Abstract
Fur mites are a persistent problem in contemporary laboratory mouse colonies. We conducted several studies to evaluate fur mite diagnostic methodologies and interpretation of results. Retrospective analysis of test results from sentinel mice exposed to soiled bedding collected from colonies infested with Myobia musculi and Myocoptes musculinus revealed the skin scrape test to be more reliable than pelt examination, provided that both the head and dorsal thoracolumbar regions were sampled. To assess their diagnostic accuracy, 3 commercial laboratories were sent positive control slides containing mites, mite parts, or eggs in sets of slides containing diagnostic skin scrapings in varying ratios. Laboratory B correctly identified the positive control slide. Laboratory A identified 1 of 3 positive control slides, whereas laboratory C failed to identify both positive control slides submitted. To determine the time required for a mouse to shed its entire hair coat, fur of Crl:CD1(ICR), BALB/cAnNCrl, and Crl:CFW(SW) albino mice was dyed black and the presence of dyed fur evaluated monthly for 8 mo. Limited dyed hair was still present at 8 mo; therefore, finding eggs or egg casings many months after treatment cessation does not necessarily imply treatment failure. To evaluate the effectiveness of soiled bedding sentinels for detection of fur mites in a mite-infested colony, we exposed naïve mice to varying amounts (100%, 50%, 25%, 2.5%, and 0%) of soiled bedding in clean bedding. As little as 2.5% soiled bedding resulted in detection of a positive sentinel within a 2-mo period.
Despite great advancements in laboratory animal science and technologic discoveries, rodent fur mites remain a persistent problem in contemporary laboratory mouse colonies. A survey conducted in 2006 found that as many as 40% of facilities were infested with either Myobia musculi, Myocoptes musculinus, or both.4 Their continued persistence, even in barrier colonies, may be related to the low sensitivity of diagnostic tests, resulting in false negative results.6
Recently we experienced a fur mite outbreak with both Myobia musculi and Myocoptes musculinus simultaneously in 3 vivaria housing approximately 30,000 mouse cages. We suspected that the fur mites entered our facility on imported animals due to failure to identify their presence during quarantine. This assumption triggered an investigation of the detection method used, evaluation of diagnostic laboratory reliability, and development of a novel treatment regime suitable for large mouse colonies. The treatment regime we developed is reported elsewhere.15,16
The difficulty of diagnosing fur mites in rodents is multifactorial. Adult rodent fur mites are approximately 250 µm in length and easily blend into the animal's fur, making it very difficult to observe them under the microscope. Mite burdens on individual mice can vary widely depending on the length of infestation, mouse strain and age, and presence of self or conspecific grooming.5,19 Several diagnostic techniques have been described, including microscopic examination of skin scrapes, tape impressions of fur, and hair plucks; pelt digestion; stereomicroscopic examination of cooled pelts; and, observation of clinical signs.2,10,18 Microscopic analysis of skin scrapes and pulled hair tufts are probably the most commonly used methods to screen for fur mites.3,12 Myobia musculi is reported to preferentially inhabit the forehead and cheeks, whereas Myocoptes musculinus reportedly prefers the ventral abdomen and inguinal areas.9,10 Therefore, the choice of anatomic area sampled likely will affect test sensitivity depending on the mite species present. In addition, because diagnostic methods rely on microscopic identification by appropriately trained personnel, all of these tests are labor-intensive, exposing personnel to fatigue, and thus are prone to error.
During the outbreak, we found that the mites were transmitted readily to sentinel mice receiving soiled bedding within a 2-mo testing cycle.16 Although the successful use of soiled bedding to transfer Myobia musculi to sentinels has been reported,17 no attempt was made to determine sensitivity or the minimal time needed for sentinels to become infested. Other published and anecdotal reports, which suggested that soiled bedding is a poor fomite for fur mite transmission and that sentinel programs using soiled bedding are an inefficient method to screen for mites, contrasted with our experience.8,10 During follow-up testing after facility-wide fur mite treatment, we occasionally (0.05% of submitted samples) found eggs and egg casings on mice from which we had failed to detect adult or larval forms for as long as 3 mo after completion of treatment.16 Others have described similar findings.12,14 We hypothesized that the presence of these eggs and casings did not reflect treatment failure but represented persistence of nonviable mite stages.
In the studies reported here, we evaluated various test methods and sample collection sites to compare detection efficiencies in mice with low to moderate mite burdens. In addition, we suspected that some false-negative results may reflect human error and therefore submitted known-positive slides with large numbers of unknown samples to 3 commercial diagnostic laboratories to determine the frequency of detection failure. Furthermore we validated the efficacy of soiled bedding sentinel health monitoring programs by exposing mice to various concentrations of soiled bedding from M. musculi and M. musculinus infested mice. Finally, we characterized the hair shedding pattern in mice and its duration to better understand the persistence of eggs and egg casings in mice treated with an acaricide.
Materials and Methods
Animals.
Mice of various stocks and strains [Crl:CD1(ICR), BALB/cAnNCrl, Crl:CFW(SW)] were obtained from Charles River Laboratories (Wilmington, MA). On arrival, animals were free of antibodies to mouse hepatitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, mouse parvovirus, minute virus of mice, murine norovirus, pneumonia virus of mice, reovirus type 3, Sendai virus, Theiler mouse encephalomyelitis virus, mouse adenovirus, K virus, polyoma virus, mouse cytomegalovirus, mouse thymic virus, Haantan virus, lactic dehydrogenase elevating virus, cilia-associated respiratory bacillus, and Mycoplasma pulmonis. Animals also were free of Helicobacter spp., Salmonella spp., Clostridium piliforme, Corynebacterium kutscheri, Citrobacter rodentium, and endoparasites and ectoparasites. All mice were housed at Animal Biosafety Level 2 in a quarantine facility in solid-bottom polysulfone shoebox containment cages maintained in an individually ventilated caging system providing 2 zones (cage and row) of differential pressure (Dual Zone, Biozone, Burton upon Trent, UK) on autoclaved aspen chip bedding (PWI Industries Canada, Quebec, Canada). Gamma-irradiated feed (LabDiet 5058, PMI, St Louis, MO) and acidified water (pH 2.5 to 2.8) were provided ad libitum. Cages were changed in a certified Class II Type A2 biological safety cabinet (NU S602-500 Series SP, Nuaire, Plymouth, MN) weekly. The holding room was ventilated with filtered (95% ASHRAE efficient), 100% outside air at 15 air changes per hour. Light:dark photoperiod cycle was maintained at 12:12 h intervals. Room temperature was maintained at 72 ± 2 °F and relative humidity at 30% to 70%.
Animal use was approved by the Memorial Sloan–Kettering Cancer Center IACUC. The animal care and use program at Memorial Sloan–Kettering Cancer Center is AAALAC-accredited, and all animals are maintained in accordance to the recommendations provided in the Guide for the Use and Care of Laboratory Animals7.
Comparison of diagnostic techniques and sample collection sites.
Skin scrape.
A no. 10 scalpel blade was used to scrape at a 90° angle to the surface of the skin an approximately 1-cm2 area of skin of the head (scalp over the calvarium between the ears), back (midline dorsal thoracolumbar junction), and ventrum (ventral inguinal at the level of the femoral triangle, bilaterally). Loose hair and debris was transferred to a 2 × 2.5 cm piece of cellophane tape and affixed to a glass slide. Slides were examined under scanning microscopy at low power (100×) by following a grid pattern until the entire piece of tape, including hair fibers extending beyond its borders, was evaluated.
Pelt examination.
The entire pelt, including the skin of the head, was collected immediately after euthanasia by CO2 inhalation. After removal, pelts were mounted in sealed culture dishes and incubated at room temperature for 3 to 4 h. Subsequently they were examined under a dissecting stereomicroscope at 50× power by following a grid pattern, until the entire pelt and dish surface were examined. A positive result from either technique was defined as identification of a mite, mite parts, egg, or egg casing.
Fur mite test results from 2 soiled bedding sentinel cohorts submitted to our diagnostic laboratory were analyzed retrospectively. Sentinels (2- to 12-mo-old female Crl:CD1[ICR] mice) were used to monitor the previously described colonies involved in the outbreak with Myobia musculi and Myocoptes musculinus. A total of 222 sentinel mice were tested once for fur mites. Cohort 1 (n = 130 mice) comprised skin scrape samples collected from 2 sites (head and back) and pelt examination. Cohort 2 (n = 92 mice) comprised skin scrape samples from 3 sites (head, back, and ventrum).
Quality assurance testing.
To monitor the reliability of various diagnostic laboratories in diagnosing fur mites, 100, 200, and 300 diagnostic skin scrape slides (collected as described earlier and including 1 to 3 positive control slides) were sent to 3 commercial diagnostic laboratories, respectively. The control slides contained mites, mite parts, or mite eggs or combinations thereof.
Coat shedding.
We speculated that mite eggs and casings can persist for months in mice presumptively treated successfully for fur mites, because eggs are attached permanently to hair shafts and potentially could remain on the animal until the respective hair is shed. Although hair growth in mice has been studied extensively,13 to our knowledge the time required for a mouse to completely shed all of its hair has not been reported. To determine the time needed for a mouse to shed its entire coat of fur, we applied black hair dye to 6-wk-old female BALB/cAnNCrl, Crl:CFW(SW), and Crl:CD1(ICR) mice (n = 4 per strain or stock). A commercial hair dye (Revlon Colorsilk, Oxford, NC) was used according to the manufacturers' instructions, except that the color was washed off the animal after 5 min (instead of the recommended 25 min). Mice were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) administered intraperitoneally in combination. After anesthesia was attained, hair dye was applied carefully, avoiding the scalp, eyes, ears, mouth, anus, and urogenital opening. Mice were washed thoroughly with warm water after 5 min to remove all dye residues and were allowed to recover from anesthesia. Mice were checked daily for 3 d after the procedure for signs of toxicity. No adverse effects were noticed. Animals were anesthetized monthly for 8 mo with isoflurane (2% to 3%), and their dorsal and ventral surfaces were photographed to monitor changes in coat color.
Soiled bedding transmission.
A pilot study was conducted to determine the efficacy of transmission of fur mites by soiled bedding transfer. Naïve 6-wk-old female Crl:CD1(ICR) mice were exposed to different concentrations of soiled bedding during weekly cage changes. Composite contaminated bedding samples obtained from 20 cages, each containing 3 to 4 mice that had been naturally infested with both Myobia musculi and Myocoptes musculinus during the outbreak were used. These mice were maintained in our quarantine facilities for the purpose of these experiments. At cage change, all contaminated bedding was emptied into a plastic bag and mixed thoroughly. Five groups, each consisting of one cage housing 2 female mice, received varying amounts of soiled:clean bedding (100%:0%, 50%:50%, 25%:75%, 2.5%:97.5%, and 0%:100% [control]). A final volume of 600 mL bedding was provided by using a calibrated medicine cup. Each cage was changed weekly in order of most soiled bedding to least to monitor for potential cage-to-cage transfer of mites. Skin scrapes (head and back) were performed weekly on every animal until both mice in each group yielded positive scrapes for 2 consecutive weeks.
Results
Comparison of diagnostic techniques and sample collection sites.
Skin scrape and pelt examination results obtained from sentinels exposed to soil bedding collected from fur mite infested colonies were evaluated over a 2-mo period. In cohort 1 (mice that underwent pelt examinations and from which skin scrape samples were obtained from 2 sites), 18 of 130 sentinels tested positive for mites by skin scrape test. In most mice, mites were detected at a single site (back, 44%; head, 39%); only 3 sentinels (17%) were mite-positive at both sites. Mites were not detected in any sentinel by pelt examination. In cohort 2 (mice from which skin scrape samples were obtained from 3 sites), 18 of 92 sentinels tested positive for mites. Of these, mites were found most frequently on the head (94%), followed by the back (39%) and ventrum (11%). In 10 animals mites were found exclusively on the head. Mites were found on the back but not the other 2 sites in a single sentinel. The 2 animals from which mites were detected on the ventrum were also mite-positive at one or more other sites.
Quality assurance testing.
Among the 3 commercial laboratories to which we sent samples, Laboratory B correctly identified the single positive control slide submitted. Laboratory A correctly identified 1 of the 3 positive control slides submitted, whereas laboratory C did not identify either of the 2 positive control slides provided. All slides were returned to us by the commercial laboratories and reevaluated by our own personnel. All slides, except for the known positive control slides, were negative for mites and eggs.
Coat shedding.
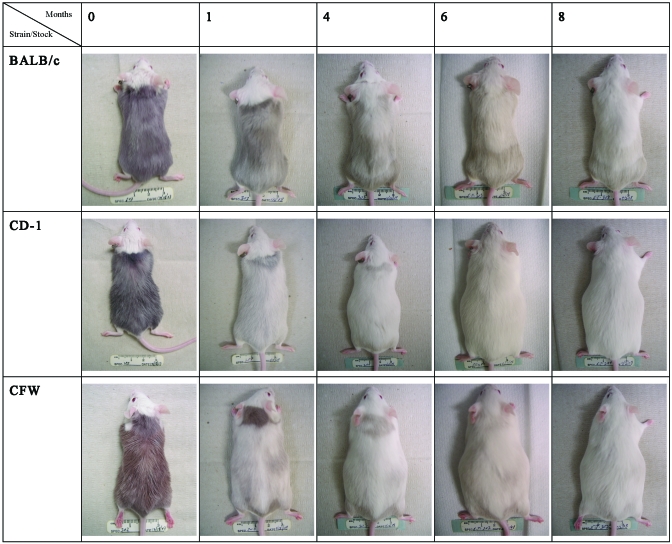
Figure 1 shows the pattern of coat color changes from representative BALB/cAnNCrl, Crl:CFW(SW), and Crl:CD1(ICR) mice on day 0 and selected months through 8 mo after dye application. Strain-dependent differences are apparent, with BALB/cAnNCrl mice retaining more dyed hair than Crl:CFW(SW) or Crl:CD1(ICR) mice at each time point. Hair appeared to be shed in multiple waves in a cranial to caudal direction. However, small areas of dyed hair, primarily in dorsal lumbar areas, were still present at the end of the 8-mo observation period in all 3 mouse strains. These results indicate that in these mouse strains and stocks, more than 8 mo is needed for a mouse to shed its entire hair coat.
Figure 1.
Representative pictures of the pelage of BALB/cAnNCrl, Crl:CFW(SW), and Crl:CD1(ICR) mice at various time points after dye application.
Soiled bedding transmission study.
Both sentinels exposed to 600 mL (100%) contaminated bedding tested positive for mites within 2 wk. One mouse exposed to 50% soiled bedding tested positive at week 2, but the second mouse in the same cage didn't test mite-positive until week 5. One of the 2 mice exposed to 25% soiled bedding tested positive at week 3, and both were positive at week 4. One mouse exposed to 15 mL (2.5%) soiled bedding tested positive at week 7, whereas the second mouse tested positive at week 8. Once an individual animal tested positive, it remained positive on the next test (performed 1 wk later). Control animals that received clean bedding only and that were handled last during cage change to monitor for potential cage-to-cage transmission of mites remained negative for the duration of the study.
Discussion
Various testing modalities have been described to diagnose murine fur mites, including microscopic examination of hair collected by removal with a forceps, applying clear cellophane tape to the fur, or scraping with a scalpel blade and postmortem stereomicroscopic examination or digestion of the pelage.2,10,18 However, only one report evaluated testing methods by comparing them with the skin scrape test, which was subjectively designated as the ‘gold standard.’3 Unfortunately, the cited report does not indicate whether any of the samples that tested negative on the skin scrape ever tested positive by using any other method, leaving this important question unanswered. Nevertheless, the previous report indicated that the direct examination of the pelt with the stereomicroscope yielded results comparable to the skin scrape method, whereas the cellophane tape method was only slightly less sensitive.
The cellophane tape method had been in use at our institution prior to the mite outbreak. We assumed that its lack of sensitivity, coupled with poor implementation (for example, incorrect sample site), may have led to diagnostic failure in our rodent quarantine program. In light of the previous report,3 we switched to the skin scrape test method combined, for terminal examinations, with direct examination of the pelt. After a 2-mo period, the results were analyzed and are reported herein. All pelt examinations were negative, whereas 13.8% of the animals tested positive by skin scrape. This finding was surprising, because many authors consider pelt examination to be superior, or at least comparable, to the skin scrape method.1,3 One possible explanation for this discrepancy could be that the mite burden on our sentinel mice was lower than that of mice examined in other studies. However, we did not quantify mite burdens to substantiate this possibility.
To increase the likelihood of detecting mites, mite parts, eggs, and egg casings with the methods we used, we switched from the cellophane tape method to skin scrape and collected samples from the animals' head, back, and ventrum. These additional locations were selected based on the published biology of the 2 fur mite species present. 10 Results from skin scrapes collected from mice with mixed-species infestations indicated that sampling 2, head and back, of the 3 sites tested is essential to avoid false-negative results. Each of these areas yielded mites at times when the other 2 sites were negative. Interestingly, the ventral inguinal area is reportedly a preferred site for Myocoptes musculinus;10 however, when mites were detected from this area, they always were present in at least one other site tested. Reported location preferences by fur mite species were from single-species infestations. In our experience with mixed infestations, Myocoptes musculinus, when given sufficient time, tends to overrun Myobia musculi and even displaces it so that either species can be found in ‘atypical’ locations, as has been described previously.8 Therefore, we recommend that when using the skin scrape method, samples should minimally be collected from both the animal's head and back.
In addition to sample collection methods and sampling location, accurate slide examination is vital for test sensitivity. Microscopic examination of skin scrape samples for ectoparasites is a tedious process, especially when examining large numbers of slides. The possibility of identifying a single mite or egg on a slide covered with hair and debris is subject to error. This propensity was confirmed by sending known positive slides with large numbers of unknown diagnostic samples to commercial laboratories that specialize in laboratory animal diagnostics. The number of positive control slides included in each submission was chosen according to their availability at the time of submission. Not all laboratories received the same number of positive control slides—a situation that we feel actually better mimics a real scenario, in which the total number of positive slides would not necessarily correspond to the total number of slides submitted. In our evaluation, 2 of the 3 laboratories failed to detect at least one of the positive slides submitted. As a result of these findings, we recommend the routine submission of control (known mite-positive) slides with diagnostic samples regardless of the diagnostic laboratory used. This practice enables the submitter to objectively assess the accuracy of the examination and provide the technician with positive feedback, especially when most of the samples examined are expected to be negative.
We obtained a small number (0.05%) of positive skin scrape samples that contained eggs or egg casings from our colonies during follow-up testing after using an acaricide. Others have reported similar findings for as long as 6 mo after treatment1,3 and have interpreted this finding to reflect treatment failure.12 We noted samples positive for eggs or egg casings for as long as 3 mo after the end of treatment. Despite following published guidelines describing specific criteria to distinguish viable from nonviable eggs,3,14 we found it extremely difficult to determine whether these mite eggs were viable and whether they had been laid before or after treatment. We speculated that all the eggs we detected were nonviable and that some could remain until the entire hair coat is shed. Although shedding patterns and differences between strains have been reported,13 the duration to shed the entire coat has not.
Using 2 outbred albino stocks and 1 inbred albino strain, we observed that at least some dyed hair remained after an 8-mo period. Therefore, it is possible to find eggs, empty casings, or mite parts attached even after 8 mo after treatment. Considering our experience and these results, we implemented a 3-mo posttreatment testing moratorium prior to initiating follow-up testing, to reduce the number of confounding results. We felt this period was a reasonable amount of time for enough hair to be shed to reduce the probability of false-positive results. Ultimately, naïve contact sentinels could be added to cages when test results are questionable, because these mice would only become positive if an active infestation is present within the cage.
The mite outbreak that led to the initiation of the studies described in the current report was discovered through our colony health monitoring program.11 When first identified, mites were found in soiled bedding sentinels from approximately 40% of the rooms tested within the same testing cycle. All sentinels were negative during testing 2 mo earlier, and only a few additional positive rooms were found over the next 2 mo. The reason for this rapid spread of mites is discussed elsewhere.16 We concluded that our soiled bedding sentinel program was highly effective in detecting fur mites from colony animals. The effectiveness of mite transmission through dirty bedding is controversial. Many cite a study by Letscher10 as evidence that dirty bedding is ineffective as a fomite for fur mites. In this study, a screen was used to separate naïve mice from mite-infested mice housed in the same cage. The screen only provided enough space at the bottom of the cage to allow movement of bedding while preventing direct contact between mice. In contrast, Myobia musculi was detected in a colony by using soiled bedding sentinels. 17 In that case, only 1 of 12 racks had mite-infested mice, but the prevalence on the affected rack was quite high (approximately 56% of cages). Unfortunately, mite testing was not initiated until 4 mo after sentinels had been exposed to soiled bedding; therefore, how soon after Myobia musculi gained access to the colony after it was transferred to the sentinels is unclear.
To further examine the effectiveness of soiled bedding transfer of mites, we exposed naïve mice to various concentrations of contaminated bedding from mice with mixed Myobia musculi and Myocoptes musculinus infestation. By diluting the contaminated bedding with clean bedding, we attempted to estimate the minimal prevalence of colony infestation that could be detected within a 2-mo testing cycle. Exposure to higher concentrations of soiled bedding resulted in earlier detection of fur mites. In our pilot study, results indicated that only 2.5% of the bedding (1 of 40 cages) needs to be mite-infested to lead to a positive soiled bedding sentinel within 8 wk, thus corresponding to a 2.5% prevalence in the colony.
Our results are in accordance with previous findings,17 and both studies support the use of soiled bedding sentinels as a method to screen colonies, even those with an extremely low prevalence of mites. We speculate that anecdotal reports suggesting that soiled bedding sentinels are ineffective for fur mite detection arise from incorrect implementation of the soiled bedding transfer, rather than from an inherent deficiency of the method.
In conclusion, we have found the skin scrape method to be the most sensitive method for the detection of fur mites and that both the head and back of a mouse should always be sampled. Even when sampling is done correctly, meticulous examination of the slides is imperative to avoid false-negative results. Hair shedding varies among strains and must be taken into consideration to avoid confounding results during posttreatment testing. Soiled bedding transfer to sentinels, as implemented in the present study, is a reliable method to screen for fur mite infestation.
Acknowledgments
We thank Dr Suzana Couto and the staff of the Laboratory for Comparative Pathology for their diagnostic support and Lisa Eldred for her invaluable help in caring for mite-infested mice.
References
- 1.Baumans V, Havenaar R, van Herck H. 1988. The use of repeated treatment with Ivomec and Neguvon spray in the control of murine fur mites and oxyurid worms. Lab Anim 22:246–249 [DOI] [PubMed] [Google Scholar]
- 2.Baumans V, Havenaar R, van Herck H, Rooymans TP. 1988. The effectiveness of Ivomec and Neguvon in the control of murine mites. Lab Anim 22:243–245 [DOI] [PubMed] [Google Scholar]
- 3.Burdett EC, Heckmann RA, Ochoa R. 1997. Evaluation of 5 treatment regimens and 5 diagnostic methods for murine mites (Myocoptes musculinus and Myobia musculi). Contemp Top Lab Anim Sci 36:73–76 [PubMed] [Google Scholar]
- 4.Carty AJ. 2008. Opportunistic infections of mice and rats: Jacoby and Lindsey revisited. ILAR J 49:272–276 [DOI] [PubMed] [Google Scholar]
- 5.Friedman S, Weisbroth SH. 1975. The parasitic ecology of the rodent mite Myobia musculi. II. Genetic factors. Lab Anim Sci 25:440–445 [PubMed] [Google Scholar]
- 6.Gaertner DJ. 2004. Speculations on why some lab rodent pathogens continue to be prevalent. Contemp Top Lab Anim Sci 43:8. [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 8.Jacoby RO, Fox JG, Davisson M. Biology and diseases of mice. : Fox JG, Anderson LC, Loew FM, Quimby FW. Laboratory animal medicine, 2nd ed Orlando (FL): Academic Press [Google Scholar]
- 9.Labrzycka A. 2004. [Ectoprasitic mites of the families Myocoptidae and Listrophoridae (Acari: Astigmata) infecting mammals in Poland] Wiad Parazytol 50:117–124 [Article in Polish] [PubMed] [Google Scholar]
- 10.Letscher RM. Observations concerning the life cycle and biology of Myobia musculi (Schrank) and Myocoptes musculinus (Koch). [MS Thesis] College Station (TX): Texas A&M University [Google Scholar]
- 11.Lipman NS, Homberger FR. 2003. Rodent quality assurance testing: use of sentinel animal systems. Lab Anim (NY) 32:36–43 [DOI] [PubMed] [Google Scholar]
- 12.Mook DM, Benjamin KA. 2008. Use of selamectin and moxidectin in the treatment of mouse fur mites. J Am Assoc Lab Anim Sci 47:20–24 [PMC free article] [PubMed] [Google Scholar]
- 13.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. 2001. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol 117:3–15 [DOI] [PubMed] [Google Scholar]
- 14.Pullium JK, Brooks WJ, Langley AD, Huerkamp MJ. 2005. A single dose of topical moxidectin as an effective treatment for murine acariasis due to Myocoptes musculinus. Contemp Top Lab Anim Sci 44:26–28 [PubMed] [Google Scholar]
- 15.Ricart Arbona RJ, Lipman NS, Wolf F. 2010. Treatment and eradicationof murine fur mites: I. Toxicologic evaluation of ivermectin-impregnated feed. J Am Assoc Lab Anim Sci 49:564–570 [PMC free article] [PubMed] [Google Scholar]
- 16.Ricart Arbona RJ, Lipman NS, Wolf F. 2010. Treatment and eradication of murine fur mites: III. Treatment of a large mouse colony with ivermectin-compunded feed. J Am Assoc Lab Anim Sci 49:633–637 [PMC free article] [PubMed] [Google Scholar]
- 17.Thigpen JE, Lebetkin EH, Dawes ML, Amyx HL, Caviness GF, Sawyer BA, Blackmore DE. 1989. The use of dirty bedding for detection of murine pathogens in sentinel mice. Lab Anim Sci 39:324–327 [PubMed] [Google Scholar]
- 18.Weisbroth SH, Friedman S, Powell M, Scher S. 1974. The parasitic ecology of the rodent mite Myobia musculi. I. Grooming factors. Lab Anim Sci 24:510–516 [PubMed] [Google Scholar]
- 19.Weisbroth SH, Friedman S, Scher S. 1976. The parasitic ecology of the rodent mite, Myobia musculi. III. Lesions in certain host strains. Lab Anim Sci 26:725–735 [PubMed] [Google Scholar]