Abstract
Buprenorphine is administered to humans and animals for postoperative pain management, although its use is associated with complications. Alternative analgesics, including the nonsteroidal antiinflammatory meloxicam, are available, but information on their postoperative effects is limited. The objective of the present study was to compare buprenorphine (0.03 mg/kg SC twice daily for 3 d) with meloxicam (2 mg/kg SC initial dose followed by 1 mg/kg SC once daily for 2 d) by assessing parameters relating to postsurgical recovery in rats that underwent surgical implantation of radiotelemetric transducers. Rats treated after surgery with buprenorphine showed greater reductions in body weight, food consumption, locomotor activity, and nighttime heart rates than did meloxicam-treated rats. Buprenorphine and meloxicam treatments both had stimulatory effects on mean arterial pressure and daytime heart rate measurements, although effects on nighttime mean arterial pressure were greater in the buprenorphine-treated rats. In summary, the lesser physiologic changes associated with meloxicam, as compared with buprenorphine, suggest that meloxicam offers advantages for use as a postoperative analgesic after laparotomy and radiotelemetric transducer implantation in rats.
Abbreviation: HR, heart rate; MAP, mean arterial pressure; WD, Western diet
In collaboration with facility veterinarians, researchers who perform invasive surgical techniques must decide on an appropriate postoperative pain management regimen. From an ethical perspective, minimizing an animal's suffering should be a primary concern. Moreover, inadequate pain management can compromise a study's outcome, because activation of the pain cascade can influence physiologic function.10 In addition, the full pharmacodynamic spectrum of the analgesic drug should be considered, because any of its activities could affect a study's endpoints. Therefore, appropriate selection of drugs for management of postoperative pain is not a trivial decision.
Implantation of radiotelemetric transducers is an invasive but necessary surgical procedure for direct and chronic measurement of hemodynamic parameters in animals. Although noninvasive techniques for blood pressure assessments are available, direct assessment by implantation of arterial catheters is generally considered to yield far superior data,13 notwithstanding the cost and surgical expertise required. In rats, implantation of transducers involves an abdominal incision of approximately 4 to 5 cm, visceral manipulation, implantation of a small catheter into the abdominal aorta, suturing the transducer to the inner muscular layer, and finally suturing the abdominal incision.
Postoperative analgesia for telemetry implantation, like many other surgeries, often consists of opioid analgesics such as buprenorphine,9,11,20 a partial µ-opioid receptor agonist. Buprenorphine treatment has been shown to improve overall indications of pain and surgical stress, including postoperative food and water intake, changes in body weight, and ambient locomotor activity, compared with those of untreated controls.20 Nevertheless, there can be unwanted effects associated with buprenorphine use, including rebound hyperalgesia,23 variability in potency between sexes and strains of rodents,7 and issues with food intake (such as anorexia and pica).6,15 Furthermore, assessment of postoperative pain and recovery are complicated, given that buprenorphine treatment affects growth rate, feeding, and locomotor activity in naive rats;4,15,19 thus, discriminating between behavioral cues caused by pain compared with those due to buprenorphine treatment per se is problematic. Finally, as an opioid analgesic, buprenorphine use is controlled in most jurisdictions, thereby complicating its availability.
Alternative therapeutics for treating postoperative pain include nonsteroidal antiinflammatory drugs such as meloxicam.21 Meloxicam acts by inhibiting cyclooxygenases and thus preventing the synthesis of prostaglandin H2, which is a precursor to mediators that elicit pain and inflammation.24 Although meloxicam is a potential alternative treatment for surgery in rats, knowledge of its effects on recovery and cardiovascular parameters after surgery is incomplete. The objective of this study was to compare the postoperative recovery in rats treated with either buprenorphine or meloxicam for management of pain after surgical implantation of telemetric transducers.
Materials and Methods
Animals and treatments.
The experimental protocols described herein were performed in the Animal Care Facilities at Queen's University (Kingston, Ontario, Canada) and were approved by the Queen's University Animal Care Committee. The animals used in the present study represent a subset of rats that were used for another study investigating the effects of in utero iron deficiency on long-term metabolic function.3 For the present study, 16 male, Sprague–Dawley untreated (control) rats were reared in the Queen's University Animal Care Facility as previously described.2 These animals represent the first-generation subline maintained internally in the Queen's University Animal Care Facility; the rats that constituted the parental line were obtained from Charles River (St Constant, Quebec, Canada). All rats were housed in solid-bottom cages (24 cm × 45 cm × 20 cm) containing Beta Chips (North Eastern Products, Warrensburg, NY). After radiotelemetry implantation, rats were housed 2 per cage, with the exception of the period between weaning and 6 wk of age, when they were housed 4 per cage. Room sentinels, which are exposed to dirty bedding, were tested quarterly and found to be serologically negative for the following agents: Sendai virus, pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, Toolan H1 virus, rat parvovirus, rat minute virus, reovirus, rat theilovirus, Mycoplasma pulmonis, lymphocytic choriomeningitis virus, hantavirus, mouse adenovirus, Encephalitozoon cuniculi, and cilia-associated respiratory bacillus. In addition, rats were free of endoparasites and ectoparasites.
At 21 d after birth, rats were weaned onto either a purified normal diet (catalog no. 5TJM, Lab Diet, St Louis, MO) or a high-calorie Western diet (WD; catalog no. 5TJN, Lab Diet) and maintained thereon throughout the study. Rats had ad libitum access to food and water and were maintained on a 12:12-h light:dark cycle and at an ambient temperature of 23 °C. At approximately 12 wk of age, rats were implanted with radiotelemetric transducers (TA11PA-C40, Data Sciences International, St Paul, MN) for direct hemodynamic assessments, as previously described.2 Briefly, rats were anesthetized with isoflurane (dosed to effect by inhalation), and a 4- to 5-cm incision was made along the ventral midline. The viscera were exteriorized temporarily onto saline soaked gauze, and the abdominal aorta was isolated from the surrounding connective tissue. A fluid-filled telemetric catheter was introduced into the lower abdominal aorta, positioned 1 cm below the left renal artery, and held in position with a small amount of cyanoacrylate glue. The body of the transducer was sutured to the muscular layer of the abdominal wall to prevent device movement. After implantation, the viscera were replaced in the abdominal cavity, and the muscular wall and skin were sutured. The incision wounds were treated topically with approximately 0.5 mL of a 5-mg/mL solution of bupivicaine hydrochloride USP (Marcaine, AstraZeneca, Mississauga, Ontario, Canada) before awakening the rats from anesthesia.
After surgery, rats were assigned randomly to receive either buprenorphine or meloxicam treatment. The buprenorphine group (normal diet, n = 4; WD, n = 4) was treated twice daily with 0.03 mg/kg SC buprenorphine (Temgesic, Schering–Plough, Pointe Claire, Quebec, Canada) for 3 d. The meloxicam group (normal diet, n = 4; WD, n = 4) was treated with 2 mg/kg SC meloxicam (Metacam, Boehringer Ingelheim Vetmedica, St Joseph, MO) immediately after surgery and once daily with 1 mg/kg SC for the next 2 d.
During the postsurgical period, rats were housed in individual cages. Food consumption and body weights were assessed daily for 10 d. Because rats were given fluids subcutaneously as needed, fluid intake was not measured during this period. Rat cages were placed on receivers (model RPC1, DSI, St Paul, MN), which convert the radiotelemetric waveform into a digital signal. This information was then transmitted through a consolidation matrix (BCM100, DSI) to a computer-based acquisition system (version 3.1, Dataquest IV, DSI) located in an adjacent room. Ambient locomotor activity, heart rate (HR), mean arterial pressure (MAP), systolic blood pressure, diastolic blood pressure, and pulse pressure were recorded for 15 s every 5 min for each animal. Daytime telemetry data presented in this study represent a mean of all data points collected between the hours of 1000 h and 1600h, and nighttime data between the hours of 2200 h and 0400 h (72 total recordings per night or day); this period corresponds to the 6-h interim period of each light cycle in the Animal Care Facility at Queen's University, which cycles from night to day at 0700 h and from day to night at 1900 h.
Statistical analyses.
Growth rates were calculated as percentage change normalized to body weight over a 24-h period. Food consumption, locomotor activity, and hemodynamic parameters were normalized to baseline nonsurgical values: in the case of food consumption, these values represent those obtained the week prior to surgeries, whereas for data obtained by radiotelemetry, normal values were taken as those obtained during postsurgical day 10 (or postsurgical night 9), because all parameters have been shown to be normalized by postsurgical day 7.22 Initially, all data were analyzed by 3-way ANOVA for the effects of Western diet, postoperative treatment, and day. Subsequently, control and WD pooled data were analyzed by repeated-measures 2-way ANOVA for postoperative analgesic treatment and day. Mean variation associated with each parameter was calculated as the absolute area under the curve (both negative and positive values were added as positive values) within a given period and subsequently divided by the number of days within that period. Deviation data were compared by 2-way ANOVA for treatment and time. When significance was found with 2-way ANOVA, Student t tests with Bonferroni correction or Dunnett analysis for multiple comparisons was used as appropriate. Data are presented as mean ± SEM, with a P value of less than 0.05 considered significant.
Results
Effect of postoperative pain treatment on body weight and food consumption.
Feeding rats a WD for 9 wk caused a significant (P < 0.01) increase in body weight (control, 587 ± 11 g; WD, 641 ± 12 g). However, despite this effect, WD- and control-fed animals were similarly affected by buprenorphine or meloxicam treatment in all parameters analyzed, albeit weight loss in the buprenorphine group was greater in animals with higher body weights (P < 0.05; see next paragraph). Therefore, the effects of diet were minor compared with the effects of postoperative treatment. Given the minor effects of diet, control and WD-fed groups were pooled according to postoperative treatment, to increase statistical power and enable us to identify more subtle differences of postoperative treatment. After combining control and WD groups, mean body weight was not different between groups selected to receive buprenorphine compared with those selected to receive meloxicam at the start of the study (buprenorphine, 624 ± 15 g; meloxicam, 635 ± 16 g; P = 0.64).
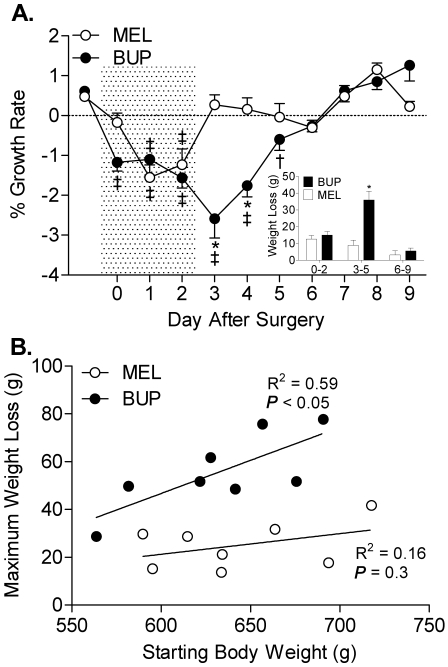
Buprenorphine treatment had a profound effect on postoperative body weight (Figure 1). Rats treated with buprenorphine lost weight until day 5 after surgery and did not reach preoperative growth rates until day 6. In contrast, rats given meloxicam lost weight until day 2 after surgery and began gaining weight by day 3 after surgery. During the postoperative period immediately after cessation of treatment, buprenorphine-treated rats lost more than 4-fold more weight compared with meloxicam-treated rats (P < 0.001; Figure 1, inset); the 2 groups were not different during the other postoperative periods. Analysis of the relationship between body weight at the time of surgery and total weight loss through the postoperative period revealed a significant (P < 0.05) correlation in rats treated with buprenorphine (Figure 1 B); no such correlation was observed in the rats treated with meloxicam.
Figure 1.
(A) Analgesic treatment affects body weight during the postoperative period; inset, accumulated weight loss (area under the curve) during the early (days 0 to 2), intermediate (days 3 to 5) and late (days 6 to 9) postoperative periods. (B) Correlation between weight loss throughout the 10-d postoperative period and body weight at the time of implantation surgery. BUP, buprenorphine; MEL, meloxicam; *, P < 0.001 compared with the meloxicam group on the same day; †, P < 0.01 compared with preoperative values in the same group; ‡, P < 0.01 compared with preoperative values in the same group. Shaded area indicates analgesic treatment period.
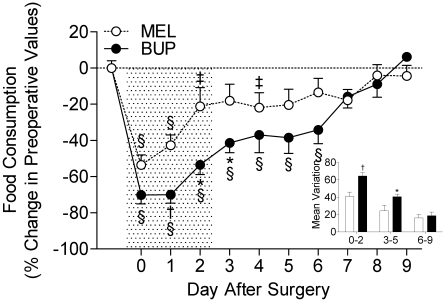
Postoperative buprenorphine treatment had a greater effect than did meloxicam on both the degree and duration of reduced food intake (Figure 2). Buprenorphine-treated rats had reduced food intake until day 6 after surgery (P < 0.01), whereas meloxicam-treated rats consumed less food until day 4 after surgery (P < 0.05). Over the course of the treatment phase and during the period immediately after cessation of treatment, the accumulated deviation from preoperative food consumption values (assessed as area under the curve) was substantially greater in the buprenorphine-treated rats compared with meloxicam-treated rats (P < 0.05 at both periods; Figure 2, inset). The groups were not different during the extended period after analgesic treatment.
Figure 2.
Analgesic treatment affects food consumption during the postoperative period; inset, mean variation during the early (days 0 to 2), intermediate (days 3 to 5), and late postoperative period. BUP, buprenorphine; MEL, meloxicam; *, P < 0.05 compared with the meloxicam group on the same day; †, P < 0.01 compared with the meloxicam group on the same day; ‡, P < 0.05 compared with postoperative values in the same treatment group; §, P < 0.01 compared with preoperative values in the same treatment group. Shaded area indicates analgesic treatment period.
Effect of postoperative treatment on activity and hemodynamic assessments.
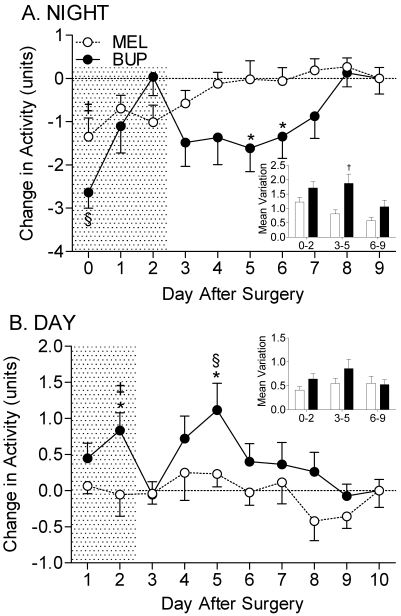
In general, rats were more active and had higher HR and hemodynamic parameters during the night phase than during the day phase (data not shown). Analysis revealed that buprenorphine had a significant inhibitory effect on nighttime activity and a stimulatory effect on daytime activity (compared with postoperative day 10; P = 0.001 for both parameters). In contrast, meloxicam had a significant inhibitory effect on nighttime activity (P = 0.001) but no effect on daytime activity (P = 0.24). Direct comparisons between treatment groups revealed that the most prominent differences existed during the intermediate postsurgical period in nighttime activity, corresponding to the 3-d period immediately after cessation of analgesic treatment (P < 0.01; Figure 3 A, inset). Although buprenorphine-treated animals had significantly higher daytime activities compared to meloxicam-treated rats at postoperative days 2 and 5 (P < 0.05 on both days; Figure 3 B), the accumulated deviation from baseline values was not significant over the course of the 10-d recovery period.
Figure 3.
(A) Analgesic treatment affects nighttime locomotor activity and, to a lesser extent, (B) daytime locomotor activity in the postoperative period. Insets, mean variation in activity during early (days 0 to 2), intermediate (days 3 to 5), and late (days 6 to 9) postoperative periods. BUP, buprenorphine; MEL, meloxicam; *, P < 0.05 compared with meloxicam group during the same time interval; †, P < 0.01 compared with meloxicam group during the same time interval; ‡, P < 0.05 compared with baseline value (postoperative day 9 or 10) in the same treatment group; §, P < 0.01 compared with baseline value (postoperative day 9 or 10) in the same treatment group. Shaded area indicates analgesic treatment period.
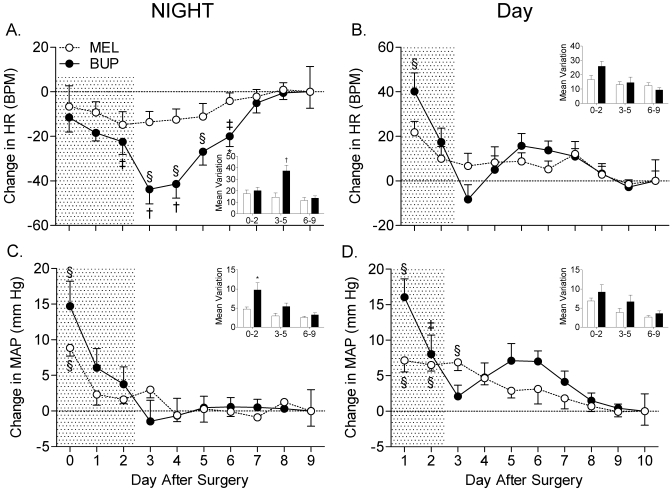
The effects of postoperative analgesia on HR and MAP are shown in Figure 4. Buprenorphine treatment had a dramatic effect on nighttime HR (P < 0.001; Figure 4 A), for which differences were most pronounced on the days after cessation of treatment (days 3 and 4). In contrast, meloxicam had no effect on nighttime HR (P = 0.55). Specifically, the decrease in HR due to buprenorphine treatment during the intermediate postoperative period was more than twice that induced by meloxicam (Figure 4 A). Postoperative buprenorphine treatment had a significant (P < 0.001) stimulatory effect on HR during the daytime period, whereas no such effect was observed in the meloxicam group (P = 0.14; Figure 4 B).
Figure 4.
Analgesic treatment affects (A) nighttime HR, (B) daytime HR, (C) nighttime MAP, and (D) daytime MAP. Insets, mean variation in MAP or HR during early (days 0 to 2), intermediate (days 3 to 5), and late (days 6 to 9) postoperative periods. BUP, buprenorphine; MEL, meloxicam; *, P < 0.05 compared with the meloxicam group during the same time interval; †, P < 0.01 compared with the meloxicam group during the same time interval; ‡, P < 0.05 compared with baseline value (postoperative day 9 or 10) in the same treatment group; §, P < 0.01 compared with baseline value (postoperative day 9 or 10) in the same treatment group. Shaded area indicates analgesic treatment period.
Both buprenorphine and meloxicam treatment had a significant effect on MAP during both night and day periods (P < 0.001 for all parameters; Figure 4 C and D). MAP was significantly elevated in the buprenorphine group compared with the meloxicam group during the first night and day after surgery (P < 0.01 for both parameters). In addition, MAP was significantly more variable in the buprenorphine-treated group during the nighttime phase but not during the daytime phase (P < 0.05). Other hemodynamic parameters, including systolic and diastolic blood pressures, had similar patterns as MAP (data not shown). Pulse pressure was not different between groups in any period.
Discussion
This study compared the effects of buprenorphine and meloxicam when used as analgesics after laporotomy in rats. The purpose was to determine whether meloxicam was advantageous in terms of its impact on various physiologic and behavioral assessments after abdominal implantation of radiotelemetric transducers. The main observations were that 1) meloxicam-treated rats ate more food during and after treatment, 2) buprenorphine-treated animals lost more weight in the immediate posttreatment period, 3) buprenorphine-treated rats were hyperactive during the day, 4) buprenorphine-treated rats had more pronounced HR decreases, and 5) the differences between the treatment groups were greatest in the 3 d after cessation of analgesic treatment, with the sole exception of food consumption, which was most evident during treatment.
Assessing the effectiveness of pain management in animals requires the use of physiologic or behavioral indices that accurately reflect pain and discomfort. We used a combination of indices of behavior (eating, locomotor activity) and physiology (body weight, hemodynamics). An ideal treatment regimen would result in normal consumption of food, maintenance of physical activity and normal sleep patterns, uninterrupted weight gain, and maintenance of normal hemodynamics. Neither drug regimen accomplished this ideal. Nevertheless some differences between the treatments are relevant for rats that receive major surgical procedures, such as laparotomy.
Compared with most other types of surgery, the implantation of radiotelemetric transducers into the abdominal cavity involves abdominal pain that is likely to affect food intake and is therefore likely to have a more dramatic effect on body weight and growth rates. Consistent with this, both treatments were associated with decreased food consumption and weight loss during the treatment period after surgery. However, the weight loss during the 3 d of drug treatment is not readily explained by the changes in food intake, because food intake was lower during drug treatment in buprenorphine-treated rats, yet weight loss was not different between groups. In contrast, during the immediate and extended periods after drug treatments, weight was consistent with the effects on food intake. Specifically, the more than 2-fold greater weight loss among the buprenorphine-treated group after day 3 coincides well with the large differences in the effect of these postoperative treatments on food intake.
Alternatively, the observed weight loss and reduced appetite in either group may not necessarily reflect a behavioral response to postsurgical pain but could result from a number of other factors, including altered water intake, physiologic effects of anesthesia, and adaptations to postsurgical handling. Although this distinction is beyond the scope of the present study, the severity of effects on food consumption and body weight are nevertheless important, as is the length of time required to reestablish normal growth patterns. These endpoints were most pronounced in the buprenorphine-treated rats, which took the longest to return to normal (preoperative) values. Interestingly, the severity of weight loss was positively correlated with body weight in buprenorphine-treated rats but not in the meloxicam-treated animals. These data suggest that body weight and composition may be an important factor to consider when choosing opioids as a postoperative analgesic. For example, buprenorphine treatment may be particularly unsuitable for obesity-related studies, in which it is less desirable to alter body mass and composition by uncontrolled factors such as postoperative treatment.
Decreased locomotor activity can be used as an indicator of postsurgical pain, because the degree of reduction from preoperative levels reflects the severity of the procedure.16,17 Consistent with this notion, activity was decreased in both groups the night after the implantation surgeries. In contrast, during the day period, activity levels were not depressed; in fact, daytime activities were significantly elevated in the buprenorphine group. This outcome may be reconciled, in part, by the observation that buprenorphine increases locomotor activity in rats that have not undergone surgery,8,15 suggesting this effect is unrelated to the pain-relieving effects of the drug. However, this hypothesis does not explain why, in the present study, this effect was only observed during the daytime period and not at night, given that buprenorphine-treated animals received a dose every 12 h. This finding may reflect, to some degree, the fact that preoperative ‘baseline’ activity levels are higher during the night phase, given that rats are nocturnal. Regardless of the underlying cause, buprenorphine clearly had a greater effect on locomotor activity than did meloxicam.
In general, the hemodynamic effects of postoperative analgesic treatment were more pronounced during the night phase compared with the day phase. Given that rats are nocturnal, this result might be expected. Therefore, the lack of clear differences in hemodynamic parameters during the day cycle could result from a lack of physical activity, and therefore lack of strenuous cardiovascular activity, which might mask the subtleties between postoperative treatment groups. It may be that only when these animals become active do the differences in overall activity, and therefore cardiovascular parameters, manifest.
The increased MAP and daytime HR values in the buprenorphine group are consistent with previous findings.12,22 We cannot ascertain definitely whether the changes in hemodynamic parameters (much like the other behavioral and physiologic parameters assessed in the present study) are a consequence of alleviating pain or are due to a secondary effect of the drug, because the control groups necessary for this determination (rats implanted with telemetry units but not treated with analgesic) were not included. However, previous studies12,22 have shown that buprenorphine treatment results in more pronounced increases in MAP and HR than those in vehicle-treated rats implanted with telemetry units; similar albeit more subtle effects on HR were observed in rats treated with the nonsteroidal antiinflammatory drug ketoprofen.22 On the basis of these studies, it appears that the hemodynamic effects of buprenorphine, and to a lesser extent nonsteroidal antiinflammatory drugs, are more marked than the hemodynamic changes in response to surgical pain during the immediate postnatal period. Therefore, the pronounced differences in hemodynamic measurements quite possibly reflect the effects of drug treatment, independent of surgical pain. The lack of nighttime increases in HR in the buprenorphine-treated group (Figure 4A) is peculiar and appears to be at odds with the aforementioned studies.12,22 For the same reason just discussed, it is unlikely that these results reflect more effective pain management. Irrespective of the underlying cause, the outcomes associated with MAP and HR less pronounced, and therefore less deleterious, with meloxicam treatment.
The differences between the treatment groups in several parameters, including growth rate (Figure 1 A), locomotor activity (Figure 3), and heart rate (Figure 4 A), being most pronounced in the period after analgesic (days 3 to 5 after surgery) treatment deserves some comment. If one assumes that the rate of healing was the same between the 2 groups, the effects observed must be attributed to residual effects of drug treatment or to effects of drug withdrawal. The buprenorphine dosage used in the present study falls within the recommended dosing range of 0.01 and 0.05 mg/kg.20 Although the dose was specifically chosen to avoid the potential confounding factor of rebound hyperalgesia (increased pain sensitivity after cessation of treatment) and other complications after discontinuation of treatment,23 it is possible that some hyperalgesia or other residual effect of removing buprenorphine was present. The other possibility of a lingering therapeutic effect of meloxicam seems less likely because the 9-h halflife of meloxicam in rats1 indicates that the analgesic effects of meloxicam should be eliminated 1 d after the last drug dose.
Rats were not treated preemptively with analgesics in the present study. Clear evidence supports the beneficial effects of preoperative treatment with opioids an nonsteroidal antiinflammatory drugs as a means to reduce postoperative pain and distress.5,14,18 Nevertheless, analgesics often are not given preemptively, either because of oversight or due to a lack of awareness of the beneficial effects associated with prior administrations. Moreover, in the case of unplanned surgeries, such as during trauma, preemptive analgesia is often not feasible. Consequently, the present results are relevant and provide valuable insight into the effects of these drugs when used postoperatively. Whether meloxicam treatment would prove to be as beneficial had these animals been treated preemptively with their respective drugs is unclear; future studies are needed to provide insights into this matter.
In summary, the data presented herein demonstrate that all the parameters assessed in the present study had a more favorable outcome with postoperative treatment with meloxicam than with buprenorphine. Specifically, meloxicam-treated rats had less severe behavioral (eating habits, locomotor activity) and physiologic (body weight loss, hemodynamic effects) manifestations than did buprenorphine-treated rats, either due to better analgesia, fewer pharmacologic side effects, or a combination thereof. In light of these observations, we suggest that meloxicam is superior to buprenorphine for postsurgical management after implantation of radiotelemetry devices in rats; its application for other surgical procedures should also be considered.
Acknowledgments
We thank Ms Corry Smallegange for implantating the radiotelemetric transducers. This study was supported by the Canadian Institutes of Health Research (MOP68993, MOP74521), and the Heart and Stroke Foundation of Canada (H&SNA5523).
References
- 1.Aguilar-Mariscal H, Patino-Camacho SI, Rodriguez-Silverio J, Torres-Lopez JE, Flores-Murrieta FJ. 2007. Oral pharmacokinetics of meloxicam in the rat using a high-performance liquid chromatography method in micro-whole-blood samples. Methods Find Exp Clin Pharmacol 29:587–591 [DOI] [PubMed] [Google Scholar]
- 2.Bourque SL, Komolova M, Nakatsu K, Adams MA. 2008. Long-term circulatory consequences of perinatal iron deficiency in male Wistar rats. Hypertension 51:154–159 [DOI] [PubMed] [Google Scholar]
- 3.Bourque SL, Komolova M, Nakatsu K, Adams MA. 2010. Unpublished data. [Google Scholar]
- 4.Brennan MP, Sinusas AJ, Horvath TL, Collins JG, Harding MJ. 2009. Correlation between body weight changes and postoperative pain in rats treated with meloxicam or buprenorphine. Lab Anim (NY) 38:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buvanendran A, Kroin JS. 2009. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol 22:588–593 [DOI] [PubMed] [Google Scholar]
- 6.Clark JA, Jr, Myers PH, Goelz MF, Thigpen JE, Forsythe DB. 1997. Pica behavior associated with buprenorphine administration in the rat. Lab Anim Sci 47:300–303 [PubMed] [Google Scholar]
- 7.Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. 2000. Sex-related differences in the antinociceptive effects of opioids: Importance of rat genotype, nociceptive stimulus intensity, and efficacy at the µ opioid receptor. Psychopharmacology (Berl) 150:430–442 [DOI] [PubMed] [Google Scholar]
- 8.Cowan A, Doxey JC, Harry EJ. 1977. The animal pharmacology of buprenorphine, an oripavine analgesic agent. Br J Pharmacol 60:547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dohoo SE, Dohoo IR. 1996. Factors influencing the postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can Vet J 37:552–556 [PMC free article] [PubMed] [Google Scholar]
- 10.Dunwoody CJ, Krenzischek DA, Pasero C, Rathmell JP, Polomano RC. 2008. Assessment, physiological monitoring, and consequences of inadequately treated acute pain. J Perianesth Nurs 23:S15–S27 [DOI] [PubMed] [Google Scholar]
- 11.Hawkins P. 2002. Recognizing and assessing pain, suffering and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab Anim 36:378–395 [DOI] [PubMed] [Google Scholar]
- 12.Ilback NG, Siller M, Stalhandske T. 2008. Effects of buprenorphine on body temperature, locomotor activity, and cardiovascular function when assessed by telemetric monitoring in rats. Lab Anim 42:149–160 [DOI] [PubMed] [Google Scholar]
- 13.Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE, Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research 2005. Recommendations for blood pressure measurement in humans and experimental animals, part 2: blood pressure measurement in experimental animals. A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Arterioscler Thromb Vasc Biol 25:e22–e33 [DOI] [PubMed] [Google Scholar]
- 14.Kurukahvecioglu O, Karamercan A, Ege B, Koksal H, Anadol Z, Tezel E, Ersoy E. 2007. Effect of meloxicam on postoperative pain relief after inguinal hernia repair with local anaesthesia. West Indian Med J 56:530–533 [PubMed] [Google Scholar]
- 15.Liles JH, Flecknell PA. 1992. The effects of buprenorphine, nalbuphine, and butorphanol alone or following halothane anaesthesia on food and water consumption and locomotor movement in rats. Lab Anim 26:180–189 [DOI] [PubMed] [Google Scholar]
- 16.Liles JH, Flecknell PA. 1993. The effects of surgical stimulus on the rat and the influence of analgesic treatment. Br Vet J 149:515–525 [DOI] [PubMed] [Google Scholar]
- 17.Liles JH, Flecknell PA. 1993. The influence of buprenorphine or bupivacaine on the postoperative effects of laparotomy and bile-duct ligation in rats. Lab Anim 27:374–380 [DOI] [PubMed] [Google Scholar]
- 18.Penderis J, Franklin RJ. 2005. Effects of pre- versus postanaesthetic buprenorphine on propofol-anaesthetized rats. Vet Anaesth Analg 32:256–260 [DOI] [PubMed] [Google Scholar]
- 19.Roughan JV, Flecknell PA. 2000. Effects of surgery and analgesic administration on spontaneous behaviour in singly housed rats. Res Vet Sci 69:283–288 [DOI] [PubMed] [Google Scholar]
- 20.Roughan JV, Flecknell PA. 2002. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating postoperative pain in animals. Lab Anim 36:322–343 [DOI] [PubMed] [Google Scholar]
- 21.Roughan JV, Flecknell PA. 2003. Evaluation of a short-duration behaviour-based postoperative pain scoring system in rats. Eur J Pain 7:397–406 [DOI] [PubMed] [Google Scholar]
- 22.Sharp J, Zammit T, Azar T, Lawson D. 2003. Recovery of male rats from major abdominal surgery after treatment with various analgesics. Contemp Top Lab Anim Sci 42:22–27 [PubMed] [Google Scholar]
- 23.St A Stewart L, Martin WJ. 2003. Evaluation of postoperative analgesia in a rat model of incisional pain. Contemp Top Lab Anim Sci 42:28–34 [PubMed] [Google Scholar]
- 24.Vane JR, Bakhle YS, Botting RM. 1998. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol 38:97–120 [DOI] [PubMed] [Google Scholar]