Abstract
Since HLA-restricted cytotoxic T-cell responses select specific polymorphisms in HIV-1 sequences and HLA diversity is relatively static in human populations, we investigated the use of peptide epitopes based on sites of HLA-associated adaptation in HIV-1 sequences to stimulate and detect T-cell responses ex-vivo. These “HLA-optimised” peptides captured more HIV-1 Nef-specific responses compared with overlapping peptides of a single consensus sequence, in interferon-γ enzyme linked immunospot assays. Sites of immune selection can reveal more immunogenic epitopes in HLA-diverse populations and offer insights into the nature of HLA-epitope targeting, which could be applied in vaccine design.
Keywords: CD8 T cells, HLA alleles, HIV-1
1.0 Introduction
One of the challenges in HIV-1 vaccine design is the immense variability of the virus. The variability over full length proteomes is a reflection of multiple forces, including neutral evolution through multiple hosts, genetic bottlenecks at new transmissions and escape mutations selected by immunological pressures within single hosts over time [1]. Among the latter, HIV mutates within or near human leukocyte antigen (HLA)-restricted epitopes to escape cytotoxic CD8 T-cell responses, either to disrupt T-cell receptor (TCR) recognition, HLA-epitope binding or epitope processing [2, 3]. Some mutations appear to create new variant “neo-epitopes” which are also subject to immune responses [4]. It is therefore the variation within or near HLA-restricted epitopes that is most functionally relevant to CD8 T-cell reactivity. As HLA distributions remain relatively stable in the face of rapid HIV-1 evolution, HLA alleles and their allele-specific selection effects on the virus make viral diversity, to some extent, predictable [1, 5-9].
These points are equally applicable to the choice of stimulating antigens in assays of HIV-1-specific CD8 T-cell responses, which continues to be of major interest for a wide range of studies spanning HIV immunobiology and T-cell based HIV vaccine design. The most commonly used capture assays of responses ex-vivo or of clonal T-cell populations rely on short or long term stimulation of circulating T-cells with peptides that contain or correspond to HLA-restricted T-cell epitopes in HIV-1. Peptides derived from HIV-1 reference sequences based on laboratory isolates or population consensus sequences have contributed a great deal to the accumulated knowledge of viral T-cell epitopes and responses to date; however, responses against variant or subdominant epitopes may be missed using these approaches [2, 10-11]. A comparison of consensus and autologous sequence peptides showed that 29% of responses were detected by autologous peptides only and that these responses were directed towards epitopes in the variable regions of the virus [2]. Though highly stringent, the cost of generating autologous peptide sets is prohibitive for large scale screening studies. More recently, peptide design strategies that explicitly account for HIV sequence diversity such as potential T-cell epitopes (PTEs), toggled peptides and peptides derived from consensus, ancestral and centre-of-tree viral sequences have been developed [10-13]. PTE peptides are derived from 110 full-length genome subtype B sequences and a stepwise algorithm which generate ninety 15-mer peptides that cover 70% of population sequence diversity [10, 12]. As HIV sequence variation can be reduced to a few co-dominant alternatives when epitope-length windows are considered, toggled peptides have epitope variation embedded by including alternative amino acids during peptide synthesis. In comparative studies using interferon (IFN)-γ enzyme linked immunospot (ELISpot) assays, HIV-specific CD8 T-cell responses of higher breadth were detected with PTEs and toggled peptides than matched consensus peptides [10, 11], and in the case of toggled peptides, this was explained by greater sequence similarity with the autologous virus [11]. Although comparable IFN-γ responses were detected with peptides derived from consensus, centre-of-tree and ancestor sequences, the range of responses detected was significantly broader when a combination of all three sets was used which was attributed to an increased coverage of viral diversity [13]. As a key principle, these studies demonstrate the functional importance of HIV sequence variation to HLA-peptide-TCR interactions mediating T-cell reactivity in immunoassays, and conceivably also important in responses to vaccines.
Here we have sought to examine whether the efficiency of diversity coverage in immunoassays (and by implication in vaccine immunogens) could be improved by an alternative approach based on optimising for HLA-restricted recognition at the population level. These “polyallelic” peptides were conceived as a HIV-1 subtype B peptide reagent set containing all well known optimal HLA-restricted CD8 T-cell epitopes as well as predicted optimal epitopes spanning sites of evident HLA class I allele-specific polymorphism in subtype B HIV-1, optimised for the HLA distribution of the tested population. By focussing on “immunologically relevant” variation, we hypothesised that a broader range of responses against optimal epitopes could be elicited with fewer assays. We compared the use of polyallelic peptides to standard overlapping subtype B consensus peptides in detection of HIV Nef-specific responses in the IFN-γ ELISpot assay. We carried out testing of both peptide sets in parallel with the same patient samples under equivalent assay conditions. Nef was chosen for the combination of high CD8 T-cell epitope density and high sequence variability, allowing for the best chance of detecting a difference between the two peptide sets in detection of variant-specific responses.
2.0 Materials and methods
2.1 Patient cohort
We studied 28 individuals with chronic HIV infection enrolled in the observational West Australian (WA) HIV Cohort Study which operates under institutional review board approval [14]. Selection of study subjects was necessarily restricted to those with stored PBMCs in sufficient numbers for simultaneous ELISpot screening with both peptide sets. Written informed consent was obtained from all patients. Medium to high resolution HLA genotyping for all class I loci, plasma HIV RNA concentrations (viral load) and the percentages of CD4 and CD8 T-cells most contemporaneous with ELISpot testing were available for all individuals.
2.2 HLA-HIV polymorphism associations and putative epitopes
We used a reference set of phylogeny-adjusted associations between HLA alleles and sequence polymorphisms across all HIV proteins computed in an independent subtype B infected cohort, in which there was good statistical power over a diverse array of HLA genotypes [15]. The specific amino acids representing the non-adapted/reversion/wild-type or adapted/escaped residue at each site of HLA association were computed by the analysis. After all viral amino acid-HLA associations were characterized, the consensus sequence of the population surrounding sites of HLA-driven polymorphism was scanned with the ‘Epi-pred’ epitope prediction program [16], http://atom.research.microsoft.com/bio/epipred.aspx]. In some cases, the epitope that was predicted to be a variant epitope induced by immune pressure but still possibly immunogenic based on its predicted HLA-peptide binding (“adapted epitopes”) was included. The prediction algorithm was trained on specified characteristics of known CD8 T-cell epitopes; including epitope length, HLA-restriction and the physicochemical properties of amino acids within and flanking epitopes [16]. Polyallelic peptides therefore included predicted and known epitopes [17], regardless of whether the epitope could be a target of selection in-vivo (non-adapted epitopes) or a variant capable of inducing a de-novo immune response (adapted epitopes). All epitopes assigned with a prediction probability score >0.4 were included in the polyallelic peptide set.
2.3 Formulation of polyallelic and overlapping peptide sets
The panel of polyallelic peptides comprising of sixty-two 8- to 11-mer peptides covering HIV-1 clade B Nef were synthesized (Invitrogen, Victoria, Australia), solubilised and tested as individual peptides. The responses to the polyallelic peptides were compared with those elicited by forty-nine overlapping 15-mer peptides spanning a subtype B consensus Nef sequence (AIDS Research and Reference Reagent Program, NIH, Maryland, United States of America). These peptides overlapped by 11 amino acids and were set up in a matrix of 14 pools. Twenty-four peptides from the polyallelic peptide panel were embedded within the overlapping peptides and the overlap between the two peptide sets is shown in Figure 1. Lyophilized optimal and overlapping peptides were reconstituted in DMSO (VWR International Ltd, Leicestershire, England) at 10 mg/mL stored at −20°C until used. The final concentration of individual peptides whether tested singly or within pools was 2 μg/mL and all peptides were tested in duplicate. Though the majority of patients screened by ELISpot assays had HLA genotyping results available, testing of peptides were not limited to those known to be restricted by the HLA genotype of each individual. The polyallelic panel was tested in a standard fashion in all individuals in keeping with a screening strategy and so that the correlation between responses and HLA genotype could be analysed post-hoc.
Figure 1. Location of polyallelic peptides in relation to overlapping peptide set for the Nef region of HIV-1.


Overlapping peptides derived from a standard consensus clade B sequence, were 15-mer in length and overlapped by 11 amino acid residues. Analyses of HLA-HIV associations were used to construct a ‘cohort specific’ consensus sequence from which the polyallelic peptides were generated. Published CD8 T-cell epitopes were also included in the panel of polyallelic peptides. IFN-γ production was quantified in response to forty-nine overlapping peptides and sixty-two polyallelic peptides. HLA-associations for each epitope in the polyallelic peptide set are shown below the peptide. Differences between the overlapping and polyallelic peptide sequences are highlighted in bold.
2.4 IFN-γ ELISpot assay
Peripheral blood mononuclear cells (PBMCs) were separated using Accuspin tubes (Sigma, Missouri, United States of America), stored and subsequently thawed when required. Cell numbers and viability were assessed on the Vi-Cell XR (Beckman Coulter, Sydney, Australia) and volume of media adjusted to give a concentration of 106 PBMCs/mL. IFN-γ ELISpot assays were performed using the Biomek FX (Beckman Coulter) [18]. Anti-CD3 antibodies and pools of CMV-, EBV- and influenza (CEF)-derived peptides (Mabtech, Victoria, Australia) were set up in duplicate or triplicate (final concentration=2 μg/mL) as positive controls while triplicate negative controls consisted of PBMCs cultured in media alone.
IFN-γ producing spots were enumerated using an AID ELISPOT reader (Autoimmun-Diagnostika, Strassberg, Germany). The number of IFN-γ producing T-cells was determined after subtraction of the background and expressed as SFUs/106 PBMCs [19].
2.5 Statistical methods
T-cell responses to both peptide sets were counted as positive if the number of spots were at least twice as high as the background and had ≥50 SFUs/106 PBMCs. In addition to fulfilling the above criteria, a peptide in the overlapping panel was included in the analysis only if it elicited a response in two pools, with the magnitude of the response taken to be the median of the two contributing pools. Peptide response rates were estimated as the proportions of the peptides eliciting an IFN-γ response over a defined responder group. To accommodate the correlation induced by multiple measures per patient, generalized estimating equations were utilized for analysing the rates of response, and generalized linear mixed effects models of log-transformed SFUs for assessing the magnitude of responses.
3.0 Results
The majority of patient in the cohort were male (89%) and all but four individuals were on antiretroviral therapy. The median viral load was <50 HIV RNA copies/mL and median percentage of CD4 and CD8 T-cells were 27% and 46% respectively. The HLA allele frequency distribution was consistent with known ethnicity (predominantly white, European ancestry; data not shown).
3.1 IFN- γ responses to polyallelic peptides
PBMCs from 61% of patients produced IFN-γ in response to stimulation by at least one polyallelic peptide, with 80% of the peptides in the panel eliciting responses. The responder and non-responder patients groups were balanced for the number per person of tested epitopes that were HLA-matched (mean [SD]: 16.1 [4.8] vs 15.1 [6.8], p=0.7). There were no significant differences between responders and non-responders in terms of percentage CD4 T-cells (mean [SD]: 26% [9%] vs 29% [13%], p=0.5), CD8 T-cells (48% [11%] vs 48% [14%], p=0.9), or the proportions with undetectable viral load (65% vs 91%, p=0.2, Fisher’s exact test). Only one of the eleven non-responders was not on therapy.
Amongst the 17 responders, the average response rate per peptide was 9.96%. Ninety-four percent of responders directed at least one response to a peptide in the region between amino acid residues 105 to 146 (HXB2 numbering). There was no observed correlation between the breadth of responses and viral suppression, percentage of CD4 and CD8 T-cells, treatment status or age.
As shown in Figure 2A, the median magnitude of the IFN-γ response to polyallelic peptides was 120 SFUs/106 PBMCs (range=50-3500 SFUs/106 PBMCs). Ten of the 17 responders had a maximum response above 200 SFUs/106 PBMCs. The magnitude of IFN-γ responses was not associated with epitope adaptation, carriage of the HLA allele restricted to the epitope, viral suppression, percentage of CD4 or CD8 T-cells, treatment status or age, however, responses were marginally higher when directed against epitopes located in the central Nef region (p=0.05).
Figure 2. Magnitude of responses (A) and response rates (B) to the set of overlapping peptides covering all of Nef and the set of polyallelic peptides identified as HLA-restricted epitopes within Nef.
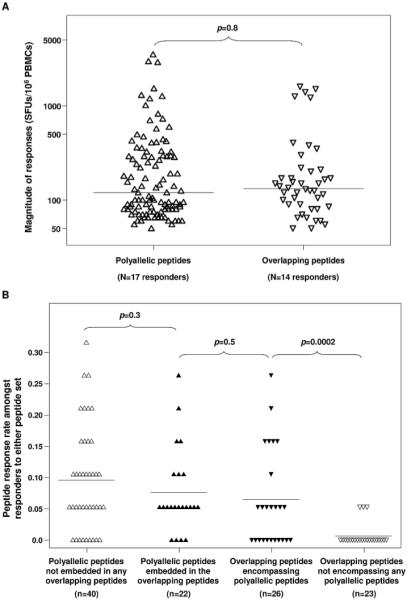
There were no significant differences in the magnitude of responses (A) detected by the panel of tested polyallelic peptides (△, median=120 SFUs/106 PBMCs) compared to the overlapping peptides (▽, median=132 SFUs/106 PBMCs). Each of the two peptide sets were split to further focus on common peptide sequences: embedded polyallelic peptides were those sequences contained within an overlapping peptide; encompassing overlapping peptides were those which contained at least one embedded polyallelic peptide within the longer sequence. Similar response rates (p=0.5) were obtained for peptide sequences common to sets (B). However, a significantly higher number of overlapping peptides elicited a response when the peptide contained an embedded polyallelic epitope than if no polyallelic peptide was present in the overlapping peptide sequence (p=0.0002).
3.2 IFN- γ responses to novel and variant (HLA-adapted) polyallelic peptides
Forty-one percent of responses were in patients carrying a matching HLA-allele restriction of the stimulating epitope as previously published or predicted by the ‘Epi-pred’ programme. A further 18% of responses were in individuals with an HLA allele from the same supertype group [20]and 26% were observed in individuals carrying an HLA allele matching the restriction of an epitope overlapping the stimulating peptide (mean overlap of 7 amino acid residues) in the Nef protein.
Among the responses directed against central Nef epitopes, there was a predominance of adapted epitopes that elicited an IFN-γ response. Seven of the 13 adapted epitopes elicited responses in more than 20% of responders (FPLTFGWCF 35%; PLTFGWCYKL and YPLTFGWCY 29%; KEKGGLEGI, KRQEILDLWVY, TRYPLTFGW and TPGPGVRYPL 24%) compared with 2 of 9 of the non-adapted epitopes (RYPLTFGWCF 29%; TQGYFPDWQNY 24%). Carriage of the restricting HLA allele increased the likelihood of response to adapted epitopes (mean response rate per adapted epitope amongst HLA-matched carriers 12% vs 5% in non-carriers, p<0.0001), but IFN-γ response rates to non-adapted epitopes did not differ significantly when carriage of the HLA-allele was considered (carriers: 6%, non-carriers: 5%, p=0.7). Similar patterns of relative responsiveness were observed when omitting the eleven non-responding patients from the analyses (adapted epitopes: 18% vs 8%, p=0.0001; non-adapted epitopes: 10% vs 8%, p=0.5) and also restricting further to include only those epitopes eliciting a response (adapted epitopes: 21% vs 11%, p=0.002; non-adapted epitopes: 12% vs 10%, p=0.6).
3.3 Polyallelic versus overlapping consensus peptides
IFN-γ responses to pools of overlapping peptides were seen in 50% of tested individuals. The magnitude of these responses (median [range] = 132 [50, 1595]) was very comparable to that observed for the polyallelic peptides (p=0.8, Figure 2A). Forty-three percent of individuals responded to both peptide sets and nine patients, all of whom were on therapy and had undetectable viral loads, did not respond to either peptide set (Figure 3).
Figure 3. Individual responses to polyallelic and overlapping peptide sets.
Responses to polyallelic ( ) and overlapping (
) and overlapping ( ) peptides are shown for all 28 patients tested in IFN-γ ELISpot assays. Of the 28 patients, 17 had responses to the polyallelic peptide set while 12 potential epitopes were identified using overlapping peptides and 12 individuals had IFN-γ responses to both peptide sets.
) peptides are shown for all 28 patients tested in IFN-γ ELISpot assays. Of the 28 patients, 17 had responses to the polyallelic peptide set while 12 potential epitopes were identified using overlapping peptides and 12 individuals had IFN-γ responses to both peptide sets.
Of the 22 non-adapted epitopes from the polyallelic peptide set embedded within the overlapping peptides, nine elicited responses in both peptide panels. There was no significant difference in their estimated response rates (p=0.5, Figure 2B) nor the magnitude of the responses (p=0.2). Notably, there was a very low response rate amongst those overlapping peptides having no embedded peptides from the polyallelic set (p=0.0002, Figure 2B).
3.4 Discussion
Polyallelic peptides have been designed to combat the problem of viral diversity by focusing on that component of viral diversity that has functional relevance for CD8 T-cell responses. Existing peptide approaches generally aim to incorporate all apparent viral variation, including the variation reflecting phylogeny, random neutral changes, and non-T-cell associated selection. These changes should not impact detection of CD8 T-cell responses at the population level systematically, unless they occur predictably or stereotypically and have functional effects on peptide presentation or HLA-peptide-TCR binding. In this study we show that Nef peptides which are explicitly based on optimal immunogenic epitopes and their variants and optimised as a group for the HLA distribution of the population are associated with detection of more IFN-γ responses overall. The predominance of responses to central Nef is consistent with previous observations on the intense clustering of CD8 epitopes [21-23] and the reason for broader reactivity to polyallelic peptides appears attributable in part, to the inclusion of putative novel epitopes which elicited detectable IFN-γ responses in the ELISpot assay (Figure 2B). These were predicted using sites of HLA allele-specific polymorphism in HIV-1 sequence detected by large scale population-based analyses, suggesting that these sites do mark targets of immune selection in-vivo and can be used to map novel epitopes in HIV. This approach could therefore be extended to other HIV proteins such as Gag and Pol as common vaccine proteins and also subject to HLA-driven selection in-vivo.
Our testing suggests that a significant proportion of epitope variants contain HLA-driven amino acid changes that do not affect HLA-peptide binding significantly. Presumably many of these are TCR escape variants which are able to stimulate IFN-γ responses from memory T-cells when in excess concentrations in a screening ELISpot assay; however more detailed testing of relative functional avidity or off-rate assays may find differences compared with the non-adapted epitopes. Notably some of these HLA-adapted epitopes (KY11 and TL10) are also published and immunodominant epitopes [17], raising the possibility that they are immunogenic by widely used immunoassays, but may alter TCR recognition for viral advantage in-vivo.
Though polyallelic peptides were shorter (8 to 11-mer) optimal length peptides and tested individually, we did not find evidence that this led to a greater magnitude of responses than observed in the pools of longer (15-mer) overlapping peptides, or when compared to the estimated individual overlapping peptide responses in cases where the same sequence was present in both peptide sets. It is possible that responses to peptides present in the overlapping peptides were potentially detectable in some individuals but were inhibited completely because of competition between pooled peptides [24]. There was a significant proportion of responses to the polyallelic peptides that could not be explained by the HLA genotype or supertype of the tested PBMCs which may reflect ‘promiscuous’ HLA-peptide binding as demonstrated in studies using overlapping peptides [21]. However the fact that carriage of HLA alleles matching the known or predicted restriction of the polyallelic peptides was associated with a greater response rate per peptide supports the proposition that polyallelic peptides can be customised for the HLA of regional populations. Though HIV diversifies rapidly at the population level, HLA distributions are relatively fixed and well documented for many global populations so the known epitopes, novel epitopes, epitope variants and supertype-cross binders most likely to stimulate the predominant CD8 T cell responses are predictable [8]. A ranking of peptides based on HLA provides some rational basis for prioritising peptides for testing when assays are limited by costs or PBMC numbers. Furthermore, the polyallelic peptides have the advantage of identifying specific epitope-specific CD8 T-cell responses at first testing, without the need for repeat confirmatory tests and tests with truncated peptides to identify the true immunoreactive epitope.
Among the current limitations of the strategy, CD4 T-cell responses are not likely to be detected, and the success of detecting CD8 T-cell responses is heavily dependent on the accuracy of the ‘Epi-pred’ program. PTEs and toggled peptides have been shown to improve detection of CD8 T-cell responses compared to consensus strain peptides, and are currently being used in variety of immunological studies. Direct head to head comparisons between these and polyallelic strategies will be limited by the need for large numbers of PBMC for parallel testing. There are immediate opportunities however to use the information on HLA-specific polymorphisms, putative epitopes and HLA distribution to customise testing of peptides within any currently available set of peptides- PTEs, toggle peptides or overlapping peptides- for HLA-diverse populations or within individuals with known HLA genotype.
Though the principles of design based on exploiting epitope clustering and population HLA distribution can be applied to HIV vaccine design, we do not argue that all the epitope-specific responses detected here should be necessarily induced by an effective preventative HIV vaccine, particularly when many epitope variants are created as a result of HLA associated polymorphism. If these represent the result of viral adaptation, then they may have a negative effect if induced by a vaccine. Nevertheless, detection and characterisation of all responses in natural HIV infection-whether effective or ineffective or virus- enhancing in some way, is still needed to fully understand the HIV-specific T-cell response. In particular, detection of responses is the first step to more detailed functional studies that could determine the qualitative properties that distinguish effective T cell responses from all others, and how best to harness these in a HIV vaccine.
Acknowledgments
The authors wish to thank Donald Cooper and Shay Leary for computing support, participants of the Western Australian HIV Cohort Study and all laboratory staff of the Centre for Clinical Immunology and Biomedical Statistics (CCIBS). This work was supported by the National Institute of Allergy and Infectious Diseases (Grant RO1 AI060460) and the Australian National Health and Medical Research 367 Council (Program grant ID 384702). Research conducted as part of the Collaboration for AIDS Vaccine Discovery with support from the Bill & Melinda Gates Foundation.
Footnotes
Disclosures
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brumme ZL, John M, Carlson JM, Brumme CJ, Chan D, Brockman MA, et al. HLA-associated immune escape pathways in HIV-1 subtype B Gag, Pol and Nef proteins. PLoS One. 2009;4(8):e6687. doi: 10.1371/journal.pone.0006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld M, Addo MM, Shankarappa R, Lee PK, Allen TM, Yu XG, et al. Enhanced detection of human immunodeficiency virus type 1-specific T-cell responses to highly variable regions by using peptides based on autologous virus sequences. J Virol. 2003;77(13):7330–7340. doi: 10.1128/JVI.77.13.7330-7340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nature Reviews. 2008;8(8):619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zimbwa P, Milicic A, Frater J, Scriba TJ, Willis A, Goulder PJR, et al. Precise identification of a human immunodeficiency virus type 1 antigen processing mutant. J Virol. 2007;81(4):20331–2038. doi: 10.1128/JVI.00968-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Novitsky V, Flores-Villanueva PO, Chigwedere P, Gaolekwe S, Bussman H, Sebetso G, et al. Identification of most frequent HLA class I antigen specificities in Botswana: relevance for HIV vaccine design. Hum Immunol. 2001;62(2):146–156. doi: 10.1016/s0198-8859(00)00236-6. [DOI] [PubMed] [Google Scholar]
- 6.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296(5572):1439–1443. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 7.Brumme ZL, Brumme CJ, Heckerman D, Korber BT, Daniels M, Carlson J, et al. Evidence of differential HLA class I-mediated viral evolution in functional and accessory/regulatory genes of HIV-1. PLoS Pathog. 2007;3(8):e121. doi: 10.1371/journal.ppat.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson JM, Brumme ZL. HIV evolution in response to HLA-restricted CTL selection pressures: a population-based perspective. Microbes Infect. 2008;10(5):455–461. doi: 10.1016/j.micinf.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Rousseau CM, Daniels MG, Carlson JM, Kadie C, Crawford H, Prendergast A, et al. HLA class I-driven evolution of human immunodeficiency virus type 1 subtype c proteome: immune escape and viral load. J Virol. 2008;82(13):6434–6446. doi: 10.1128/JVI.02455-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra U, Li F, Nolin J, Allison M, Zhao H, Mullins JI, et al. Enhanced detection of human immunodeficiency virus type 1 (HIV-1) Nef-specific T cells recognizing multiple variants in early HIV-1 infection. J Virol. 2007;81(10):5225–5237. doi: 10.1128/JVI.02564-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frahm N, Kaufmann DE, Yusim K, Muldoon M, Kesmir C, Linde CH, et al. Increased sequence diversity coverage improves detection of HIV-specific T cell responses. J Immunol. 2007;179(10):6638–6650. doi: 10.4049/jimmunol.179.10.6638. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Malhotra U, Gilbert PB, Hawkins NR, Duerr AC, McElrath JM, et al. Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine. 2006;24(47-48):6893–6904. doi: 10.1016/j.vaccine.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Frahm N, Nickle DC, Linde CH, Cohen DE, Zuñiga R, Lucchetti A, et al. Increased detection of HIV-specific T cell responses by combination of central sequences with comparable immunogenicity. AIDS. 2008;22(4):447–456. doi: 10.1097/QAD.0b013e3282f42412. [DOI] [PubMed] [Google Scholar]
- 14.Mallal SA. The Western Australian HIV Cohort Study, Perth, Australia. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S23–27. doi: 10.1097/00042560-199801001-00008. [DOI] [PubMed] [Google Scholar]
- 15.John M, Heckerman D, James I, Park LP, Carlson JM, Chopra A, et al. Adaptive interactions between HLA and HIV-1: Highly divergent selection imposed by HLA Class I molecules with common supertype motifs. J Immunol. 2010;184(8):4368–4377. doi: 10.4049/jimmunol.0903745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heckerman D, Kadie C, Listgarten J. Leveraging information across HLA alleles/supertypes improves epitope prediction. J Comput Biol. 2007;14(6):736–746. doi: 10.1089/cmb.2007.R013. [DOI] [PubMed] [Google Scholar]
- 17.Korber BTM, Brander C, Haynes BF, Koup R, Moore JP, Walker BD, et al. HIV Molecular Immunology 2006/2007. Los Alamos National Laboratory, Theoretical Biology and Biophysics; Los Alamos, United States of America: [Google Scholar]
- 18.Almeida CA, Roberts SG, Laird R, McKinnon E, Ahmed I, Pfafferott K, et al. Automation of the ELISpot assay for high-throughput detection of antigen-specific T-cell responses. J Immunol Methods. 2009;344(1):1–5. doi: 10.1016/j.jim.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson AC, Martin JN, Younger SR, Bredt BM, Epling L, Ronquillo R, et al. Comparison of the ELISPOT and cytokine flow cytometry assays for the enumeration of antigen-specific T cells. J Immunol Methods. 2003;283(1-2):141–153. doi: 10.1016/j.jim.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Sidney J, Peters B, Frahm N, Brander C, Sette A. HLA class I supertypes: a revised and updated classification. BMC Immunol. 2008;9:1. doi: 10.1186/1471-2172-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frahm N, Korber BT, Adams CM, Szinger JJ, Draenert R, Addo MM, et al. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J Virol. 2004;78(5):2187–2200. doi: 10.1128/JVI.78.5.2187-2200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann DE, Bailey PM, Sidney J, Wagner B, Norris PJ, Johnston MN, et al. Comprehensive analysis of human immunodeficiency virus type 1-specific CD4 responses reveals marked immunodominance of gag and nef and the presence of broadly recognized peptides. J Virol. 2004;78(9):4463–4477. doi: 10.1128/JVI.78.9.4463-4477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turk G, Gherardi MM, Laufer N, Saracco M, Luzzi R, Cox JH. Magnitude, breadth, and functional profile of T-cell responses during human immunodeficiency virus primary infection with B and BF viral variants. J Virol. 2008;82(6):2853–2866. doi: 10.1128/JVI.02260-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Precopio ML, Butterfield TR, Casazza JP, Little SJ, Richman DD, Koup RA, et al. Optimizing peptide matrices for identifying T-cell antigens. Cytometry A. 2008;73(11):1071–1078. doi: 10.1002/cyto.a.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]



