Abstract
A cynomolgus macaque presented with an ecchymotic and edematous left leg approximately 1 wk after a blood sample had been collected from the left femoral vein. Ecchymosis was noted in the femoral triangle, prepuce, and scrotum. The animal was not febrile or exhibiting signs of pain or distress. Duplex Doppler ultrasound imaging was used to evaluate the area. An arteriovenous fistula between the femoral artery and vein, accompanied by a pseudoaneurysm arising from the femoral artery, was identified. Various invasive and noninvasive treatment options for the pseudoaneurysm, including surgical repair, thrombin injection, stent placement, and ultrasound-guided compression repair (UGCR), were considered. UGCR was chosen as the first option for treatment. After a total of 20 min of UGCR at the neck of the pseudoaneurysm, complete thrombosis was achieved. Subsequent imaging of the lesion revealed resolution of the pseudoaneurysm. Because of the risks involved with invasive management techniques for this vascular lesion, UGCR is a valuable noninvasive treatment option for the repair of pseudoaneurysms.
Abbreviation: CDI, color Doppler imaging; UGCR, ultrasound-guided compression repair
Vascular malformations have been reported to occur in a wide variety of species.2,3,5-7,10,11,13,15-22,24,25,27-35 These abnormalities include arteriovenous fistulas, vascular shunts, hemangiomas, vascular atresias, aneurysms, pseudoaneurysms, telangectasia, and lymphangiomas, with the overwhelming majority of reports describing arteriovenous fistulas.2,3,5,6,11,15-19,25,27,30,33,35 Aneurysms have occurred in several primates including chimpanzees, gorillas, squirrel monkeys, howler monkeys, owl monkeys, African green monkeys, spider monkeys, patas monkeys, and capuchin monkeys.24 Pseudoaneurysms are reported infrequently. Pseudoaneurysms (or false) aneurysms are the result of leakage of blood from an artery into a defined space. A pseudoaneurysm associated with the femoral artery in a black and white colobus monkey was detected 6 d after manual restraint for routine blood collection from the femoral artery.32 The pseudoaneurysm was excised, and the resulting vascular defect was closed with an autologous graft. Another report10 describes a pseudoaneurysm associated with the femoral artery in a rhesus monkey. The diagnosis was obtained interoperatively, and excision of the defect was successful. Because surgical treatment of pseudoaneurysm has inherent risks and requires considerable surgical skill for a successful outcome, a noninvasive approach would be a valuable and cost-effective treatment option. This report is the first to describe the clinical presentation, diagnosis, and nonsurgical treatment of a pseudoaneurysm in a cynomolgus macaque.
Case Study
Case History.
An adult male cynomolgus monkeys (Macaca fascicularis) was transferred to our AAALAC-accredited facility in November 2005. At the time of his arrival, he was in good health. Previous records indicated negative serological status for macacine herpesvirus 1(formerly cercopithecine herpesvirus 1), simian retrovirus, simian T-lymphotropic virus, and SIV. During the quarantine period, the animal was consistently negative on intradermal tuberculin testing.
After the quarantine period, the animal was individually housed in accordance with standards published in the Guide for the Care and Use of Laboratory Animals14 in a stainless-steel cage in a room with other cynomolgus macaques. The room was environmentally controlled (temperature, 21 °C; humidity, 45% to 55%; photoperiod, 12:12 h light:dark). The animal was fed a commercial monkey chow (Lab Diet 5045, PMI Nutrition International, Brentwood, MO.) and received water by means of an automated water system. The diet was supplemented with fresh fruit and other enrichment items on a daily basis.
The macaque was assigned to an IACUC-approved behavioral assessment protocol. As part of the protocol, the animal was fitted with a metal collar and was trained by using the pole-and-collar technique to facilitate transfer to the testing chamber. Once the macaque was acclimated to this procedure, behavioral testing paradigms were initiated.
On 27 November 2006, the macaque was determined to have become proficient at the various behavior testing protocols, and as such, the second phase of the protocol was initiated. Biweekly intravenous injections of manganese sulfate into the saphenous veins were initiated. Manganese sulfate injections were given to create a disease model of neurotoxicity due to chronic manganese exposure. To monitor manganese levels, blood samples were collected weekly from the femoral veins, alternating between left and right legs. Prior to the macaque's presentation to veterinary services, the last blood sample had been collected from the left leg on 10 January 2007. No abnormalities were detected in either leg at the time of collection.
Clinical findings.
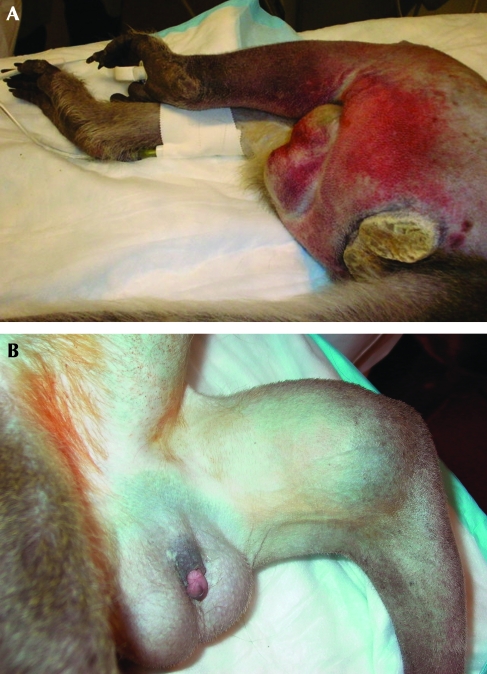
On 15 January 2007, the macaque presented with a swollen left leg, which was edematous and ecchymotic. Bruising was noted in the femoral triangle, prepuce and scrotum (Figure 1 A, B). The macaque was not showing signs of pain and distress as evidenced by a lack of change in behavior, attitude, appetite, or mobility. The macaque was sedated with ketamine (6 mg/kg; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) to allow for a more complete physical examination. The leg was grossly enlarged, and circumferential measurements were 23.5 cm at the proximal thigh (right leg, 23 cm), 23.3 cm at the stifle (right leg, 16 cm), and 25 cm at a point directly over a palpable mass (right leg, 21 cm). No pulse was palpated at the site of the mass, nor was the mass freely movable. Pulses distal to the lesion were normal. The remainder of the physical exam was within normal limits. Blood was drawn from the right femoral vein for a complete blood count and serum chemistry panel. After the blood draw, the venipuncture site was monitored for signs of a clotting defect; no abnormalities were noted. Due to the unknown nature of the lesion at this time, the macaque was treated with 81 mg aspirin orally as an antiinflammatory.
Figure 1.
Left leg of cynomologus macaque at presentation. Note the (A) bruising in the caudal thigh and scrotum and (B) diffuse edema in the inner thigh and upper leg.
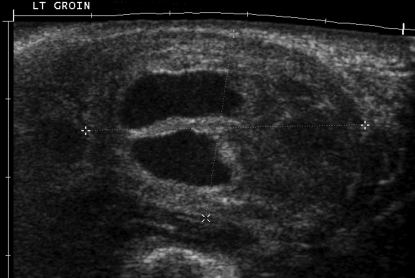
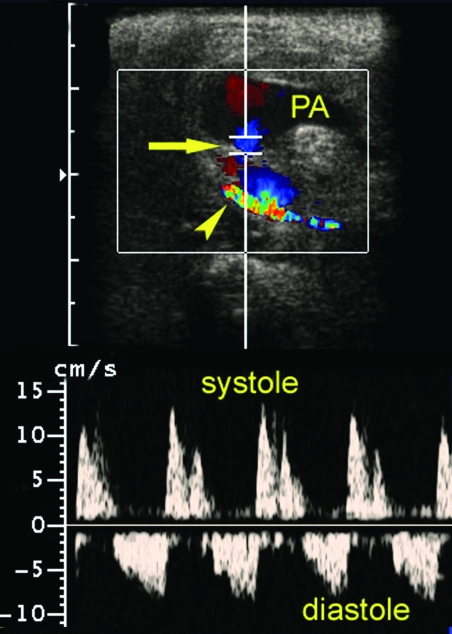
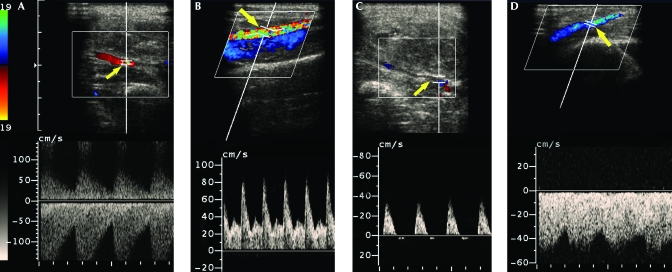
Duplex Doppler ultrasound imaging of the left groin and leg was performed the next day by an experienced sonographer using a Sonoline Elegra scanner equipped with a 7.5-MHz flat linear array transducer (Siemens Medical Solutions, Issaquah, CA). Gray-scale sonography demonstrated a complex mass (36 × 24 × 30 mm) superficial and slightly lateral to the femoral artery, consistent with a hematoma (Figure 2). Evaluation of the mass with color Doppler imaging (CDI) and pulsed Doppler spectral analysis demonstrated blood flow into a portion of the mass during systole and reversal of flow during diastole (Figure 3). These findings were consistent with a pseudoaneurysm. In addition, CDI demonstrated a focal region of high-velocity flow, with color aliasing between the femoral artery and femoral vein (Figure 4 A). CDI-guided spectral Doppler analysis of blood flow in this region demonstrated high-velocity flow with low downstream resistance. Spectral Doppler analysis of blood flow in the femoral artery above the region of high-velocity flow demonstrated a monophasic–continuous wave form (Figure 4 B). Spectral Doppler analysis of the femoral artery below the region demonstrated a biphasic waveform (Figure 4 C). Spectral analysis of blood flow in the femoral vein demonstrated increased velocities and flow that was pulsatile with the cardiac cycle (arterialized venous flow; Figure 4 D). These findings were consistent with an arteriovenous fistula between the femoral artery and femoral vein at the level of the femoral triangle.
Figure 2.
Gray-scale sonography of the palpable mass (between calipers) in the left groin. The mass measured approximately 36 × 24 × 30 mm and had anechoic as well as echogenic areas (that is, it was a complex mass).
Figure 3.
Evaluation of the groin mass (a pseudoaneurysm [PA]) with color Doppler imaging (CDI) and pulsed Doppler spectral analysis. CDI demonstrated blood flow in a portion of the mass and in a track (arrow) leading from the femoral artery (arrowhead) into the mass. Pulsed Doppler with spectral analysis demonstrated blood flow from the femoral artery into the mass during systole and reversal of flow in diastole (that is, the ‘to-and-fro sign’). These findings were consistent with a diagnosis of a pseudoaneurysm.
Figure 4.
A) Color Doppler imaging (CDI) demonstrated a focal region of high-velocity flow with color aliasing (arrow) between the femoral artery and femoral vein. CDI-guided pulsed Doppler with spectral analysis of this region demonstrated high-velocity flow with a high level of diastolic flow (indicating low downstream resistance). (B) Spectral Doppler analysis of blood flow in the femoral artery (arrow) above the region of high-velocity flow demonstrated a monophasic–continuous wave form, whereas (C) spectral analysis of the femoral artery below the region demonstrated a normal biphasic waveform. (D) Spectral analysis of blood flow in the femoral vein (arrow) central to this region demonstrated elevated velocities and flow that was pulsatile with the cardiac cycle (that is, ‘arterialized venous flow’). These findings are consistent with an arteriovenous fistula between the left femoral artery and femoral vein.
Results of the initial hematologic and serologic samples revealed an anemia characterized by a low total red blood cell count (2.28 × 106/µL; normal, 6.31 to 7.55 × 106/µL), hemoglobin (5.8 g/dL; normal, 12.7 to 14.7 g/dL), and hematocrit (17.9%; normal, 42.0 to 49.2%). Remarkable serum chemistry values included mild hypoproteinemia (6.1 g/dL; normal, 6.3 to 8.2 g/dL) and hypoalbuminemia (3.3 g/dL; normal, 3.9 to 5.0 g/dL) and markedly elevated creatine kinase (2099 U/L; normal, 200 to 800 U/L).
Discussions with a vascular surgeon indicated that surgical repair had a high risk of marked blood loss, possibly resulting in the death of the animal. A nonsurgical approach was suggested. Ultrasound-guided compression repair (UGCR) of pseudoaneurysms and arteriovenous fistulas is used routinely as a noninvasive alternative to conventional surgery in humans. The decision was made to attempt UGCR the next day. Because aspirin therapy results in hypocoagulation, aspirin was discontinued, and the staff was notified to monitor for signs of pain and distress, at which time the veterinarians would initiate an analgesic regimen.
On 17 January 2007, the animal was sedated with ketamine (6 mg/kg) and anesthetized with isoflurane by endotracheal tube for the USGR procedure. A circulating- water heating pad was used to facilitate thermoregulation. CDI confirmed that the pseudoaneurysm and arteriovenous fistula were still present. First, the track between the femoral artery and pseudoaneurysm (the neck) was located with CDI. Then, by using the ultrasound probe, pressure was applied to the neck of the pseudoaneurysm and continuously held in place for 10 min. Distal pulses were monitored manually to verify continued patency of the femoral artery. Subsequent ultrasound evaluation showed partial thrombosis of the pseudoaneurysm, and the arteriovenous fistula remained patent. A second UGCR procedure was performed as described. After the second 10-min compression session, CDI demonstrated complete thrombosis of the pseudoaneurysm. However, the arteriovenous fistula was still present. After redefining the precise location of the arteriovenous fistula, ultrasound-guided probe compression was applied and held in place for 10 min. After this third UGCR session, the arteriovenous fistula was no longer present. Spectral Doppler analysis of blood flow in the femoral artery above the region of the previously identified arteriovenous fistula demonstrated a monophasic, noncontinuous wave form, and spectral analysis of flow in the femoral vein above this region did not demonstrate arterialized flow. The macaque was maintained under isoflurane anesthesia for 3 h to allow the thromboses to solidify.
The macaque recovered from the procedure without incident and continued to exhibit normal behaviors and mobility. A follow-up duplex Doppler sonography evaluation on 19 January 2007 revealed a hematoma in the left groin, but CDI did not display any flow in the mass (Figure 5). Spectral Doppler analysis of flow in the femoral artery and femoral vein appeared within normal limits. These findings suggested that the UGCR procedures had been successful in eliminating the pseudoaneurysm and closing the arteriovenous fistula. Measurements of the leg were improved, with the groin being 22 cm in diameter, the knee 19 cm, and the midpoint (directly over the hematoma) 22 cm.
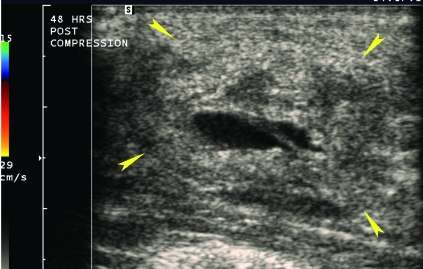
Figure 5.
Color Doppler imaging performed approximately 48 h after UGCR procedures identified a mass without internal blood flow (arrowheads) consistent with a hematoma in the location of the previously identified pseudoaneurysm.
One week after presentation, the animal was observed ambulating normally with markedly decreased swelling of the affected leg. No bruising was visualized in any area of the leg. Results from a repeat blood sample collected on 25 January 2007 revealed improvement in the anemia with a red blood cell count of 3.51 M/µL and hematocrit of 27.4% and a decrease in the creatine kinase level (346 U/L). Technical staff was instructed to avoid performing vascular access on the left leg.
During subsequent ultrasound imaging in May 2007, no evidence of the pseudoaneurysm was seen. Unfortunately, recurrence of the arteriovenous fistula between the femoral artery and femoral vein was detected, but the degree of flow between the 2 vessels was less than it had been prior to UGCR procedures. A complete physical exam performed on 11 July 2007 showed no clinical evidence of recurrence of the pseudoaneurysm.
Discussion
A pseudoaneurysm (false aneurysm) is a collection of blood that results from leakage from a vessel into adjacent tissues. It differs from a true aneurysm in that its wall does not contain the components of an artery but consists of fibrous tissue that usually continues to enlarge, creating a pulsating hematoma. False aneurysms may be due to penetrating or blunt trauma or as a complication to an endovascular procedure. Differential diagnoses to be considered are hematoma, abscess, arteriovenous fistula, lymphadenopathy, lymphocoele, deep venous thrombosis, and compartment syndrome. Presenting signs typical of a primate with a pseudoaneurysm include a mass that is pulsatile, painful, and warm. It may be possible to feel movement of blood (thrill) within the mass.
In this presentation, a blood sample had been collected from the femoral vein 5 d prior to the observation of a swollen leg. It is probable that the pseudoaneurysm was formed as a result of this procedure. Although the technician performing the blood sampling had been trained by the veterinary staff in an approved method9 and had collected samples weekly for a period of 2 y with no adverse events, phlebotomy in primates from the femoral vein has an inherent risk of inadvertently causing trauma to the femoral artery. Appropriate training and the use of correct technique should be stressed in an effort to avoid iatrogenic vascular lesions.
In this case, an arteriovenous fistula also was noted. Whether the fistula was present prior to the diagnosis of the pseudoaneurysm is unknown. An arteriovenous fistula may be congenital, spontaneous (associated with natural disease), or iatrogenic. Iatrogenic arteriovenous fistulas in nonhuman primates have occurred as a result of attempted venipuncture.33 There are reports in the human medical literature of pseudoaneurysm and formation of an arteriovenous fistula after femoral artery catheterization.8,12,23
Diagnosis of a pseudoaneurysm can be achieved by using duplex Doppler ultrasonography, arteriography, computed tomographic angiography, and magnetic resonance angiography. A more invasive diagnostic technique that can be used is a needle aspirate. This procedure has limitations in that it cannot differentiate between an acute hematoma and an aneurysm. In addition, the procedure may result in considerable blood loss.
In the present case, duplex Doppler ultrasonography was diagnostic in detecting both the pseudoaneurysm and arteriovenous fistula. Treatment options for pseudoaneurysms include surgical repair, endovascular procedures, ultrasound-guided compression repair and ultrasound-guided thrombin injection. Marked blood loss and other complications are possible sequellae to surgery and should be taken into consideration. Typically, surgical repair is indicated only if the blood supply to the distal tissues is compromised and reconstruction of damaged arteries is necessary to restore adequate blood supply. A less invasive option is to use an endovascular approach to place a covered stent within the artery. The stent can be placed to block the hole that allows blood to reach the pseudoaneurysm.
Another procedure that does not require accessing the artery is ultrasound-guided compression repair of the pseudoaneurysm. This technique relies on the ability to obstruct the flow of blood from the injured artery into the pseudoaneurysm sack, thus allowing a clot to form. By using ultrasound guidance to locate the connection (neck) between the artery and pseudoaneurysm, compression can be applied to stem the flow and monitor the effectiveness of the technique both during probe compression and immediately afterward. If ultrasonography demonstrates that the pseudoaneurysm is still present, additional UGCR procedures can be performed. This technique has a reported success rate in the human population of 74% to 95% if the patient is not on anticoagulation and is most successful in small (diameter, less than 3 cm) pseudoaneurysms.8
The use of ultrasound-guided thrombin injections has been described as a means to treat pseudoaneurysms.1,4 After the pseudoaneurysm is localized by using ultrasonography, thrombin is injected directly into the pseudoaneurysm. Although published reports1,4 indicate that this procedure is very successful for the treatment of pseudoaneurysms, it cannot be used if an arteriovenous fistula exists because the injected thrombin may migrate into the venous system and have serious deleterious effects, creating pulmonary or brain ischemia.
In the case we present, surgical repair of the pseudoaneurysm and arteriovenous fistula posed considerable risk to the survival of the primate and was considered an option of last resort. The use of stents may have been successful; however, the surgical team had little experience in placing stents in similar situations. The use of ultrasound-guided thrombin injections into the pseudoaneurysm was deemed inappropriate because of the presence of the arteriovenous fistula. UGCR was successful, thereby avoiding more invasive procedures. This technique is a viable option for the repair of peripheral pseudoaneurysms in primates.
References
- 1.Allison SJ, Merton DA, Needleman L, Polak JF. 2008. Peripheral vascular system, p 495–522 : McGahan JP, Goldberg BB. Diagnostic ultrasound: a logical approach. New York (NY): Informa Healthcare [Google Scholar]
- 2.Bailey MQ, Willard MD, McLoughlin MA, Gaber C, Hauptman J. 1988. Ultrasonographic findings associated with congenital hepatic artiovenous fistula in three dogs. J Am Vet Med Assoc 192:1099–1101 [PubMed] [Google Scholar]
- 3.Blankenship-Paris TL, Schenkman DI. 1997. What's your diagnosis? Arteriovenous abnormality in a baboon (Papio spp.). Lab Anim (NY) 26:19–20 [Google Scholar]
- 4.Brophy DP, Sheiman RG, Amatulle P, Akbari CM. 2000. Iatrogenic femoral pseudoaneurysms: thrombin injection after failed US-guided compression. Radiology 214:278–282 [DOI] [PubMed] [Google Scholar]
- 5.Butterfield AB, Hix WR, Pickrel JC, Johnson KE. 1980. Acquired peripheral arteriovenous fistula in a dog. J Am Vet Med Assoc 176:445–448 [PubMed] [Google Scholar]
- 6.Cho CY, Cook JE, Leopold HW. 1979. Angiomatous vascular malformation in the spinal cord of a Hereford calf. Vet Pathol 16:613–616 [DOI] [PubMed] [Google Scholar]
- 7.Cordy DR. 1979. Vascular malformations and hemangiomas of the canine spinal cord. Vet Pathol 16:275–282 [DOI] [PubMed] [Google Scholar]
- 8.Corriere MA, Guaman RJ. 2005. True and false aneurysms of the femoral artery. Semin Vasc Surg 18:216–223 [DOI] [PubMed] [Google Scholar]
- 9.Fortman JD, Hewett TA, Bennett BT. 2002. Experimental methodology, p 162–164 : Suckow MA. The laboratory nonhuman primate. Boca Raton (FL): CRC Press [Google Scholar]
- 10.Gralla EJ, Coleman GL. 1969. False aneurysm in a rhesus monkey—surgical removal and description of pathology. Vet Med Small Anim Clin 64:888–893 [PubMed] [Google Scholar]
- 11.Guglielmini C, Bernardini D. 2003. Echo-Doppler findings of a carotid–juglar fistula in a foal. Vet Radiol Ultrasound 44:310–314 [DOI] [PubMed] [Google Scholar]
- 12.Gur O, Canbaz S, Karaca OG, Duran E. 2007. Iatrogenic femoral arteriovenous fistula and pseudoaneurysm following catheter insertion for hemodialysis. J Cardiovasc Surg (Torino) 48:257–258 [PubMed] [Google Scholar]
- 13.Hattangady SR, Wadia DS. 1965. Aneurysmal varies in a bullock—a case report. Indian Vet J 42:622–623 [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 15.Jackson RK, Motzel SL, Corrigan JE. 1996. Diagnostic exercise: cutaneous lesions and unilateral hind limb swelling in a rhesus monkey. Lab Anim Sci 46:444–447 [PubMed] [Google Scholar]
- 16.Jones DG, Allen WE, Webbon PM. 1981. Arteriovenous fistula in the metatarsal pad of a dog: a case report. J Small Anim Pract 22:635–639 [DOI] [PubMed] [Google Scholar]
- 17.Koide K, Koide Y, Wada Y, Nakaniwa S, Yamane Y. 2004. Congenital hepatic arteriovenous fistula with intrahepatic portosystemic shunt and aortic stenosis in a dog. J Vet Med Sci 66:299–302 [DOI] [PubMed] [Google Scholar]
- 18.Latendresse JR, Ngampochjama M, Ward GS. 1987. Port-wine nevus-like artiovenous malformation in a rhesus macaque (Macaca mulatta). Vet Pathol 24:197–199 [DOI] [PubMed] [Google Scholar]
- 19.Legendre AM, Krahwinkel DJ, Carrig CB, Michel RL. 1976. Ascites associated with intrahepatic arteriovenous fistula in a cat. J Am Vet Med Assoc 168:589–591 [PubMed] [Google Scholar]
- 20.Lourenco ML, Vailati MC, Luis Junior AM, Jr, Sequeira JL, Peres JA, Gimenes SM. 2002. Dissecting aortic aneurysm in a cat. Can Vet J 43:720–721 [PMC free article] [PubMed] [Google Scholar]
- 21.Marr CM, Reef VB, Brazil TJ, Thomas WP, Knottenbelt DC, Kelly DF, Baker JR, Reimer JM, Maxson AD, Crowhurst JS. 1998. Aortocardiac fistulas in 7 horses. Vet Radiol Ultrasound 39:22–31 [DOI] [PubMed] [Google Scholar]
- 22.Meurs KM, Miller MW, Handon C, Honnas C. 1997. Tricuspid valve atresia with main pulmonary artery atresia in an Arabian foal. Equine Vet J 29:160–162 [DOI] [PubMed] [Google Scholar]
- 23.Mitchell DG, Needleman L, Bezzi M, Goldberg BB, Kurtz AF, Pennell RG, Rifkin MD, Vilar M, Baltarowich OH. 1987. Femoral artery pseudoaneurysm: diagnosis with conventional duplex and color Doppler US. Radiology 165:687–690 [DOI] [PubMed] [Google Scholar]
- 24.Moore DM, Brown RJ, Carraway JH, Gibbs CJ. 1982. Aneurysm of a brachiocephalic artery in a capuchin monkey (Cebus paella). Lab Anim Sci 32:289–290 [PubMed] [Google Scholar]
- 25.Olson LC, Walker DM, Hall WC. 1991. Portal–caval vena caval–renal–hepatic artiovenous malformation in a dog. Lab Anim Sci 41:635–638 [PubMed] [Google Scholar]
- 26.O'Sullivan GJ, Ray SA, Lewis JS, Lopez AJ, Powell BW, Moss AH, Dormandy JA, Belli AM, Buckenham TM. 1999. A review of alternative approaches in the management of isotrogenic femoral pseudoaneurysms. Ann R Coll Surg Engl 81:226–234 [PMC free article] [PubMed] [Google Scholar]
- 27.Parks AH, Guy BL, Rawlings CA, Constantino MJ. 1989. Lameness in a mare with signs of arteriovenous fistula. J Am Vet Med Assoc 194:379–380 [PubMed] [Google Scholar]
- 28.Platt H. 1987. Vascular malformations and angiomatous lesions in horses: a review of 10 cases. Equine Vet J 19:500–504 [DOI] [PubMed] [Google Scholar]
- 29.Rogers KS, Butler LM, Edwards JF, Brassard J, Boothe H, Cargile J. 1992. Rectal hemorrhage associated with vascular ectasia in a young dog. J Am Vet Med Assoc 200:1349–1350 [PubMed] [Google Scholar]
- 30.Rosenberg DP, Link DP, Prahalada S. 1983. Arteriovenous malformation in a rhesus monkey (Macaca mulatta). Lab Anim Sci 33:183–186 [PubMed] [Google Scholar]
- 31.Sleeper MM, Durando MM, Miller M, Habecker PL, Reef VB. 2001. Aortic root disease in 4 horses. J Am Vet Med Assoc 219:491–496 [DOI] [PubMed] [Google Scholar]
- 32.Stetter MD, Wells SK, Kerstein MD, Soroyan M, Schwedler M. 1992. Femoral artery pseudoaneurysm in a monkey. J Am Vet Med Assoc 201:1091–1092 [PubMed] [Google Scholar]
- 33.Streett JW, Lord PF, Schwartz A. 1980. Iatrogenic arteriovenous fistula in a cynomolgus macaque (Macaca fascicularis): a case report. Lab Anim Sci 30:1012–1015 [PubMed] [Google Scholar]
- 34.Tidwell AS, Ross LA, Klein LJ. 1997. Computed tomography and magnetic resonance imaging of cavernous sinus enlargement in a dog with unilateral exophthalmos. Vet Radiol Ultrasound 38:363–370 [DOI] [PubMed] [Google Scholar]
- 35.Tuttle AD, MacLean RA, Linder K, Cullen JM, Wolfe BA, Loomis M. 2009. Acquired arteriovenous fistula in a grizzly bear (Ursus arctos horribilis). J Zoo Wildl Med 40:193–195 [DOI] [PubMed] [Google Scholar]