Abstract
The purpose of this study was to determine the level of pain elicited by mammary fat pad removal surgery and the effects of postoperative analgesics on recovery. Female FVB mice were anesthetized, and mammary fat pad removal was performed. After surgery, mice received carprofen, buprenorphine, a combination of carprofen and buprenorphine, or saline treatment. Additional mice received anesthesia but no surgery or treatment. Food and water intake, body weight, wheel running activity, and a visual assessment score were recorded daily for 4 d after surgery and compared with presurgical findings. Corticosterone metabolites in fecal samples were analyzed at 12 and 24 h postsurgically and compared with baseline values. All surgical groups had significantly decreased food intake at 24 h, with a return to baseline by 48 h. The combination treatment resulted in a significantly decreased water intake and body weight at 24 h. All surgical groups had significantly decreased wheel running activity at 24 h only. The visual assessment scores indicated mild pain for all surgical groups, with the buprenorphine treated mice showing the highest pain index scores, as compared with nonsurgical controls. Fecal corticosterone metabolite levels did not differ significantly between any of the groups or across time. The parameters used in this study did not indicate that administration of these analgesic regimens improved recovery as compared with that of saline-treated mice. Care should be taken when using visual assessment scores to evaluate pain in mice, given that analgesics may have side effects that inadvertently elevate the score.
The recognition, prevention, and alleviation of pain are common challenges in laboratory animal medicine, particularly when working with rodents. The ILAR Guide for the Care and Use of Laboratory Animals states that, “In general, unless the contrary is known or established it should be assumed that procedures that cause pain in humans also cause pain in animals.”15 However, despite this guideline, the reported use of analgesics in rodents is still fairly low. Only 19.8% of randomly selected papers published from 2000 to 2002 reported analgesic use in rodents undergoing experimental surgical procedures.23 Of those papers, 70% of studies in which analgesics were not administered indicated that either no signs of pain were observed or that analgesics were considered unnecessary. Many do not believe that minor surgical procedures commonly performed in research, such as simple skin incisions and skin biopsies, cause sufficient pain to warrant analgesic administration.23 Our personal experience indicates that only mice in considerable pain will show signs of pain during observation by laboratory personnel. In addition, rodents may be most likely to show signs of pain, if present, after lights-out, when they are most active and research and husbandry staff are not present.9,22 This behavior can easily lead to the assumption that mice undergoing minor operative procedures are feeling normal and do not need to be given analgesics.
Another difficulty faced by laboratory animal veterinarians is investigator concerns that analgesic use will negatively affect their research and compromise a proven successful model. For example, a number of negative side effects are associated with nonsteroidal antiinflammatory drugs, including gastrointestinal tract ulceration, impairment of platelet aggregation, blood dyscrasias, nephrotoxicity, hepatotoxicity, and bone healing impairment, and these drugs have recently been shown to induce apoptosis in cancer cells.16,17 Various opioids have demonstrated antiinflammatory, antifibrotic, antitumor, cardioprotective, and renoprotective effects.8 Given the potential side effects of these analgesics, investigators should know what level of pain their study animals are most likely to experience during their specific experimental surgery. Previous studies have evaluated the use of analgesics for rodents undergoing major surgeries, but few publications assess rodents after minor surgeries or procedures.6,11,18,26,31
Mammary fat pad removal surgery is defined as a minor surgical procedure by laboratory animal medicine guidelines,15 although it requires a fairly large abdominal skin incision and various levels of tissue manipulation and cauterization. This common experimental procedure is used to study mammary gland biology and breast cancer, and thousands of mice undergo this surgery every year.3,7,20 Analgesics generally are not administered to these mice, because mammary fat pad removal is considered a minor procedure. Direct observation of these mice postsurgically does not reveal obvious signs of pain or distress, yet under the guidelines established in the Guide for the Care and Use of Laboratory Animals,15 these mice should be given analgesics.
The objective of this study was to determine the level of pain or distress associated with the mammary fat pad removal procedure and the effects of opioid, nonsteroidal antiinflammatory, and combination postoperative analgesic regimens on recovery. A second objective was to determine whether the classic outcome measures used to study postoperative pain in rodents would be sufficiently sensitive to evaluate recovery after a minor surgical procedure.
Materials and Methods
Animals.
Female FVB (n = 34; age, 3 wk) mice were purchased from Charles River Laboratories (Hollister, CA). Mice were vendor-designated as SPF for Sendai virus, pneumonia virus of mice, mouse hepatitis virus, minute virus of mice, mouse parvovirus, Theiler murine enchephalomyelitis virus, reovirus 3, rotavirus, mouse adenovirus 1 and 2, polyoma virus, K virus, mouse cytomegalovirus, mouse thymic virus, lymphocytic choriomeningitis virus, Hantaan virus, ectromelia virus, lactate dehydrogenase-elevating virus, mouse norovirus, cilia-associated respiratory bacillus, Encephalitozoon cuniculi, Mycoplasma pulmonis, Helicobacter spp., Bordetella bronchiseptica, Citrobacter rodentium, Corynebacterium kutscheri, Salmonella spp., Streptobacillus moniliformis, Tyzzer disease virus, and endo- and ectoparasites. They were singly housed on CareFRESH Pet Bedding (Absorption Corp, Ferndale, WA) in rat microisolation cages (Alternative Design, Siloam Springs, AR) equipped with mouse running wheels. The mice were fed irradiated laboratory rodent chow (Lab Diet no. 5011, Purina, Richmond, IN) and provided sterilized water. All procedures were approved by the University of California–Davis Animal Care and Use Committee in accordance with the Guide for the Care and Use of Laboratory Animals.15
After receipt, mice were singly housed in standard rat cages equipped with mouse running wheels. Rat cages were used to allow for the addition of the running wheels (diameter, 6 in.), which were too large to fit into standard mouse cages. The room temperature was 68 to 72 °F (20.0 to 22.2 °C) with approximately 50% humidity. The photoperiod was maintained at on 12:12-h light:dark cycle, but the lights were changed to turn on at 0900 and off at 2100 to accommodate collaborators and facilitate video recording. The mice had ad libitum food and water and were handled twice daily for preconditioning for 5 d prior to surgery. Each mouse was given 3 food pellets (more than enough to cover their daily food consumption) initially, and these pellets were refreshed daily during the acclimation period and postoperatively, to account for any effect the addition of new food might have on food intake. Mice were acclimated to the containers used for fecal collection by gently placing them in their individual container for 10 min in the morning and evening, and body weight was recorded each morning. The mice were handled twice daily for approximately 10 to 15 min; each handling mimicked the restraint that took place postsurgery in order to collect feces, obtain body weight, and administer treatments.
Experimental groups.
Mice were randomly assigned into 1 of 5 groups. All groups received sodium pentobarbital (60 mg/kg IP) for anesthesia, and groups 2 to 5 underwent mammary fat pad clearance surgery 6 d after receipt. Group 1 (n = 6) served as a nonsurgical control and did not undergo surgery or receive any treatment. Group 2 (n = 7) was a surgical, no-analgesic group and received an injection of 0.1 mL saline SC (all treatments were approximately 0.1 mL in volume). Group 3 (n = 7) was treated with 5 mg/kg carprofen SC immediately after surgery. Mice in group 4 (n = 7) received buprenorphine 0.2 mg/kg SC immediately after surgery and again 12 h postoperatively. Group 5 (n = 7) received both carprofen and buprenorphine as described for groups 3 and 4. A red light was used after lights out to facilitate 12-h treatments and data collection.
Surgical procedure.
Surgeries were performed at 1000 ± 15 min (approximately 1 h after lights on) on the 6th day after arrival and lasted approximately 10 min with a 30- to 45-min recovery time. Mice were anesthetized with 60 mg/kg IP sodium pentobarbital and prepared for surgery in an aseptic manner. A 1.5-cm midline abdominal incision was made beginning between the no. 4 mammary nipples and extending toward the thorax. Two contralateral incisions were made, beginning at the lower end of the previous incision, ending between the no. 4 and no. 5 mammary nipples, with the incision resembling an inverted Y. The skin was pulled back laterally, exposing the no. 4 inguinal fat pads. The no. 4 nipples, cranial superficial epigastric artery and vein branches near the inguinal lymph node in the no. 4 fat pads, and caudal superficial epigastric artery and vein branches which course between the no. 4 and no. 5 fat pads were cauterized. The triangular area described by the cautery points was removed surgically, resulting in complete clearance of the no. 4 mammary gland, because at 3 wk of age, the no. 4 mammary gland in mice has not grown beyond the lymph node. Stainless steel Michel wound clips (length, 7.5 mm) were used to close the skin incision. Analgesics or saline were administered at this time, before mice fully recovered from anesthesia. Animals were kept warm under a heat lamp and monitored until they were awake and ambulatory, at which time they were returned to their home cage.
Outcome measures.
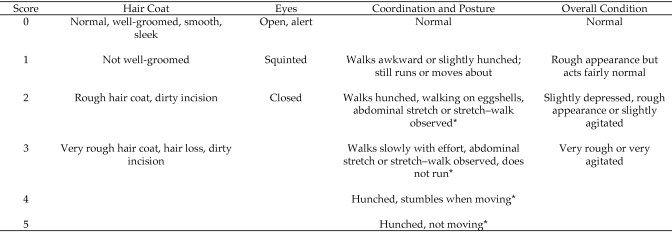
As previously mentioned, 3 food pellets were placed in each cage initially and refreshed daily. The weight of these pellets was recorded before placement in the cage and after 24 h (in conjunction with placement of fresh feed into the cage). To determine food intake, the weight of the remaining uneaten pellets was subtracted from their initial weight and recorded. Water bottles were filled with fresh autoclaved water and weighed daily to determine water intake in a similar fashion as food intake. Baseline food and water intake were recorded 24 h before surgery, and baseline body weight was recorded the morning of surgery and after recovery (to account for surgical staple weight). The scale (model APX1502, Apex Balance, Denver Instruments, Arvada, CO) used for all weight measurements had readability of 0.01 g ± 0.01 g. Running wheels were equipped with an odometer (model CY1156, Cat Eye Micro Wireless, Osaka, Japan) to record wheel revolutions, and calculations were made to adjust for running wheel size. Baseline locomotor activity (wheel running) was recorded for the 24 h before surgery. Body weight, food intake, water intake, and wheel running activity were recorded at 24, 48, 72, and 96 h after surgery. A visual assessment score modified from a previously published method for mice was used to evaluate pain level and calculate a pain index score6 (Figure 1). Video recordings (5 to 7 min) were made at 12, 24, 48, 72, and 96 h postsurgery, and a treatment-blinded observer well trained in the assessment of murine behavior calculated a pain index score for each mouse. The video recordings taken at 12 h after surgery were made 1 to 2 h after lights out, whereas all other recordings were made 1 to 2 h after lights on. Appearance of hair coat, eyes, coordination or posture, and overall condition was evaluated and scored. The pain index score was calculated by adding together the scores for each parameter listed and calculating the average for each group at each time point. For corticosterone metabolite analysis, feces were collected from individual animals on the morning of surgery (baseline samples) and then at 12 and 24 h after surgery. Mice were acclimated to the plastic containers used for fecal collection during the 5 d prior to surgery. The mice were placed gently into their individual containers for 10 min to allow time for defecation. Feces were collected and placed in microfuge tubes on crushed ice until transfer to –80 °C for storage prior to analysis.
Figure 1.
Visual assessment scoring system. Each characteristic was scored independently and then added together to obtain the final pain index score for each mouse at each time point. This scoring system was modified slightly from a previously published visual assessment score for mice.6 Changes from the published scoring system are indicated with *.
Fecal corticosterone metabolite analysis.
All steroid measurements were performed in duplicate by means of an enzyme immunoassay system developed in-house. The collected fecal samples were analyzed for immunoreactive corticosterone metabolites by using a 5α-pregnane-3β,11β,21-triol-20-one-based assay. Details regarding the development, biochemical characteristics, and physiologic validation of this assay are described elsewhere.29,30 Before analysis, the fecal samples were homogenized, and aliquots of 0.05 g were extracted with 1 mL of 80% methanol. If the sample's size was less than 0.05 g, 80% methanol was added in an appropriate amount to maintain the appropriate feces:methanol ratio. A sample size of at least 0.02 g was needed to run the assay; therefore, samples less than 0.02 g were combined for analysis. A detailed description of the assay performance has been published elsewhere.30 Briefly, the enzyme immunoassay used a double-antibody technique and was performed on antirabbit-IgG-coated microtiter plates. After overnight incubation (at 4 °C) of standards (range, 0.8 to 200 pg/well) and samples with steroid antibody and biotinylated label, the plates were emptied, washed, and blotted dry, before a streptavidin–horseradish peroxidase conjugate was added. After 45 min incubation, plates were emptied, washed, and blotted dry. The substrate (tetramethylbenzidine) was added and incubated for another 45 min at 4 °C before the enzymatic reaction was stopped with 1 mol/L sulphuric acid. Then, the optical density (at 450 nm) was recorded with an automatic plate reader, and the hormone concentrations were calculated. The intra- and interassay coefficients of variation were 8.8% and 13.4%, respectively.
Data analysis.
Statistical analysis was performed by using standard statistical software (STATA v10, Stata Corp, Bryan, TX). ANOVA within groups and across time was done by using one-way repeated-measures ANOVA and an appropriate post hoc test (pairwise Student Newmans–Keuls), if applicable. If variance was not equal, a nonparametric analysis was done using the Kruskal–Wallis test. All data are presented as mean ± SE. A P value of less than 0.05 was considered significant. Fecal corticosterone metabolites results were analyzed both with and without combined sample data.
Results
Food intake.
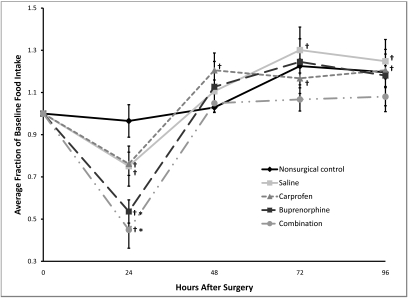
The baseline food intake was 3.62 ± 0.11 g per 24 h for all mice, with no significant differences in baseline levels between groups (P = 0.080). Food intake was significantly (P < 0.01) decreased at 24 h postoperatively for the mice treated with buprenorphine only (mean decrease of 46.4% ± 5.5%) and those treated with both carprofen and buprenorphine (mean decrease of 55.1% ± 8.7%) when compared with the nonsurgical control (Figure 2). Both groups returned to baseline by 48 h, with no other significant differences between any of the groups at any other time point with respect to food intake. The mean decrease in food intake was 24.9% ± 9.5% in the saline group and 23.8% ± 5.5% in the carprofen group. None of the analgesic regimens led to a blunting of the reduction seen in surgical mice (saline-treated mice had less of a reduction in food intake than did other surgically treated mice). When compared with baseline, all surgical groups had significantly (P ≤ 0.006) decreased food intake at 24 h. At 48 h, the carprofen group had a significantly (P = 0.016) increased food intake (mean increase of 20.5% ± 8.2%) when compared with baseline, and this elevation continued through 96 h (P ≤ 0.029). The saline group had an increased food intake when compared with baseline at both 72 h (mean increase of 30.1% ± 10.9%, P = 0.006) and 96 h (24.8% ± 5.6%, P = 0.015). In summary, all surgical groups had a decreased food intake when compared with baseline, and the buprenorphine- and combination-treated mice had an acute drop in food intake as compared with the nonsurgical controls. All groups returned to baseline in 48 h.
Figure 2.
Postoperative changes in food intake. Baseline values are represented as 1.0, and subsequent values are given as a change relative to baseline values. Each point represents the mean ± SE. *, P < 0.01 compared with value for nonsurgical control group at the same time point. †, P < 0.03 compared with baseline.
Water intake.
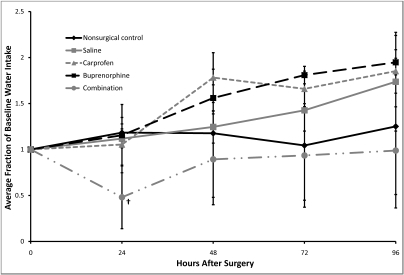
The baseline water intake was 3.61 ± 0.51 mL per 24 h for all mice, with no significant differences in baseline levels between groups (P = 0.755). The combination-treated mice had significantly (P = 0.003) lower water intake at 24 h when compared with baseline (Figure 3), with no other significant differences between any of the groups or across time for any of the groups with respect to water intake. The water intake of the combination-treated mice decreased by an average of 52% ± 34%. Water intake data showed extreme variability.
Figure 3.
Postoperative changes in water intake. Baseline values are represented as 1.0, and subsequent values are given as a change relative to baseline values. Each point represents mean ± SE. †, P = 0.003 compared with baseline value.
Body weight.
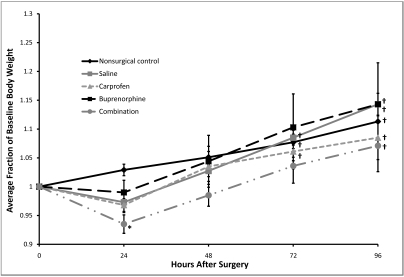
The baseline body weight was 14.67 ± 0.33 g for all mice, with no significant differences in baseline levels between groups (P = 0.188). The combination-treated mice weighed significantly (P = 0.003) less at 24 h postoperatively than did the nonsurgical control (a loss of 6.5% ± 1.6% compared with a gain of 2.9% ± 1.0; Figure 4), with no other differences between groups throughout the postoperative period. Body weight showed no immediate (within 24 or 48 h) changes as compared with baseline. By 72 h the nonsurgical control, saline-treated, and carprofen-treated mice had body weights that were significantly (P ≤ 0.045) higher than baseline, and by 96 h, the body weight of all groups was significantly (P ≤ 0.05) higher than their baseline level.
Figure 4.
Postoperative changes in body weight. Baseline values are represented as 1.0, and subsequent values are given as a change relative to baseline values. Each point represents mean ± SE. *, P = 0.003 compared with value for nonsurgical control group at the same time point; †, P ≤ 0.045 compared with baseline.
Wheel running activity.
Baseline levels of wheel running were quite variable between mice (average baseline of all groups was 24.69 ± 3.56 km per 24 h, with a range of 13.76 ± 3.86 to 42.82 ± 9.74 km/24 h), with no statistically significant differences in average baseline levels between groups (P = 0.057). A pilot study revealed that female FVB mice required about 3 d to acclimate to the wheels. After this time, each mouse consistently ran a similar distance, but this distance was variable between mice.
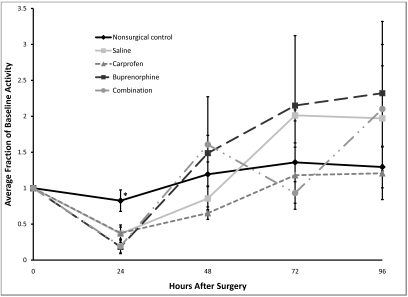
Compared with the nonsurgical control, all surgical groups had significantly (P < 0.02) decreased wheel running activity at 24 h postsurgery (Figure 5). Mean decreases for the saline-and carprofen-treated mice were 63.2% ± 12.0% and 62.5% ± 8.3% respectively, whereas the decreases for buprenorphine- and combination-treated mice were 81.7% ± 9.4 and 80.7% ± 9.5 respectively, with no other significant differences between any of the groups or across time for any of the groups with respect to activity. So in summary, at 24 h only, all surgical groups had significantly decreased wheel running activity when compared with the nonsurgical control.
Figure 5.
Postoperative changes in voluntary wheel running activity. Baseline values are given as 1.0, and subsequent values are given as a change relative to baseline values. Each point represents the mean ± SE. *, P < 0.02 compared with values from all other groups at the same time point.
Visual assessment score.
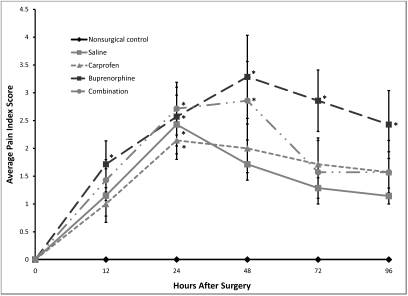
The buprenorphine-treated mice were the only surgical group to have a significantly (P = 0.046) increased pain index score (1.7 ± 0.4) at 12 h after surgery when compared with the nonsurgical controls, whereas all surgical groups had an increased pain index score (2.1 to 2.7 ± 0.3 to 0.5, P = 0.005 to 0.042) at 24 h after surgery when compared with the nonsurgical control group (Figure 6). At 48 h, the pain index score of both the buprenorphine- and combination-treated mice remained significantly elevated (3.3 ± 0.7 [P = 0.006] and 2.9 ± 0.7 [P = 0.021], respectively). Only the pain index score of the buprenorphine-treated mice remained significantly increased through 96 h, with a pain index score of 2.9 ± 0.6 (P = 0.003) at 72 h and 2.4 ± 0.7 (P = 0.013) at 96 h. The highest pain index score recorded was 6 (maximum possible, 13) in a buprenorphine-treated mouse at 48 h. All surgical groups had a significantly (P ≤ 0.019) elevated pain index score at all time points when compared with baseline, with no recovery by 96 h.
Figure 6.
Postoperative pain index scores in female FVB mice. The maximum pain index score possible was 13. Values are given as the mean pain index score ± SE. All surgical groups were significantly (P < 0.02) different from baseline at all time points. *, P < 0.05 compared with value from the nonsurgical control group at the same time point.
Fecal corticosterone metabolite levels.
Fecal corticosterone metabolites levels showed no clinically significant differences between any of the groups or across time when assessed as either independent or combined levels (Table 1).
Table 1.
Fecal corticosterone metabolites (5α-3β, 11β -corticosterone metabolites [ng/0.05g feces; mean ± SE]) before surgery (baseline) and at 12 and 24 h postoperatively
| Group | Baseline | 12 h after surgery | 24 h after surgery |
| Nonsurgical | 97.9 ± 8.4 (n = 6) | 254.6 ± 60.5 (n = 4) | 95.2 ± 14.4 (n = 6) |
| Saline | 113.6 ± 11.0 (n = 7) | 265.9 ± 38.1 (n = 5) | 152.2 ± 18.5 (n = 6) |
| Carprofen | 105.9 ± 9.8 (n = 7) | 241.6 ± 23.5 (n = 5) | 145.4 ± 20.5 (n = 6) |
| Buprenorphine | 103.0 ± 21.4 (n = 7) | 268.9 ± 54.8 (n = 4) | 166.3 ± 20.2 (n = 6) |
| Buprenorphine + carprofen | 109.4 ± 28.1 (n = 7) | 235.3 ± 27.8 (n = 5) | 219.3 ± 80.1 (n = 5) |
Due to lack of fecal production at 12 and 24 h, some samples were necessarily combined to facilitate analysis. Statistical analysis was performed on both sets of data, with no differences between analyses. There were no clinically significant differences between groups or over time.
Discussion
This study is one of the few to evaluate postoperative pain in mice after a minor surgical or experimental procedure. Several studies have evaluated pain in mice after major surgical procedures such as splenectomies, laparotomies, and vasectomies (abdominal approach),1,6,11,12,31 but few have assessed the possible pain or distress associated with a minor one. Assuming that a minor procedure would cause only mild pain or distress, we did not expect to see large changes in outcome measures used to assess postsurgical recovery. Therefore, multiple parameters were used in an attempt to effectively evaluate recovery in mice after mammary fat pad removal surgery. Food intake, water intake, body weight, wheel running activity, and visual assessment score showed statistically significant changes postoperatively, but the surgery did not elicit significant changes in fecal corticosterone metabolites.
Food intake in our mice was decreased, as is expected after most surgical procedures. All groups had significantly less food intake when compared with their baseline levels; however, only the buprenorphine- and combination-treated groups showed significantly lower food consumption than that of the nonsurgical control. Food intake returned to baseline levels in all surgical groups by 48 h. Surgery alone did not cause a significant decrease in food intake as compared with that of the nonsurgical controls, and carprofen treatment did not blunt the reduction in food intake that occurred without analgesic treatment. Buprenorphine at doses ranging from 0.05 to 2 mg/kg alone and postsurgically has been shown to suppress appetite,1,6,11,12,18,19 corresponding to the findings in our study. The decrease in food intake in the buprenorphine group was greater than that in mice that did not receive pain medication postsurgically. Although analgesic treatment is important for humane treatment and recovery of animals, overaggressive administration of analgesics to rodents could potentially lead to unexpected detrimental effects.
Water intake showed a significant decrease only in the combination-treated mice. High variability in the water intake data may have occurred due to the manner in which we measured water intake. Although our method was essentially the same as that previously reported in a number of other studies,1,6,11 it did not account for water spillage or water that may have been removed by the mouse but not consumed.
Only the body weight of the combination-treated group showed a statistically significant decrease. A marked decrease in body weight would not be expected for a minor surgical procedure. However, the mice used in this study were young. Mammary fat pad removal must be performed by 4 wk of age to ensure complete clearance; otherwise, the gland may have already grown into the inguinal lymph node. At this age, the mice are not fully grown and are still undergoing the exponential portion of their growth curve. This growth may have blunted any postsurgical decreases in body weight.
The first study to use wheel running to assess postoperative pain in mice involved a major operative procedure (splenectomy) and showed that liposome-encapsulated oxymorphone improved postsurgical recovery compared with that associated with saline or buprenorphine at 0.2 mg/kg.6 Buprenorphine did, however, lead to improved recovery when compared with that of mice only given saline. In our study, buprenorphine did not improve postoperative recovery and, in fact, seemed to inhibit recovery. Perhaps the dose of 0.2 mg/kg was too high for mice undergoing only a minor experimental procedure, and the sedative and appetite suppressant effects at this dose outweighed the need for pain relief. Wheel running in our study revealed a decrease in postsurgical activity, but mice given analgesics were not different from those given only saline. Possible explanations for this include: 1) wheel running activity is not sensitive enough to evaluate mice after a minor experimental procedure, or 2) none of the analgesic regimens tested was able to eliminate the pain or distress associated with the procedure. Given the grouping of the buprenorphine and combination groups (approximately an 80% decrease in activity) and the carprofen- and saline-treated mice (approximately a 60% decrease in activity), the wheel running did appear to reveal the sedative effects of opioids on activity. Therefore a logical conclusion is that wheel running activity would have been sensitive enough to reveal any alleviation of pain or distress caused by carprofen, had those treatments been effective. The surgical staples used for skin closure may have been another factor contributing to the decrease in wheel running seen postoperatively. Comparing closure with tissue glue, the sparing procedure for mammary fat pad clearance described in 2008 which only requires 2 staples2, and the standard technique used for the mammary fat pad surgery in this study (which typically requires 6 staples) could be informative in this regard.
Observing signs of pain in mice can be difficult. The visual assessment score used was one previously published for mice6 and was chosen due to its ease of use and apparent adherence to previously published signs of pain in rodents.17,28 Those who published the score questioned its worth, given that in their study it did not seem to be a sensitive indicator of pain.6 However, our study used remote recording and had a single assessment period after lights out (assumed to be the most likely timeframe to identify signs of pain in mice), so the visual assessment score was performed similarly as previously described. Throughout the current study, the mice showed no sign of pain if an observer was in the room, perhaps accounting for the low pain index scores in the previous study of splenectomy. However, our remote recording did result in visual assessment scores that showed significant differences, albeit mild. None of the analgesic treatments alleviated the behavioral signs of pain associated with the surgery. One behavior commonly noted in surgical mice across all groups was the stretch and stretch–walk sequence known to be an indicator of pain or discomfort in rodents.24,27,31 Not all surgical mice showed this sign of pain, but it was seen in mice from all surgical groups. The original visual assessment score proposed for use in this study did not specifically assess this behavior, so we added it under the coordination–posture category when mice were scored (Figure 1). It would be interesting to see whether a more detailed visual assessment score or use of sophisticated software, such as HomeCageScan, would show similar results to our study.27
Another possible limitation of our visual assessment score is the timing of the video recording. The first video recording was taken at 12 h after surgery. Others who successfully use video recording of rodents to monitor pain after surgery typically start recording behavior 1 h after surgery.24,27,31 However, in their studies isoflurane was used to induce anesthesia, rather than pentobarbital, as in the current study. Pentobarbital has a prolonged recovery time compared with the rapid recovery time of isoflurane anesthesia.13 For our study, mice recorded at 1 h after surgery would have still been under the influence of pentobarbital, but perhaps a recording period before 12 h would have been more reflective of any acute pain associated with the procedure and given more reliable results.
Surgical pain typically causes an endocrine response resulting in the elevation of the hormone corticosterone. This hormone causes many physiologic changes such as tachycardia, hypertension, suppression of the immune system, hyperglycemia, lipolysis, and a negative nitrogen balance, and all of these may affect study results.17 The goal of analgesic administration is not only to decrease pain felt by research animals but also to decrease the effects that the pain response may have on experimental data. Fecal corticosterone metabolites did not differ significantly among groups, indicating that mammary fat pad removal surgery did not induce pain or distress sufficient to stimulate the hypothalamic–pituitary axis. Greater elevations in fecal corticosterone may have occurred but been missed due to the timing of sample collection. The highest levels of fecal corticosterone seen after surgery in mice by others occurred at 6 and 9 h after vasectomy, depending on the mouse strain.31 The fecal corticosterone immunoassay used in our study was developed and thoroughly validated for use in mice.29,30 Peak levels of fecal corticosterone metabolites were reported at about 10 h after intraperitoneal injection of radiolabelled corticosterone or saline, but changes were evident between 6 and 16 h. Ideally, fecal pellets would have been collected at 10 h after surgery, but we felt that a 10-h time point could interfere with the behavioral assessment performed at 12 h. Therefore, feces were collected at 12 h, to coincide with the video recording and treatment administration. As a side note, the decreases in food intake seen after surgery led to a notable decrease in fecal production. Several mice did not produce enough fecal pellets during the 10-min collection time to allow for independent assay testing. This situation necessarily led to the combining of small samples in order to facilitate analysis. As was stated previously, statistical analysis was performed on the independent samples alone and on the combined sample data, with no differences in the results between the 2 analyses.
Our data revealed that analgesics after a minor procedure must be chosen thoughtfully and that the level of pain expected from a particular experimental procedure must be considered, to minimize negative side effects that may inhibit postoperative recovery. The mammary fat pat removal surgery did elicit changes in mouse behavior associated with pain, and these changes were seen in all surgical groups. However, as indicated by our outcome measures, buprenorphine at 0.2 mg/kg SC actually seemed to inhibit postsurgical recovery. The 0.2-mg/kg dose is on the lower end of the published dosing range of buprenorphine in mice (0.05 to 2.5 mg/kg4), but perhaps an even lower dose given more often would have provided sufficient analgesia without leading to the negative side effects noted by our outcome measures. Buprenorphine is one of the most widely used analgesics in mice,5,25 but its use in laboratory animal medicine is frequently inappropriate given its half-life and duration of action. The half-life of buprenorphine after intravenous administration in mice is only about 3 h.32 The drug is generally administered every 12 h, but its duration of action is reported as only 3 to 5 h in mice and 6 to 8 h in rats.10 By 12 h after surgery, the analgesic properties of buprenorphine may have already dissipated. The dosing regimen was chosen to closely mimic current laboratory practices at our institution and because of its reasonable dosage compliance, but perhaps our expectations must change if an investigator wants to use buprenorphine to alleviate postprocedural pain in mice. Buprenorphine is a partial agonist and has ceiling effects on its analgesia,10 so perhaps a pure μ-agonist such as morphine would provide effective measurable analgesia. However, considering the mild nature of the mammary fat pad removal surgery, we would be surprised if potent analgesics would be required. Although, given the known strain differences in pain threshold,17,31 perhaps FVB mice require more aggressive pain medication after surgery than do other strains of mice. A nonsteroidal antiinflammatory drug would be the most logical choice for pain alleviation after a mild procedure, but carprofen at 5 mg/kg SC did not improve the recovery of FVB mice after mammary clearance. This dose of carprofen and saline treatment essentially led to identical postsurgical recovery in FVB mice. As with buprenorphine, perhaps a different dose of carprofen or the 5 mg/kg dose given more frequently would have led to more adequate analgesia. The half-life of carprofen and similar nonsteroidal antiinflammatory drugs varies considerably among different species, and to our knowledge the half-life and duration of action of carprofen in mice has not yet been reported.14 The dosing regimen was based on the published duration of action for other species, and the recommended dosing found in the literature.21 Further studies need to focus on higher doses of carprofen, other nonsteroidal antiinflammatory drug treatments with drugs such as meloxicam and indomethacin, and novel medications such as tramadol for alleviation of pain, if needed, after minor surgical procedures. Clearly we still have quite a way to go to optimize pain control for laboratory animals.
Acknowledgments
We thank Janet Palomares for her excellent and devoted assistance throughout the completion of this study and Dr Terry Hewett for her guidance and editorial review of this manuscript prior to submission. We also thank AALAS for their support of this project through their Grants for Laboratory Animal Science.
References
- 1.Blaha MD, Leon LR. 2008. Effects of indomethacin and buprenorphine analgesia on the postoperative recovery of mice. J Am Assoc Lab Anim Sci 47:8–19 [PMC free article] [PubMed] [Google Scholar]
- 2.Brill B, Boecher N, Groner B, Shemanko CS. 2008. A sparing procedure to clear the mouse mammary fat pad of epithelial components for transplantation analysis. Lab Anim 42:104–110 [DOI] [PubMed] [Google Scholar]
- 3.Cardiff RD, Anver MR, Boivin GP, Bosenberg MW, Maronpot RR, Molinolo AA, Nikitin AY, Rehg JE, Thomas GV, Russell RG, Ward JM. 2006. Precancer in mice: animal models used to understand, prevent, and treat human precancers. Toxicol Pathol 34:699–707 [DOI] [PubMed] [Google Scholar]
- 4.Carpenter JW. 2005. Exotic animal formulary, 3rd ed, p 390 St Louis (MO): WB Saunders [Google Scholar]
- 5.Christoph T, Kogel B, Schiene K, Meen M, De Vry J, Friderichs E. 2005. Broad analgesic profile of buprenorphine in rodent models of acute and chronic pain. Eur J Pharmacol 507:87–98 [DOI] [PubMed] [Google Scholar]
- 6.Clark MD, Krugner-Higby L, Smith LJ, Heath TD, Clark KL, Olson D. 2004. Evaluation of liposome-encapsulated oxymorphone hydrochloride in mice after splenectomy. Comp Med 54:558–563 [PubMed] [Google Scholar]
- 7.Deome KB, Faulkin LJ, Jr, Bern HA, Blair PB. 1959. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res 19:515–520 [PubMed] [Google Scholar]
- 8.Dinda A, Gitman M, Singhal PC. 2005. Immunomodulatory effect of morphine: therapeutic implications. Expert Opin Drug Saf 4:669–675 [DOI] [PubMed] [Google Scholar]
- 9.Furuzawa M, Kuwahara M, Ishii K, Iwakura Y, Tsubone H. 2002. Diurnal variation of heart rate, locomotor activity, and body temperature in interleukin 1α/β doubly deficient mice. Exp Anim 51:49–56 [DOI] [PubMed] [Google Scholar]
- 10.Gades NM, Danneman PJ, Wixson SK, Tolley EA. 2000. The magnitude and duration of the analgesic effect of morphine, butorphanol, and buprenorphine in rats and mice. Contemp Top Lab Anim Sci 39:8–13 [PubMed] [Google Scholar]
- 11.Goecke JC, Awad H, Lawson JC, Boivin GP. 2005. Evaluating postoperative analgesics in mice using telemetry. Comp Med 55:37–44 [PubMed] [Google Scholar]
- 12.Hayes KE, Raucci JA, Jr, Gades NM, Toth LA. 2000. An evaluation of analgesic regimens for abdominal surgery in mice. Contemp Top Lab Anim Sci 39:18–23 [PubMed] [Google Scholar]
- 13.Hayward AM, Lemke LB, Bridgeford EC, Theve EJ, Jackson CN, Cunliffe-Beamer TL, Marini RP. 2007. Biomethodology and surgical techniques, p 437–488 : Fox JG, Davisson MT, Newcomer CE, Quimby FW, Smith AL. The mouse in biomedical research. San Diego (CA): Academic Press [Google Scholar]
- 14.Heavner JECD. 2008. Pharmacology of Analgesics, p 97–123 : Fish RE, Danneman PJ, Karas AZ. Anesthesia and analgesia in laboratory animals. San Diego (CA): Academic Press [Google Scholar]
- 15.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals, p 64–65 Washington (DC): National Academies Press [Google Scholar]
- 16.Khwaja FS, Quann EJ, Pattabiraman N, Wynne S, Djakiew D. 2008. Carprofen induction of p75NTR-dependent apoptosis via the p38 mitogen-activated protein kinase pathway in prostate cancer cells. Mol Cancer Ther 7:3539–3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohn DF, Martin TE, Foley PL, Morris TH, Swindle MM, Vogler GA, Wixson SK. 2007. Public statement: guidelines for the assessment and management of pain in rodents and rabbits. J Am Assoc Lab Anim Sci 46:97–108 [PubMed] [Google Scholar]
- 18.Liles JH, Flecknell PA. 1994. A comparison of the effects of buprenorphine, carprofen, and flunixin following laparotomy in rats. J Vet Pharmacol Ther 17:284–290 [DOI] [PubMed] [Google Scholar]
- 19.Liles JH, Flecknell PA, Roughan J, Cruz-Madorran I. 1998. Influence of oral buprenorphine, oral naltrexone or morphine on the effects of laparotomy in the rat. Lab Anim 32:149–161 [DOI] [PubMed] [Google Scholar]
- 20.Parmar H, Cunha GR. 2004. Epithelial–stromal interactions in the mouse and human mammary gland in vivo. Endocr Relat Cancer 11:437–458 [DOI] [PubMed] [Google Scholar]
- 21.Pollock C. 2002. Postoperative management of the exotic animal patient. Vet Clin North Am Exot Anim Pract 5:183–212 [DOI] [PubMed] [Google Scholar]
- 22.Ray B, Mallick HN, Kumar VM. 2004. Changes in thermal preference, sleep–wakefulness, body temperature, and locomotor activity of rats during continuous recording for 24 hours. Behav Brain Res 154:519–526 [DOI] [PubMed] [Google Scholar]
- 23.Richardson CA, Flecknell PA. 2005. Anaesthesia and postoperative analgesia following experimental surgery in laboratory rodents: are we making progress? Altern Lab Anim 33:119–127 [DOI] [PubMed] [Google Scholar]
- 24.Roughan JV, Flecknell PA. 2001. Behavioural effects of laparotomy and analgesic effects of ketoprofen and carprofen in rats. Pain 90:65–74 [DOI] [PubMed] [Google Scholar]
- 25.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343 [DOI] [PubMed] [Google Scholar]
- 26.Roughan JV, Flecknell PA. 2004. Behaviour-based assessment of the duration of laparotomy-induced abdominal pain and the analgesic effects of carprofen and buprenorphine in rats. Behav Pharmacol 15:461–472 [DOI] [PubMed] [Google Scholar]
- 27.Roughan JV, Wright-Williams SL, Flecknell PA. 2009. Automated analysis of postoperative behaviour: assessment of HomeCageScan as a novel method to rapidly identify pain and analgesic effects in mice. Lab Anim 43:17–26 [DOI] [PubMed] [Google Scholar]
- 28.Stasiak KL, Maul D, French E, Hellyer PW, VandeWoude S. 2003. Species-specific assessment of pain in laboratory animals. Contemp Top Lab Anim Sci 42:13–20 [PubMed] [Google Scholar]
- 29.Touma C, Palme R, Sachser N. 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22 [DOI] [PubMed] [Google Scholar]
- 30.Touma C, Sachser N, Mostl E, Palme R. 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278 [DOI] [PubMed] [Google Scholar]
- 31.Wright-Williams SL, Courade JP, Richardson CA, Roughan JV, Flecknell PA. 2007. Effects of vasectomy surgery and meloxicam treatment on faecal corticosterone levels and behaviour in two strains of laboratory mouse. Pain 130:108–118 [DOI] [PubMed] [Google Scholar]
- 32.Yu S, Zhang X, Sun Y, Peng Y, Johnson J, Mandrell T, Shukla AJ, Laizure SC. 2006. Pharmacokinetics of buprenorphine after intravenous administration in the mouse. J Am Assoc Lab Anim Sci 45:12–16 [PubMed] [Google Scholar]