazelton, PA) on autoclaved aspen chip bedding (PWI Industries Canada, Quebec, Canada). Feed and acidified water (pH 2.5 to 2.8) were provided ad libitum. Control mice received unmedicated Purina 5058 feed (PMI, Richmond, IN). Treated mice received ivermectin medicated feed as described following. All feed was stored at 72 ± 2 °F (21.5 ± 1 °C) at 30% to 70% relative humidity until used. Cages were changed in a certified class II type A2 biological safety cabinet (NU S602-500, series SP, Nuaire, Plymouth, MN) weekly. The holding room was ventilated with filtered 100% outside air at 15 air changes hourly and maintained on a 12:12-h light:dark cycle. Room temperature was maintained at 72 ± 2 °F (21.5 ± 1 °C) and relative humidity at 30% to 70%. Animal use was approved by the Memorial Sloan–Kettering Cancer Center IACUC. The animal care and use program at Memorial Sloan–Kettering Cancer Center is AAALAC-accredited, and all animals are housed in accordance to the recommendations provided in the Guide for the Use and Care of Laboratory Animals.15
Medicated feed.
Ivermectin contains 2 chemical components, B1a and B1b. They differ by the substitution of a methyl group (C2H5) in B1a by an ethyl group (CH2) in B1b at the 26-carbon position. However, their biological activity remains almost identical and are considered equivalent. 21 Pharmaceutical-grade ivermectin containing a minimum of 90% B1a (Spectrum Chemical, Gardena, CA) was used for our experiments.
Medicated feed was formulated by Test Diet (PMI). Pelleted rodent feed (LabDiet 5058, PMI) was granulated and mixed with powdered ivermectin to obtain final concentrations of 12, 24, and 48 ppm. Each batch also contained 0.3 ppm of nontoxic Blue #2/Indigo Carmine dye (Colorcon, West Point, PA) for easy identification. Feed was repelleted, placed into clear plastic bags (2 kg each), boxed, trucked by commercial ground carrier to a γ irradiator, exposed to 1 to 4 mRad, and subsequently trucked to our institution. Samples were collected as described following.
Blood and tissue sample collection and analysis.
At each experimental time point, mice were euthanized by CO2 inhalation (or by decapitation with a sharp blade if 7 d of age or younger). Blood was collected by cardiocentesis with a 25-gauge needle and 1-mL tuberculin syringe immediately after euthanasia. Blood for hematology (0.5 mL) was placed in tubes precalibrated with EDTA (Microtainer, Becton–Dickinson, Franklin Lakes, NJ). Hematology was performed on the day of collection by Memorial Sloan–Kettering Cancer Center's Laboratory of Comparative Pathology using an automated analyzer with veterinary software package (Cell-Dyn 3700, Abbott Laboratories, Abbott Park, IL). Blood for serum chemistry analysis was placed in preservative-free serum separator tubes (Microtainer, Becton–Dickinson). After a 30-min incubation period at room temperature, the tubes were spun for 10 min at 8,000 × g. Serum was transferred to a new preservative-free tube and sent on the day of collection to a reference laboratory (ALX Laboratories, New York, NY) for analysis using a clinical chemistry analyzer (AU400, Olympus, Center Valley, PA). Blood for measuring ivermectin concentration was collected in tubes with EDTA (Microtainer, Becton–Dickinson). Plasma was separated by centrifugation and stored at −80 °C until analysis.
In addition, skin samples of approximately 1 cm2, collected from the dorsal midlumbar and abdominal ventral regions, were harvested at the time of euthanasia. Subcutaneous fat and fascia were removed carefully by using a clean scalpel blade. Samples were placed in preservative-free tubes and maintained at −80 °C until analyzed.
Determination of ivermectin concentration in feed, plasma, and skin.
Ivermectin concentration was determined by HPLC and electrospray ionization mass spectroscopy. Five pellets from each batch of feed containing 12, 24, or 48 ppm ivermectin were granulated individually by using a mortar and pestle, and 200, 100, or 50 mg, respectively, was transferred to a 15 mL polypropylene tube, mixed with 5 mL 100% methanol, shaken, and sonicated for 15 min. The samples were centrifuged at 1,860 × g for 5 min at room temperature and supernatants transferred into 96-well plates. Skin samples were weighed and 50 mg transferred to a 1.0 mL tube containing 0.3 mL acetonitrile:methanol (3:1 v/v) solution. Tubes were vortexed for 60 s and stored at −20 °C for 30 min. Tubes then were centrifuged for 10 min at 5,170 × g and the supernatant transferred into a 96-well plate by using a 0.22-µm filter syringe. Plasma samples were prepared by pipetting 100 µL into 1-mL tubes containing 400 µL acetonitrile. The solutions were vortexed for 30 s, stored at −20 °C for 30 min, and centrifuged for 10 min at 5170 × g. The supernatant was transferred into a 96-well plate by using a 0.22-µm filter syringe. For each sample, 1 to 5 µL was injected into the HPLC system; an Inertsil C8 (5 µm; 3.0 × 50 mm) HPLC column (GL Sciences, Torrance, CA) was used for the separation of ivermectin from feed, skin, and plasma samples. A Sciex API 4000 triple-quadrupole mass spectrometer using a Turbospray ion source (Applied Biosystems, Foster City, CA) was used for quantification of ivermectin in samples. The detection limit was 300 pg/mL.
Experimental plan.
Determining ivermectin concentration in feed.
To determine the potential loss of ivermectin activity during formulation and storage, feed samples were collected immediately after pelleting (wet pellet), after drying at low (68 °F [20 °C]) or high (225 °F [107 °C]) temperature, and after receiving 1 to 4 mRad γ irradiation. In addition, we measured ivermectin at monthly intervals for 6 mo after milling. All samples were stored at −80 °C until analysis.
Toxicity in mice.
C57Bl/6NTac mice were housed as breeding pairs (n = 288) and randomly assigned to 1 of 4 groups, each with 72 cages (P0 generation). The control group received unmedicated feed while the other 3 groups received medicated feed compounded with 12, 24, or 48 ppm ivermectin and manufactured by using the high-temperature drying method. Eight breeding pairs from each group were euthanized and samples collected, as previously described, on days 1, 3, 7, 10, 14, 21, and 43 after initiation of treatment with the test diet. All remaining animals were switched to unmedicated feed on day 57. An additional 8 pairs of mice were each euthanized at 65 and 181 d after initiation of treatment. At each time point, body weight was recorded, and abdominal and thoracic organs were examined for gross abnormalities. All tissues were archived in 10% neutral buffered formalin. A full microscopic examination was performed on any mouse with macroscopic abnormalities or signs of ivermectin toxicity.
Reproductive performance parameters (numbers of litters and pups born, pup mortality, weanling weight) were recorded for each group during the treatment and posttreatment periods until the time of euthanasia. Neonatal deaths and live pups euthanized at 1, 3, and 7 d of age were evaluated for macroscopic developmental abnormalities (for example, cleft palate), and tissues were archived in 10% formalin.
To determine long-term effects on fertility, mice were examined over 2 generations. From the F1 generation born during the treatment period, 8 breeding pairs per treatment group were established and maintained as described for the P0 generation for a maximum of 34 wk of age before euthanasia. The same procedure was repeated with animals selected from the F2 generation. Body weight was recorded at the times of weaning and euthanasia. Reproductive performance parameters were evaluated as described for the P0 generation.
Statistical analysis.
Differences in body weight and ivermectin levels in feed and plasma among treatment groups were compared by using the Kruskal–Wallis test when testing for overall differences among the treatment groups. The Wilcoxon rank sum test was used when 2 groups were compared. The associations of treatment on number of litters per breeding pair and number of pups born per breeding pair were analyzed by using Poisson regression. The association of treatment on the mortality rate per litter was analyzed by using logistic regression with generalized estimating equations to correct for correlations among litters within a breeding pair. Differences in ivermectin levels between the ventral and dorsal skin samples were compared within each time group by using the Wilcoxon signed rank test, whereas differences in levels across time points were compared by using the Wilcoxon rank sum test. All computations were done by using SAS 9.1 (SAS Institute, Cary, NC. A P value of less than or equal to 0.05 was considered statistically significant.
Results
Ivermectin concentration in feed.
The quantity of ivermectin in feed measured after the first step of the manufacturing process (wet pellets) was 33.6% lower than expected. Further processing of the feed, by using 2 different drying options (low or high temperature) or irradiation, yielded concentrations 10.6%, 17.5%, and 20.5% lower than expected, respectively. There was considerable variation from the expected ivermectin concentration among pellets assayed from the same batch at the same point in the manufacturing process. For example, a single pellet from the low-temperature-dried sample contained 4 times the expected concentration whereas single pellets from both the high-temperature and irradiated samples contained 2.0 and 1.4 times the expected concentrations, respectively.
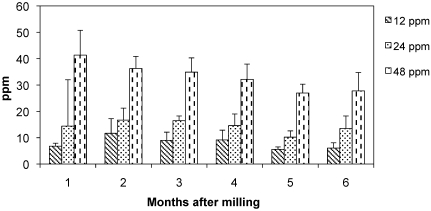
Ivermectin concentration remained stable in irradiated feed for 6 mo (Figure 1). A slight decrease in ivermectin concentration was detected in the 48-ppm feed over the 6 mo period; however, the decreases were not statistically significant when data were compared month-to-month or over the entire 6-mo period. In the 12- and 24-ppm groups, no statistically significant differences were observed in feed analyzed at months 1 and 6.
Figure 1.
Ivermectin concentration (ppm; mean ± 1 SD) in feed analyzed at various time points after milling.
Toxicity study.
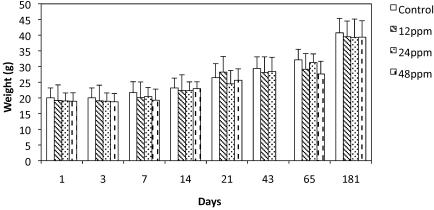
Body weight increased at the same rate for mice in all groups over the course of the study. No significant weight differences were detected when comparing each of the 3 ivermectin-treated groups and the controls for the P0 generation for the initial 3 wk of treatment (Figure 2). At day 65 (9 d after the treatment ended), mice in the 48-ppm group were significantly (P = 0.01) smaller (26.6 g) than were control mice (32.1 g). This weight reduction was transient, because the difference was no longer evident at day 181. The weights of the mice in the 12- and 24-ppm groups were not significantly different from those of the control group at any time point.
Figure 2.
Weights (mean ± 1 SD) of P0 mice during (1 to 43 d) and after (65 to 181 d) treatment with ivermectin-compounded or control feed. At day 65, mice in the 48-ppm group were significantly (P < 0.05) smaller than control mice.
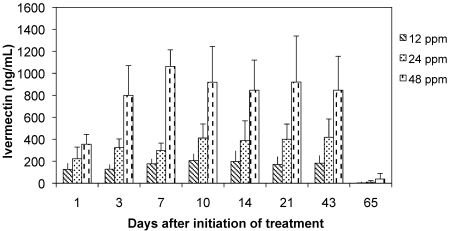
On initiation of providing medicated feed, plasma ivermectin levels increased and subsequently plateaued at dose-dependent levels in approximately 7 to 10 d (Figure 3). Levels remained stable at approximately 180, 420, and 840 ng/mL for the 12-, 24-, and 48-ppm treatment groups, respectively, for the duration of the treatment period. At day 65, 9 d after the end of treatment, ivermectin was almost completely eliminated from plasma.
Figure 3.
Plasma ivermectin concentration (mean ± 1 SD) in mice treated with ivermectin-compounded feed.
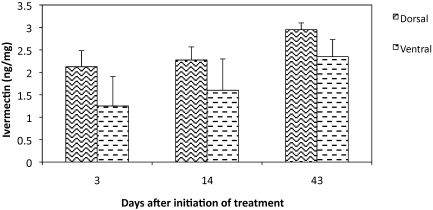
Ivermectin levels in skin samples from mice treated with 12-ppm feed (Figure 4) were approximately 100-fold lower (2.35 ng/mL) than those in the plasma of the same animals. The differences between dorsal and ventral locations at days 3, 14, and 43 were not statistically significant. However, ivermectin concentration in dorsal samples was significantly (P = 0.03) higher after 43 d than 3 d of treatment.
Figure 4.
Skin ivermectin concentration (mean ± 1 SD) in mice treated with 12-ppm ivermectin-compounded feed. There is a significant (P < 0.05) difference between days 3 and 43 of the dorsal samples.
RBC, WBC, and platelet counts and measured concentrations of hemoglobin, albumin, blood urea nitrogen, alanine aminotransferase, alkaline phosphate, γ-glutamyl transpeptidase, and bilirubin were within the established laboratory reference ranges in animals from all groups and time points (data not shown). Overall, no adverse clinical effects were noted, except that a single adult mouse in the 48-ppm feed group developed tremors and ataxia 2 d after initiation of treatment and subsequently was euthanized. Macroscopic and microscopic pathology performed on this animal were unremarkable.
Reproductive performance.
No significant differences were observed in either the numbers of litters or pups born per breeding pair in any group during the 8-wk treatment period (Table 1). However, pup mortality in the 24- and 48-ppm groups was significantly (P < 0.05) and dose-dependently higher than that in the control group, reaching 100% in the 48-ppm group. This effect was transient; all reproductive parameters returned to baseline in all groups during the 18 wk-period that followed the 8-wk treatment period (Table 1).
Table 1.
P0 generation breeding parameters during and after treatment with ivermectin-containing feed
| Ivermectin | No. of litters per breeding pair (mean ± 1 SD) | No. of pups born per breeding pair (mean ± 1 SD) | No. of pups weaned | Pup mortality (%) | Weight (g; mean ± 1 SD) of F1 weanlings |
| During treatment | |||||
| 0 ppm (control) | 0.8 ± 0.8 | 6.5 ± 6.4 | 32 | 67.8 | 10.4 ± 1.4 |
| 12 ppm | 1.1 ± 0.6 | 7.0 ± 4.4 | 23 | 77.8 | 10.6 ± 1.8 |
| 24 ppm | 1.2 ± 0.7 | 8.0 ± 4.8 | 17 | 89.1a | 9.8 ± 2.1 |
| 48 ppm | 1.0 ± 0.6 | 7.4 ± 4.5 | 0a | 100a | not applicable |
| After treatment | |||||
| 0 ppm (control) | 0.7 ± 0.6 | 5.8 ± 4.4 | 33 | 70.1 | 10.1 ± 1.5 |
| 12 ppm | 1.3 ± 0.8 | 10.3 ± 6.0 | 65 | 60.3 | 9.5 ± 2.1 |
| 24 ppm | 1.3 ± 0.8 | 10.1 ± 6.1 | 62 | 56.4 | 10.1 ± 1.0 |
| 48 ppm | 0.9 ± 0.7 | 6.0 ± 4.8 | 42 | 60.8 | 9.8 ± 1.4 |
P < 0.05 when compared with value for control group
F1 mice born to P0 parents during treatment with 24-ppm feed had significantly (P < 0.05) higher average numbers of litters and pups born per breeding pair (3.7 ± 1.5 and 30.3 ± 14.2, respectively) compared with those of descendants of the control group (1.5 ±1.3, 11.5 ± 9.5; Table 2). The 12-ppm group had an average of 2.6 ± 2.0 litters and 19.6 ± 15.2 pups born per breeding pair. These differences were not significant when compared with values for the control group. Pup mortality (65.35, 80.55, 67.3% for control, 12-ppm, and 24-ppm groups) did not differ among groups. The number of litters per breeding pair, pups born per breeding pair, or pup mortality percentage did not differ among all groups in the F2 generation (Table 2).
Table 2.
Breeding parameters for F1 and F2 generationsa
| Ivermectin | F1 | F2 | |
| No. of litters | 0 ppm | 1.5 ± 1.3 | 1.4 ± 1.1 |
| born per | 12 ppm | 2.6 ± 2.0 | 1.3 ± 0.7 |
| breeding pair | 24 ppm | 3.7 ± 1.5b | 1.4 ± 1.4 |
| No. of pups | 0 ppm | 11.5 ± 9.5 | 10.4 ± 8.3 |
| born per | 12 ppm | 19.6 ± 15.2 | 8.9 ± 5.6 |
| breeding pair | 24 ppm | 30.3 ± 14.2b | 9.4 ± 10.1 |
| Pup mortality | 0 ppm | 65.3% | 83.8% |
| 12 ppm | 80.5% | 80.2% | |
| 24 ppm | 67.3% | 46.8% |
Data for no. of litters and pups per breeding pair are given as mean ± 1SD.
F1 pairs were born during the treatment period and did not receive ivermectin after weaning. F2 pairs were born after the treatment period.
P < 0.05 when compared with value for the control (0 ppm) group.
In addition, average weights for weanlings from the F3 generations originating from 12- and 24-ppm ivermectin-treated mice were 11.6 and 10.5 g, respectively. Weanlings from the F1 generation weaned during treatment were 10.6 and 9.8 g for the 12- and 24 ppm ivermectin-treated mice, respectively(Table 3). Eight pairs of F1 and F2 pups were maintained as breeders until they were 34 wk old and then euthanized and their body weights and those of the P0 generation measured (Table 4). F1 and F2 mice born to the control group mice were significantly (P < 0.05) larger (50.5 ± 5.9 and 46.9 ± 6.1 g, respectively) than their P0 predecessors (40.8 ± 4.6 g). F2 mice born to the 12-ppm treated lineage were significantly (P < 0.05) larger (48.9 ± 9.2 g) than their P0 predecessors (39.5 ± 5.9 gm). F1 mice in the 24-ppm group were significantly (P < 0.05) smaller than the control group of the same generation, whereas there was no significant difference among mice in the F2 generation.
Table 3.
Mean weights (g; mean ± 1 SD) of 3-wk-old weanlings
| F1 |
||||
| During | After | F2 | F3 | |
| 0 ppm | 10.4 ± 1.4 | 10.5 ± 1.5 | 9.6 ± 1.9 | 12.3 ± 1.5 |
| 12 ppm | 10.6 ± 1.8 | 9.5 ± 2.1 | 10.9 ± 1.5 | 11.6 ± 1.4 |
| 24 ppm | 9.8 ± 2.1 | 10.1 ± 1.0 | 10.2 ± 1.7 | 10.5 ± 1.2 |
| 48 ppm | not done | 9.8 ± 1.4 | not done | not done |
All pups born during treatment (During) with the 48-ppm feed died before weaning. We did not assess generations born after treatment (After) with 48-ppm feed.
Table 4.
Mean weight (g; mean ± 1 SD) at 32 wk of age for each generation
| Ivermectin | P0 | F1 | F2 |
| 0 ppm | 40.8 ± 4.6 | 50.5 ± 5.9a | 46.9 ± 6.1a |
| 12 ppm | 39.5 ± 5.9 | 43.8 ± 8.5 | 48.9 ± 9.2a |
| 24 ppm | 39.4 ± 5.8 | 36.2 ± 5.1b | 42.5 ± 7.4 |
P < 0.05 when compared with value for P0 on the same row.
P < 0.05 when compared with value for control (0 ppm) in the same column.
Discussion
The concentration of ivermectin in compounded feed was determined during and after manufacturing. Previous reports have demonstrated that compounded medicated feeds often contain lower-than-expected drug concentrations, which have been attributed to the manufacturing process.1,25 Heat, water, and γ irradiation can cause declines in drug concentration in feed.27,38 Our results are consistent, in that we measured a lower-than-expected concentration of ivermectin in feed compounded with 3 different drug concentrations.
Pelleting involves forcing moistened feed meal through a dye in the presence of steam. We found that ivermectin concentration declined significantly during the initial step of the manufacturing process which results in a wet pellet. Ivermectin concentration in pellets dried at either low or high temperature declined on average 10.6% and 17.5%, respectively, from expected. Although the dried samples contained more ivermectin than that observed after the earlier manufacturing step (that is, the wet pellets) ivermectin concentration varied considerably between pellets dried at either temperature; individual pellets in the low and high temperature dried samples contained as much as 409% and 220% more ivermectin than expected, respectively. Similarly, the irradiated sample contained a single pellet with 140% more ivermectin than expected. The large variability in ivermectin concentrations between pellets likely was due to uneven distribution of drug within the meal mixture prior to pelleting. It is important to note that prolonging mixing time can potentiate this problem by redistributing the mixture components based on particle size, resulting in a more uneven mixture. Only 5 pellets were evaluated per sample. If we had tested more pellets from each group, we expect there would have been less of a difference in ivermectin concentration between sample groups. Excluding single pellets with unexpectedly high levels of ivermectin from each group, the average decline of ivermectin for the low-temperature-dried, high-temperature-dried, and irradiated samples would have been 40.6%, 34.6%, and 32.0%, thus paralleling the 33.6% decline measured in the wet pellets. Because ivermectin concentration essentially did not differ between the high and low drying temperatures, all feed used for the toxicity study was manufactured by using the high drying temperature which is the standard manufacturing protocol.
Given the dose dependent distribution evident in Figure 3 and the fact that toxicity in adult mice was not a problem in our study, we speculate this high variability among pellets did not have a significant effect on the doses ingested by mice fed ivermectin-medicated feed. This lack of effect likely is related to the fact that mice, rather than eating an entire pellet at a time, forage and nibble on various random pellets during feeding hours. Assuming that a 30-g mouse consumes 5 g feed daily on average,2 providing diets compounded with 12, 24, and 48 ppm ivermectin would be expected to deliver approximately 1.3 mg/kg, 2.7 mg/kg, and 5.4 mg/kg ivermectin, respectively, when drug loss during manufacturing is taken into consideration.
In our study, ivermectin levels in the plasma plateaus at 7 to 10 d after initiation of oral treatment. Our 12-ppm (1.3 mg/kg) group yielded a plasma concentration of 180 ng/mL, which is somewhat proportional to the 90-ng/mL plasma concentration achieved after oral administration of 0.45 mg/kg ivermectin.9 We did not observe a concentration plateau in the skin. We believe that such a plateau would have been achieved if the administration duration was longer, given that the transfer of drug from plasma to skin is slow and time was not sufficient to reach equilibrium between skin and plasma levels during the 8 wk of treatment. Future studies could compare the skin concentration achieved with oral administration to that resulting from topical exposure.
Previously published toxicologic testing of ivermectin yielded conflicting results. One study reported daily exposure of adult CD1 mice to doses as high as 12 mg/kg for as long as 94 d resulted in only a 29% decreased body weight and no other clinical signs.21 In another study, doses as low as 0.2 mg/kg resulted in mortality, convulsion, and coma in pregnant female mice, and 0.4 mg/kg caused cleft palate in pups exposed in utero.22 This finding established a noneffect level in mice at 0.1 mg/kg and the belief that mice are uniquely sensitive to ivermectin toxicity. As a comparison, noneffect levels in rats are 5 mg/kg.21 However, oral ivermectin at doses as high as 2.0 mg/kg have been used to treat mice without adverse effects.11 Most published treatment regimes for mice range from 1 to 1.9 mg/kg,7,9,19,23,24 all of which are clearly above the 0.1-mg/kg noneffect level cited.
To maximize the probability that we would successfully eliminate fur mites from our large mouse colony, we used the highest dose tested that lacked adverse effects in our toxicity study, given that the literature provided little information that could be used to establish an appropriate dose. Because most colonies in our vivarium are maintained on a C57BL/6 (B6) background, toxicity experiments were conducted on mice of this strain. We found adult B6 mice to be relatively resistant to high doses of ivermectin; only 1 of 144 mice receiving 5.4 mg/kg ivermectin daily (48 ppm diet) demonstrated clinical signs. Although gross necropsy and histologic evaluation of tissues from this mouse were unremarkable, the nature of the clinical presentation (tremors, ataxia) and the timing (2 d after initiation of treatment) are suggestive of ivermectin toxicity. Most reports do not describe mortality at doses equivalent to our 12- and 24-ppm feed (1.3 and 2.7 mg/kg), but one study reported that 2 of 22 129/SvSj mice died after receiving a dose of 1 to 2 mg/kg.36 Pathology was not performed and clinical signs were not observed in the cited study; it is possible that the mice died of unrelated causes, especially because an additional 10 129/SvSj mice receiving 5 times the original dose did not demonstrate toxicity.32 Furthermore, 129/SvEv mice treated with oral ivermectin in water for 8 wk at doses between 1.2 and 1.9 mg/kg demonstrated no adverse effects.10
In our study, body weight gain among the P0 generation remained constant through the first 3 wk of treatment in all groups. Nine days after the cessation of treatment (day 65), mice receiving 5.4 mg/kg (48 ppm diet) ivermectin were smaller than control mice. Unfortunately, the day 43 weight for the 48-ppm group was not recorded, so these animals may have gained less weight during the second half of the treatment period than during the first half; however, at day 181, there were no weight differences between groups. Weight loss was the only adverse finding reported in the initial toxicology study of ivermectin in adult CD1 mice.20 Even though the dose used in the cited study (12 mg/kg) was more than 2-fold greater that the highest dose we evaluated here (5.4 mg/kg), our findings are consistent.
Comparing body weight at 32 wk of age among mice in the P0, F1 (control diet), and F2 (control and 12-ppm diets) generations revealed that F1 and F2 mice were significantly larger than their P0 counterparts. This result may reflect the feed these animals had received. The P0 generation was obtained from Taconic Farms at 6 to 8 wk of age and had been fed NIH no. 31M Rodent Diet prior to their arrival. This diet has a considerably lower fat content (5.3%) than does the Purina 5058 feed (9% crude fat content) we used.34 However, weight gain was not observed in the group that received 2.7 mg/kg ivermectin. There was no weight increase over subsequent generations, and the treated F1 mice were significantly smaller than those in the control F1 group. Exposure to ivermectin as neonates apparently can reduce the animals’ adult weight, even after drug exposure had ceased. Interestingly, the average weaning weight of F1 mice born during ivermectin treatment was very similar between dosage groups and to the weaning weight of animals born after the end of treatment. During the preweaning period, all pups gained weight at the same rate regardless of ivermectin exposure. The preweaning growth rate most likely reflects the dam's milk production. Despite identical weaning weights, the ability of these animals to gain weight throughout their adult lives may be impaired by neonatal ivermectin exposure.
The main adverse effects of ivermectin in the original toxicology study were seen in pregnant dams, embryos, and neonatal pups.21 We were unable to duplicate most of these effects. None of the pregnant mice in our studies showed any clinical signs or mortality in any of the treatment groups. In addition, we observed no teratogenic effects (for example, cleft palate) in any of the pups that had been exposed to ivermectin at any dose in utero. Moreover, we found no difference in the numbers of litters or pups born to dams of the various treatment groups. Together, these findings indicate that ivermectin at doses as high as 5.4 mg/kg has no effect on the fertility of C57BL/6NTac mice. However, we noted dose-dependent increases in mortality in neonatal mice in the 2.7- and 5.4-mg/kg groups. In the highest dose group, none of the pups survived past the fourth day. This effect was transient, and litters born in the 12- and 48-ppm groups after cessation of treatment had identical survival rates. Even pups born immediately after treatment ended and that therefore had been exposed to ivermectin in utero for most of the pregnancy survived. This finding indicates that there was no measurable intrauterine effect of ivermectin on the pups.
In light of these results, we propose that feed containing ivermectin at dose levels of 2.7 mg/kg and higher can be toxic to neonates. The increased mortality in neonates in the higher dose groups is most likely due to 2 processes. Nursing pups are exposed to ivermectin through milk. The amount of ivermectin transferred to pups is dependent on the dose exposure of the dam.21 Because ivermectin is lipophilic, it concentrates in the milk, and the pups’ oral exposure level is higher than that of the dam. In addition, the blood–brain barrier of rodents is not fully developed until approximately 10 d of age. One study21 reports similar findings in rats exposed to ivermectin and attributes neonatal mortality to the accumulation of ivermectin to toxic levels in the brain. Although high doses of ivermectin do affect pup survival adversely, we were unable to duplicate the severe maternotoxicity and teratogenic effects described in the original toxicology study,21 even at dose levels 25-fold higher than originally reported. This finding is supported by subsequent published treatment protocols. Although adult toxicity was studied in CD1 mice, reproductive toxicity was studied in CF1 mice. The CF1 stock later was found to have a high percentage of mice with a P-glycoprotein deficiency due to a natural mutation.20 This deficiency markedly increases the susceptibility of these mice to ivermectin toxicity. The unfortunate use of this highly susceptible stock in the toxicity study gave rise to the idea that mice in general are highly susceptible to ivermectin toxicity.
We did not observe any adverse effects of ivermectin exposure on the fertility or fecundity of offspring of C57BL/6NTac mice treated with ivermectin. Interestingly, we observed the opposite effect. Animals that had been exposed to 2.7 mg/kg ivermectin as neonates produced significantly more litters and pups than did animals in the control group. We hypothesize this difference is most likely due to the leaner body weight induced by neonatal ivermectin exposure.
In conclusion, our results suggest that ivermectin can be compounded in rodent feed and, although a decline in drug concentration occurs due to manufacturing, the remaining drug is stable for at least 6 mo postmilling. Ivermectin can be safely administered to neonatal, juvenile, adult, and pregnant C57BL/6NTac mice at 1.3 mg/kg daily for an 8-wk period. Further studies should be conducted to assess safety in other strains of mice, including those known to be highly susceptible to ivermectin toxicity.
Acknowledgments
We thank Dr Suzana Couto and the staff of the Laboratory for Comparative Pathology for their diagnostic support, Lisa Eldred for her invaluable help in caring for mite-infested mice, Dr Nian Wu and the staff of the Analytical Pharmacology Core Facility at MSKCC for their help with spectrometry analysis, Dr Carrie Schultz and Dr Kristi Thompson (Purina Test Diet, St Louis, MO) for their help with feed production, and Dr George Conder (Pfizer) for his helpful insight during the early stages of this project.
References
- 1.Altholtz LY, La Perle KM, Quimby FW. 2006. Dose-dependant hypothyroidism in mice induced by commercial trimethoprim–sulfamethoxazole rodent feed. Comp Med 56:395–401 [PubMed] [Google Scholar]
- 2.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. 2002. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32:435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumans V, Havenaar R, van Herck H. 1988. The use of repeated treatment with Ivomec and Neguvon spray in the control of murine fur mites and oxyurid worms. Lab Anim 22:246–249 [DOI] [PubMed] [Google Scholar]
- 4.Baumans V, Havenaar R, van Herck H, Rooymans TP. 1988. The effectiveness of Ivomec and Neguvon in the control of murine mites. Lab Anim 22:243–245 [DOI] [PubMed] [Google Scholar]
- 5.Bissonnette S, Paradis M, Daneau I, Silversides DW. 2009. The ABCB1 1δ mutation is not responsible for subchronic neurotoxicity seen in dogs of noncollie breeds following macrocyclic lactone treatment for generalized demodicosis. Vet Dermatol 20:60–66 [DOI] [PubMed] [Google Scholar]
- 6.Blakley BR, Rousseaux CG. 1991. Effect of ivermectin on the immune response in mice. Am J Vet Res 52:593–595 [PubMed] [Google Scholar]
- 7.Burdett EC, Heckmann RA, Ochoa R. 1997. Evaluation of 5 treatment regimens and 5 diagnostic methods for murine mites (Myocoptes musculinus and Myobia musculi). Contemp Top Lab Anim Sci 36:73–76 [PubMed] [Google Scholar]
- 8.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276 [DOI] [PubMed] [Google Scholar]
- 9.Conole J, Wilkinson MJ, McKellar QA. 2003. Some observations on the pharmacological properties of ivermectin during treatment of a mite infestation in mice. Contemp Top Lab Anim Sci 42:42–45 [PubMed] [Google Scholar]
- 10.Davis JA, Paylor R, McDonald MP, Libbey M, Ligler A, Bryant K, Crawley JN. 1999. Behavioral effects of ivermectin in mice. Lab Anim Sci 49:288–296 [PubMed] [Google Scholar]
- 11.Flynn BM, Brown PA, Eckstein JM, Strong D. 1989. Treatment of Syphacia obvelata in mice using ivermectin. Lab Anim Sci 39:461–463 [PubMed] [Google Scholar]
- 12.Gaertner DJ. 2004. Speculations on why some lab rodent pathogens continue to be prevalent. Contemp Top Lab Anim Sci 43:8. [PubMed] [Google Scholar]
- 13.Gönenç B, Sarimehmetoğlu HO, Iça A, Kozan E. 2006. Efficacy of selamectin against mites (Myobia musculi, Mycoptes musculinus and Radfordia ensifera) and nematodes (Aspiculuris tetraptera and Syphacia obvelata) in mice. Lab Anim 40:210–213 [DOI] [PubMed] [Google Scholar]
- 14.Huerkamp MJ, Zitzow LA, Webb S, Pullium JK. 2005. Cross-fostering in combination with ivermectin therapy: a method to eradicate murine fur mites. Contemp Top Lab Anim Sci 44:12–16 [PubMed] [Google Scholar]
- 15.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 16.Jacoby RO, Fox JG, Davisson M. Biology and diseases of mice. : Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed Orlando (FL): Academic Press [Google Scholar]
- 17.Jacoby RO, Lindsey JR. 1998. Risks of infection among laboratory rats and mice at major biomedical research institutions. ILAR J 39:266–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston NA, Trammell RA, Ball-Kell S, Verhulst S, Toth LA. 2009. Assesment of immune function in mice before and after eradication of mite infestation. J Am Assoc Lab Anim Sci 48:371–377 [PMC free article] [PubMed] [Google Scholar]
- 19.Klement P, Augustine JM, Delaney KH, Klement G, Weitz JI. 1996. An oral ivermectin regimen that eradicates pinworms (Syphacia spp.) in laboratory rats and mice. Lab Anim Sci 46:286–290 [PubMed] [Google Scholar]
- 20.Lankas GR, Cartwright ME, Umbenhauer D. 1997. P-glycoprotein deficiency in a subpopulation of CF1 mice enhances avermectin-induced neurotoxicity. Toxicol Appl Pharmacol 143:357–365 [DOI] [PubMed] [Google Scholar]
- 21.Lankas GR, Gordon LR. Toxicology. : Campbell WC. Ivermectin and abamectin. New York (NY): Springer–Verlag [Google Scholar]
- 22.Lankas GR, Wise LD, Cartwright ME, Pippert T, Umbenhauer DR. 1998. Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod Toxicol 12:457–463 [DOI] [PubMed] [Google Scholar]
- 23.Levee EM, Klinger MM, Kaiser CC, Serrano LJ. 1994. A practical delivery method for oral administration of ivermectin to large colonies of rodents. Contemp Top Lab Anim Sci 33:68–70 [PubMed] [Google Scholar]
- 24.Lipman NS, Dalton SD, Stuart AR, Arruda K. 1994. Eradication of pinworms (Syphacia obvelata) from a large mouse breeding colony by combination oral anthelmintic therapy. Lab Anim Sci 44:517–520 [PubMed] [Google Scholar]
- 25.McIntyre AR, Lipman NS. 2007. Amoxicillin–clavulanic acid and trimethoprim– sulfamethoxazole in rodent feed and water: effects of compounding on antibiotic stability. J Am Assoc Lab Anim Sci 46:26–32 [PubMed] [Google Scholar]
- 26.Mook DM, Benjamin KA. 2008. Use of selamectin and moxidectin in the treatment of mouse fur mites. J Am Assoc Lab Anim Sci 47:20–24 [PMC free article] [PubMed] [Google Scholar]
- 27.Moore TD, Horton R, Utrup LJ, Miller LA, Poupard JA. 1996. Stability of amoxicillin–clavulanate in BACTEC medium determined by high-performance liquid chromatography and bioassay. J Clin Microbiol 34:1321–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papini R, Marconcini A. 1991. Treatment with ivermectin in drinking water against Myobia musculi and Myocoptes musculinus mange in naturally infected laboratory mice. Angew Parasitol 32:11–13 [PubMed] [Google Scholar]
- 29.Pollicino P, Rossi L, Rambozzi L, Farca AM, Peano A. 2007. Oral administration of moxidectin for treatment of murine acariosis due to Radfordia affinis. Vet Parasitol 151:355–357 [DOI] [PubMed] [Google Scholar]
- 30.Ricart Arbona RJ, Lipman NS, Wolf F. 2010. Murine fur mite treatment and eradication III. Treatment of a large mouse colony with ivermectin-compounded feed. J Am Assoc Lab Anim Sci 49:633–637 [PMC free article] [PubMed] [Google Scholar]
- 31.Schinkel AH, Smit JJ, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CA, van der Valk MA, Robanus-Maandag EC, te Riele HP, Berns AJM, Borst P. 1994. Disruption of the mouse Mdr1a P-glycoprotein gene leads to a deficiency in the blood–brain barrier and to increased sensitivity to drugs. Cell 77:491–502 [DOI] [PubMed] [Google Scholar]
- 32.Silverman J, Blatt H, Lerro A. 1983. Effect of ivermectin against Myobia musculi. Lab Anim Sci 33:487 [Google Scholar]
- 33.Skopets B, Wilson RP, Griffith JW, Lang CM. 1996. Ivermectin toxicity in young mice. Lab Anim Sci 46:111–112 [PubMed] [Google Scholar]
- 34.Support TFT Taconic inquiry response 7.7.09. [Google Scholar]
- 35.Takano N, Arai I, Kurachi M. 2006. A method to induce stable atopic dermatitis-like symptoms in NC/Nga mice housed with skin-lesioned mice. Br J Dermatol 154:426–430 [DOI] [PubMed] [Google Scholar]
- 36.Toth LA, Oberbeck C, Straign CM, Frazier S, Rehg JE. 2000. Toxicity evaluation of prophylactic treatments for mites and pinworms in mice. Contemp Top Lab Anim Sci 39:18–21 [PubMed] [Google Scholar]
- 37.Turner MJ, Schaeffer JM. Mode of action of ivermectin. : Campbell WC. Ivermectin and abamectin. New York (NY): Springer–Verlag [Google Scholar]
- 38.Valvo L, Manna L, Alimenti R, Alimonti S, Bertocchi P, Ciranni E. 1999. Amoxicillin sodium–potassium clavulanate: evaluation of γ-radiation-induced effects by liquid chromatography on both the individual drugs and their combination. J Pharm Biomed Anal 21:9–14 [DOI] [PubMed] [Google Scholar]
- 39.Watson DP. 1961. The effect of the mite Myocoptes musculinus (CL Koch 1840) on the skin of the white laboratory mouse and its control. Parasitology 51:373–378 [DOI] [PubMed] [Google Scholar]
- 40.Whiteley HJ, Horton DL. 1965. Further observations on the effect of Myobia musculi on the skin of the mouse. J Pathol Bacteriol 89:331–335 [PubMed] [Google Scholar]
- 41.Wing SR, Courtney CH, Young MD. 1985. Effect of ivermectin on murine mites. J Am Vet Med Assoc 187:1191–1192 [PubMed] [Google Scholar]