Abstract
Immunodeficient mice bearing components of a human immune system present a novel approach for studying human immune responses. We investigated the number, phenotype, developmental kinetics and function of developing human immune cells following transfer of CD34+ hematopoietic stem cell (HSC) preparations, originating from second trimester human fetal liver (HFL), umbilical cord blood (UCB), or granulocyte colony-stimulating factor-mobilized adult blood (G-CSF-AB) delivered via intrahepatic injection into sublethally irradiated neonatal NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/−, and C.B-17-scid/bg mice. HFL and UCB HSC provided the greatest number and breadth of developing cells. NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− harbored human B and dendritic cells as well as human platelets in peripheral blood, whereas NOD-scid/γc−/− mice harbored higher levels of human T cells. NOD-scid/γc−/− mice engrafted with HFL CD34+ HSC demonstrated human immunological competence evidenced by white pulp expansion and increases in total human immunoglobulin following immunization with T-dependent antigens, and delayed type hypersensitivity-infiltrating leukocytes in response to antigenic challenge. In conclusion, we describe an encouraging base system for studying human hematopoietic lineage development and function utilizing human HFL or UCB HSC-engrafted NOD-scid/γc−/− mice that is well suited for future studies toward the development of a fully competent humanized mouse model.
Keywords: hematopoietic stem cell, mouse model, human immune system development, delayed-type hypersensitivity, isotype switching
Introduction
Immunodeficient mice harboring human cells or tissues, frequently referred to as “humanized mice”, are promising tools for studying complex processes in human biology. Mice bearing human immune systems, in particular, are being developed to investigate immune-mediated disease pathogenesis (1,2) and allogeneic tissue rejection and tolerance in vivo (3–5). Two decades ago, the original “SCID-hu” model was developed by McCune and colleagues, using C.B-17-scid mice as recipients for human hematopoietic tissues including fetal liver, bone, and/or thymus originating from second trimester human fetuses (6–8). The engrafted human hematopoietic tissues gave rise to low levels of human T and B cells that were capable of producing a primary antibody response when autologous fetal skin, serving as an additional source of dendritic cells, was co-engrafted along with thymus, bone marrow, and lymph node (9). Adoptive transfer of peripheral blood mononuclear cells (PBMC) in this same mouse strain supported engraftment of T, B, and dendritic cells (10). Further compromising the innate immune system of C.B-17-scid animals by either introducing a beige mutation or crossing with mice of the NOD background allowed recipients to accept higher levels of mature human T and B cells (11,12).
Two major technical advances enhance the applicability of humanized mice for studying the human immune system. First, isolated hematopoietic stem cells (HSC) were shown to engraft in C.B-17-scid (13) and NOD-scid mice (14). Secondly, the development of more severely immunodeficient mouse lines lacking the common cytokine receptor gamma chain (γc) (15,16) and thus more profoundly deficient in the innate immune system, supported even higher levels of HSC engraftment and differentiation of T, B, and dendritic cells (17). Most recent work has centered on HSC engraftment in neonatal γc-deficient mice on either the NOD-scid (18) or Balb/c-Rag1−/− (19) background. Though such animals show varying levels of splenic lymphoid development, human immune system function is incomplete. Nevertheless, these systems offer great promise for routine use as humanized mouse models, especially if HSC can be reproducibly generated from embryonic stem cells (20,21) or inducible pluripotent stem cells (iPSC) (22). In addition to the requirement of severe defects in both innate and adaptive immunity in the recipient mouse, engraftment efficiency also appears dependent on the genetic background and age of the recipient mouse, route of engraftment and conditioning regime, as well as the type and individual aliquot of injected HSC (for review, see Shultz et al.) (2). We were interested in comparing NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice for preparing humanized mice for our ongoing transplant immunology and disease pathogenesis studies. To this end, we performed a side-by-side comparison of the kinetics and breadth of the developing immune system following intrahepatic, neonatal transplantation using matched aliquots of HSC originating from human fetal liver (HFL), umbilical cord blood (UCB) and granulocyte colony-stimulating factor-mobilized adult blood (G-CSF-AB) in NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice and also considered engraftment of our currently-utilized strain, C.B-17-scid/bg.
Materials and Methods
Mice
Mice were housed in the approved facilities in the Yale Animal Resources Center and handled according to Guide for the Care and Use of Laboratory Animals. All experimentation was approved by the Yale Institutional Animal Care and Use Committee. The original breeding pairs of NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice were generous donations from Dr. Leonard Shultz at the Jackson Laboratories (Bar Harbor, ME) and from Dr. Drew Pardoll, Johns Hopkins University, respectively. C.B-17-scid/bg breeders were purchased from Taconic (Germantown, NY). Animals were housed in autoclaved microisolator cages and fed autoclaved food and hyperchlorinated water. In addition, NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice were maintained on trimethoprim-sulfamethoxazole (80/140 mg/ml; Hi-Tech Pharmacal, Amityville, NY) in the drinking water.
HSC
All protocols involving collection and use of HFL, UCB and G-CSF-AB HSC were approved by the Yale Human Investigative Committee prior to commencing the study. In addition, the collection and use of HFL HSC was approved by the Albert Einstein College of Medicine. Either prior to and/or following enrichment, HSC were cryopreserved in 10% DMSO/90% fetal calf serum (FCS). All HSC types were thawed rapidly, immediately treated with DNase (150 IU, Qiagen, Valencia, CA) to inhibit cell clumping, washed in RPMI containing 10% FCS, and incubated at 37°C for 1–2 hours to allow re-expression of surface markers and/or membrane stabilization prior to phenotyping, enrichment, or transplantation, as described specifically for each HSC type in the following sections.
Discarded anonymized human leukapheresis collections from blood of healthy adult donors enriched for HSC through mobilization by G-CSF were obtained from the Yale New Haven Hospital Blood Bank. Leukapheresis packs were stored at liquid nitrogen until use. Peripheral blood mononuclear cells were isolated following a rapid thaw using Lymphocyte Separation Medium (MP Biochemicals, Irvine, CA), and then G-CSF-AB HSC were enriched using anti-human CD34 microbeads and either midiMACS columns or the isolex magnetic cell selection system (all from Miltenyi Biotec, Auburn, CA), according to the manufacturer’s instructions. Fresh human cord blood was obtained from healthy full-term newborns and UCB HSC were isolated using anti-human CD34 microbeads and Minimacs columns (Miltenyi Biotec). For the retrieval of HFL HSC, liver tissue from second trimester (14–17 gestational week) abortuses was collected from elective terminations following maternal consent by the Human Fetal Tissue Repository staff (hFTR, Albert Einstein College of Medicine). Tissue was transported to the laboratory in ice-cold Hank’s Balanced Salt Solution (HBSS) containing 100 units/ml penicillin and 100 μg/ml streptomycin. HFL tissue was minced into small pieces, then further disrupted by digesting with 5 mg/ml collagenase type D (Roche, Indianapolis, IN) in buffer containing 70 mM NaCl, 6 mM KCl, 4.8 mM CaCl2.2H2O, and 10 mM Hepes at 37°C for 15–30 minutes with gentle agitation. Dispersed cell clumps were washed in EGTA-containing buffer (5mM EGTA in HBBS, containing 1% bovine serum albumin (BSA), 100 mM Hepes, and 14 mM glucose) by centrifuging at 50 × g for 5 minutes to pellet contaminating parenchymal cells. The supernatant was then centrifuged at 200 × g for 10 minutes and cells cryopreserved in 10% DMSO/90% FCS. Cell clumps were allowed to attach to cationic tissue culture plates (Primaria, Becton-Dickinson, San Jose, CA), and additional non-adherent HSC produced by the clumps were collected for up to 48 hours.
Phenotyping of HSC preparations
Each HSC preparation was enriched as described above, and cell suspensions were stained with a FITC-labeled mouse monoclonal antibody against human CD34 (Miltenyi-Biotec) and with one of the following PE-labeled mouse antibodies to human surface markers: CD45, CD3, CD11c, CD19, and CD56 (Beckman Coulter, Fullerton, CA). Percentages of various hematopoietic stem cells (CD34+lin-) in addition to mature (CD34-lin+) leukocytes were calculated using CellQuest software after acquisition on a FACsort flow cytometer (Becton Dickinson).
Transplantation of human CD34+ cells and characterization of human cell engraftment
Neonatal mice (less than 48 hours old) were irradiated with 1 Gy (scid/bg and NOD-scid/γc−/− litters) or 2 × 2 Gy (3 hours apart; Balb/c-Rag1−/−γc−/− litters) from a cesium-137 source (Mark I irradiator, J.L. Shepherd and Associates, San Fernando, CA). Three to five hours later, thawed CD34-enriched HSC preparations originating from either G-CSF-AB (0.5 or 1.0 × 106 cells), UCB (1.0 × 105 cells), or HFL (1.5–5.0 × 104 cells) were transplanted in a 50 ml volume in 2 injections into 2 liver lobes of anesthetized (hypothermic) neonates via intrahepatic injection using a Hamilton syringe and 30 gauge × ½ inch needle. Beginning at 6 weeks of age, mice were bled approximately every 2 weeks by retro-orbital centesis until at least 12 weeks of age. Heparinized peripheral blood was treated with red cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM EDTA, pH 7.2) and analyzed for relative percentages of murine and human CD45+ cells by flow cytometry. Once human CD45+ cells were detected, analysis was extended to include human CD3+, CD11c+, and CD19+ on all subsequent retro-orbital blood collections. A human CD45+ level of >2% was considered positive. Reconstituted mice became candidates for functional analyses of the immune system (see methods below) when at least several members of the cohort showed discrete and measurable levels of engrafted cells in the circulation; this time point varied among the specific mouse strain/HSC combinations being investigated.
At the time of euthanasia, single cell suspensions were prepared from spleen and bone marrow, as well as peripheral blood. Cells were harvested from bone marrow by flushing femurs and tibias and splenocytes expressed from the splenic capsule using small volumes of PBS/1%BSA (FACS buffer). Percentages of various human immune cell subsets compared to total gated leukocytes were characterized by 4-color flow cytometry using the following antibodies: anti-mouse CD45-PE, anti-human CD45-APC, CD19-FITC, CD56-PE-Cy7 (BD Pharmingen), anti-human CD45-FITC, CD11c-PE, CD3-FITC, CD8-FITC, (Beckman Coulter), anti-human CD3-PE, CD4-PE-Cy7, CD5-PE-Cy7, CD41a-APC, anti-mouse CD41-FITC (BD Biosciences, San Jose, CA), and anti-human CD34-PE (Miltenyi Biotec). Non-engrafted, non-irradiated littermates served as negative controls in all analyses.
KLH and influenza immunization
Mice were immunized with keyhole limpet hemocyanin (KLH) according to previously published methods (23). In brief, mice were injected intradermally with 100 μg KLH (Chemicon, Temecula, CA) emulsified in complete Freund’s adjuvant in the abdominal skin at 4 sites (50 μl volume per site). Twelve days later, mice were boosted with 100 μg KLH emulsified in incomplete Freund’s adjuvant at the same site. To immunize mice with influenza antigen, mice received 10 μg purified, inactivated H5N1 influenza virus (A/VN1203/PR8) on 2 occasions, 3 weeks apart, as described above. To analyze formation of lymph node-like structures, mice were again boosted with 20 μg influenza antigen administered intradermally into the abdominal and footpad skin, as well as intraperitoneally, 5 days prior to euthanasia. Lymph nodes and spleens were harvested and tissue sections analyzed by hematoxylin and eosin (H&E) and various immune cell markers, as described below.
Measurement of human immunoglobulin
Total plasma human immunoglobulin (IgM and IgG) following KLH and influenza antigen exposure was measured using ELISA kits, according to the specification of the manufacturer (Bethyl Laboratories, Montgomery, TX). Human KLH-specific antibody was quantitated as previously described (24). In brief, KLH (test antigen; 1.0 μg/well), human IgM and IgG, or mouse IgM and IgG (positive and negative control antigens, respectively; 5.0 ug/well), were added to appropriate wells in 0.05 M carbonate-bicarbonate buffer, pH 9.6. After overnight incubation at 4°C, wells were washed three times and blocked with tris-buffered saline (TBS) containing 1.25% gelatin. Following incubation for 1 hour at 37°C, test plasma diluted in 1.25% gelatin in TBS/0.05% Tween (TBS-T) was added and incubated at 37°C for 90 minutes. After washing, bound antibody was detected with horse radish peroxidase (HRP)-conjugated anti-human IgM or IgG antibodies (Bethyl Laboratories) diluted in 1.25% gelatin in TBS-T followed by TMB substrate. Human influenza-specific antibody was detected in a direct ELISA format. Briefly, wells were coated with 2 ug purified influenza virus (A/VN/1203/04) in 0.05 M carbonate-bicarbonate buffer, pH 9.6. After overnight incubation at 4°C, wells were washed three times with PBS and blocked with PBS containing 1% BSA. Following incubation for 1 hour at 37°C, test plasma diluted in 0.5% BSA in PBS/0.05% Tween 20 (PBS-T) was added and incubated at 37°C for 90 minutes. After washing, bound antibody was detected with HRP-conjugated anti-human immunoglobulin (IgM+IgG+IgA; Southern Biotech, Birmingham AB) at a 1:3000 dilution in 0.5% BSA/PBS-T followed by OPD substrate.
In vitro blastogenesis assays
Splenocytes from reconstituted and non-reconstituted mice, as well as human PBMC positive control cells, were seeded into wells of a U-bottom plate at 5 × 105 viable cells per well and cultured in RPMI plus 20% FCS and antibiotics, either with or without 2 μg/ml phytohemagluttinin (PHA, Sigma, St. Louis, MO), 80 μg/ml KLH, or 10 μg/ml influenza antigen. Following incubation for 48 hours (PHA) or 6 days (KLH/influenza) at 37°C in 5% CO2, 1 μCi 3H-thymidine was added per well and allowed to incubate for an additional 18 hours. Cells were harvested using a Skatron cell harvester (Analis, Ghent, Belgium), and incorporated radioactivity was measured using a beta-scintillation counter (Wallac, Waltham, MA).
In vivo delayed type hypersensitivity response
Mice were immunized with KLH as described above. Eighteen days following the initial injection, mice were challenged with 25 μg KLH (25 μl volume) diluted in saline vehicle by injecting into the dorsal footpad and ear. Saline alone (25 μl volume) was similarly injected into the contralateral paw. Footpad and ear swelling were measured with calipers at 24, 48, and 72 hours, and then mice were euthanized and footpads analyzed for various human immune cell markers by immunohistochemistry (IHC), as described below. Immunocompetent NOD or Balb/c and non-engrafted NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− mice served as positive and negative controls, respectively.
Immunohistochemistry (IHC)
Tissues were fixed in 10% formalin in PBS at room temperature overnight then transferred to PBS. Paraffin-embedded sections (5 μm) were de-paraffinized with xylene and then re-hydrated in ethanol and water. For antigen retrieval, sections were subjected to 95°C for 15 minutes in 10 mM sodium citrate buffer, pH = 6.0. Sections were then treated with 20 μg/ml proteinase K (Sigma) in 0.1 M Tris, pH 8.0, for 10 minutes at 37°C. Slides were blocked in PBS/5% normal goat serum for 30 minutes at room temperature. Primary antibodies, which included mouse anti-human CD45 (clones RP2/18 or RP2/22, Vector Laboratories, Burlingame, CA), CD68 (clone KP1, Vector Laboratories), CD20 (Clone L26, Dako, Carpinteria, CA), and CD3 (Clone UCHT1 for footpads, Dako; Clone F7.2.38 for spleens, Dako) diluted in blocking buffer was added and slides were incubated overnight at 4°C. After washing in PBS, bound antibody was detected with biotin-labeled goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), Vectastain ABC reagent system, and DAB substrate (Vector Laboratories). Finally, slides were counterstained with methylene green or hematoxylin (Sigma). Positive controls for IHC staining of human antigens included sections from discarded de-identified adult human splenic and liver tissue from the Yale-New Haven Hospital, which were obtained under a protocol approved by the Yale Human Investigative Committee.
Results
Characterization of HSC preparations
To assess the potential origin of human leukocytes detected in HSC-reconstituted mice, each inoculum was analyzed for lineage marker expression (Table 1). Eighty-four percent (84%) of G-CSF-AB-column cells expressed human CD34, and 9.3% and 6.3% of cells in the preparation were CD3+ and CD11c+, reflecting contaminating T cells and DCs, respectively. Fewer numbers of cells in the preparation (<2%) were either CD19+ B cells or CD56+ natural killer (NK) cells (Table 1). In contrast, 92% of G-CSF-AB-isolex cells expressed CD34, and most lacked other lineage markers, although 7.2% of cells were contaminating B cells. Even higher numbers of UCB HSC expressed CD34+ (consistently >96% for all cords tested). Low levels of T and B cells (1.5% and 1.4%, respectively) were the only observed contaminants in these preparations. The purity of HFL HSC preparations from second trimester fetuses was consistently greater than 95%. Levels of various subpopulations of contaminating cells varied between second trimester livers, perhaps as a function of gestational age of the fetal liver analyzed. HFL HSC preparations harbored both CD19+ B cells (1.0%) and CD56+ natural killer cells (0.7%, respectively) and lower levels (<0.5%) of CD3+ T cells and CD11c+ DCs.
Table 1. Characterization of human HSC preparations by flow cytometry.
HSC inocula were prepared as described in the Materials and Methods. Following a 2-hour incubation at 37°C to allow surface marker re-expression, cells were stained with anti-human CD34-FITC and one of several PE-labeled antibodies to various lineage markers and analyzed by flow cytometry. Numbers represent percentages of cells of a specific phenotype in the total viable cell population from a representative preparation of each type of HSC inoculum.
| Human surface marker | MidiMACS column- purified G-CSF-AB | Isolex 300i- purified G-CSF-AB | MiniMACS column- purified UCB | MiniMACS column- purified HFL |
|---|---|---|---|---|
| CD34 | 84.2 | 91.9 | 97.0 | 98.0 |
| CD3 | 9.3 | 0 | 1.5 | 0.2 |
| CD19 | 1.4 | 7.2 | 1.4 | 1.0 |
| CD11c | 6.3 | 0.2 | 0.1 | 0.1 |
| CD56 | 0.3 | 0 | 0 | 0.7 |
Engraftment of HFL CD34+ HSC preparations mice
Table 2 provides a summary of the numbers of engrafted NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/− or C.B.-17-scid/bg mice receiving matched HSC inocula by intrahepatic injection as neonates. High levels of human CD45+ cell engraftment were achieved by inoculating either HFL or UCB HSC in NOD-scid/γc−/− mice. With the HFL and UCB HSC- NOD-scid/γc−/− mouse strain combinations, at least 60% of mice in all litters were successfully engrafted. There was rarely a death due to maternal rejection or hemorrhage following injection. An occasional highly reconstituted NOD-scid/γc−/− mouse died following routine retro-orbital centesis by an experienced operator. Otherwise, mice appeared healthy until at least 6 months of age; no evidence of lymphoma or graft-versus-host-disease was apparent following HFL HSC engraftment in NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− mice. We also analyzed CD34+ engraftment using neonatal C.B-17-scid/bg mice, since our laboratory routinely utilizes this mouse strain for adoptive transfer of mature human lymphocytes (4,25). However, no human cells engrafted in this strain following inoculation with HFL HSC (Table 2).
Table 2. Engraftment of CD34+ HSC preparations in neonatal NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/− and C.B-17-scid/bg mice.
Prior to 2 days of age, NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/, or C.B-17-scid/bg mice were irradiated and injected 3–4 hours later intrahepatically with various HSC preparations. At 6–10 weeks of life, percentages of human CD45+ cells of total leukocytes in peripheral blood were determined by flow cytometry.
| Mouse strain | Type HSC | # donors | # cells per mouse | Frequency of mice harboring human CD45+ cells | Range of human CD45+ cells at 6 – 10 weeks post -engraftment (%) | Mean % human CD45+ cells at 6 –10 weeks post engraftment# |
|---|---|---|---|---|---|---|
| NOD-scid/γc−/− | Adult - isolex | 1 | 0.5 × 106 | 4/4 | 9.8 – 18.1 | 12.7 |
| NOD-scid/γc−/− | Cord | 2 | 1.0 × 105 | 8/9 | 6.9 – 89.7 | 51.8 |
| NOD-scid/γc−/− | Fetal liver | 2 | 1.5 – 5.0 × 104 | 12/18 | 0.00 – 63.9 | 15.5 |
| Balb -Rag1−/−γc−/− | Adult - isolex | 1 | 0.9 × 106 | 0/4 | 0.0 | 0.00 |
| Balb -Rag1−/−γc−/− | Cord | 3 | 1.0 × 105 | 4/10 | 0.00 – 29.0 | 10.2 |
| Balb -Rag1−/−γc−/− | Fetal liver | 2 | 4.0 × 104 | 1/14 | 0.00 – 32.4 | 32.4 |
| C.B-17- scid/bg | Adult - column | 2 | 0.5 – 1.0 × 106 | 4/9 + | 0.00 – 60.8 | 32.5 |
| C.B-17- scid/bg | Adult - isolex | 1 | 0.5 – 1.0 × 106 | 0/9 | 0.0 – 0.1 | 0.00 |
| C.B-17- scid/bg | Cord | 3 | 0.7 – 1.0 × 105 | 0/8 | 0.0 – 1.0 | 1.00 |
| C.B-17- scid/bg | Fetal liver | 1 | 4.0 × 104 | 0/5 | 0.00 – 0.1 | 0.00 |
Three mice required euthanasia at 6 weeks of age due to graft -versus-host symptomatology
Successfully -engrafted mice only were used to compute mean values
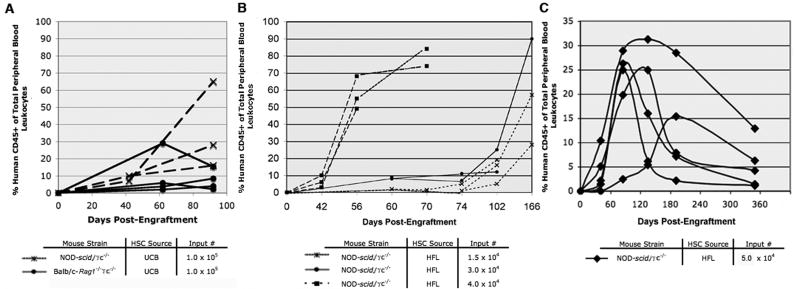
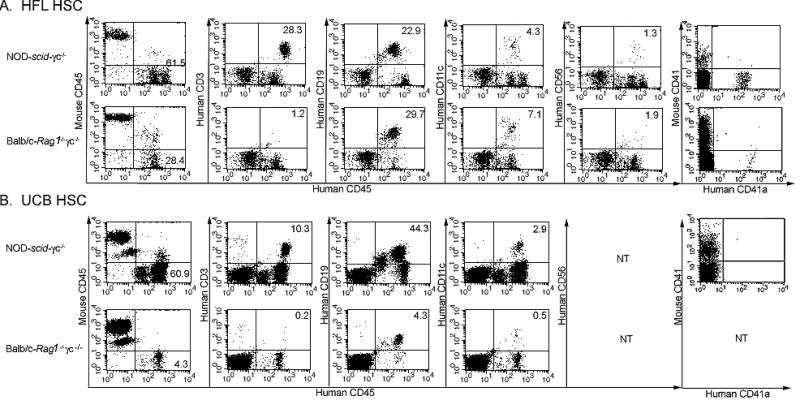
Kinetic analysis of the reconstitution process revealed that detectable levels of human CD45+ cell engraftment were achievable by 42 days of age in irradiated, neonatal NOD-scid/γc−/− or mice that received UCB or HFL CD34+ cells (Fig. 1A and 1B). By 8 weeks post-engraftment in a Balb/c-Rag1−/−γc−/− mouse that received 4.0 × 104 HFL HSC, ~30% of leukocytes expressed human CD45 whereas levels ranged from ~50–64% in a matched set of engrafted NOD-scid/γc−/− mice. Higher input numbers of HFL HSC were associated with a more rapid expansion of peripheral blood human CD45+ cells (Fig. 1B). Though engraftment levels remained stable for the duration of most experiments (<15wks, Fig. 1B), levels in some mice began to decline over longer periods of time, particularly those exhibiting faster engraftment kinetics (Fig. 1C). NOD-scid/γc−/− mice engrafted with at least 3.0 × 104 HFL HSC consistently harbored CD3+ T cells, with CD4/CD8 ratios ranging from 2 to 6 (data not shown), CD19+ B cells, CD11c+ DCs, and CD56+ NK cells in the peripheral blood (Fig. 2, top plots), bone marrow, and spleen (data not shown). Furthermore, CD41a+ platelets were observed in the peripheral blood of NOD-scid/γc−/− mice (Fig. 2, upper right plot). Compared to NOD-scid/γc−/− mice, a greater proportion of human CD45+ cells in Balb/c-Rag1−/−γc−/− mice generally expressed CD19 and CD11c, while a significantly smaller proportion (if any at all) expressed CD3. Platelets were also observed in the peripheral blood of Balb/c-Rag1−/−γc−/− mice engrafted with HFL HSC (Fig. 2A, bottom plots).
Figure 1. Kinetics of human CD45+ cell development in NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/−mice injected as neonates.
Prior to 2 days of age, NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− mice were irradiated and then administered HFL or UCB HSC preparations by intrahepatic injection. The percentage of human CD45+ cells of the total leukocyte population in peripheral blood was determined in successive post-engraftment dates by flow cytometry in various cohorts of mice. A: 3 month comparative analysis of individual NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− receiving 1.0 × 105 UCB HSC; B: 6 month comparative analysis of individual NOD-scid/γc−/− mice receiving either 1.5 × 104, 3.0 × 104 or 4.0 × 104 HFL HSC; C: 12 month comparative analysis of individual NOD-scid/γc−/− mice receiving 5.0 × 104 HFL HSC.
Figure 2. Development of human immune cell subsets in NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice receiving HFL HSC or UCB HSC.
At 1 day of age, NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− mice were irradiated and then injected intrahepatically with 4.0 × 104 HFL HSC (A) or 1.0 × 105 UCB HSC (B). The phenotype of human CD45+ cells expressing CD3, CD19, CD11c, or CD56 (at 8 or 13 weeks of age in A or B, respectively) as well as CD41a+ platelets (at 15 weeks or 28 weeks of age in A or B, respectively), in peripheral blood from HFL HSC- or UCB HSC-engrafted mice was determined by flow cytometry. Representative sets of plots from a NOD-scid/γc−/− and a Balb/c-Rag1−/−γc−/− mouse are shown in the top and bottom row of panel A (HFL HSC-engrafted mice) and panel B (UCB HSC-engrafted mice), respectively. NT = not tested.
Intriguingly, though all HFL HSC-engrafted NOD-scid/γc−/− mice receiving higher numbers (at least 3.0 × 104) of HFL HSC harbored both T and B cells whereas mice engrafted with fewer cells (1.5 × 104) effectively harbored no CD3+ T lymphocytes in the peripheral blood (Fig. 3A). Though some variability was noted, a positive correlation between T cell engraftment and HFL HSC input number was observed; as the number of HFL CD34+ cells transferred increased, so did the ratio of human T cells to B cells in the peripheral blood of NOD-scid/γc−/− reconstituted mice (Fig. 3B).
Figure 3. Dependence of human T and B lymphocyte development on HFL HSC inocula number in NOD-scid/γc−/− mice.
At 1 day of age, NOD-scid/γc−/− mice were irradiated and then injected intrahepatically with 1.5 – 5.0 × 104 HFL HSC. A: Flow cytometric plots showing human CD45+CD3+ cells in peripheral blood at 10 weeks of age from individual mice injected with either 1.5 × 104 or 3.0 × 104 HFL HSC. B: Percentages of CD45+CD3+ and CD45+CD19+ cells of total lymphocytes determined by flow cytometric analysis of peripheral blood at 10 – 12 weeks of age from individual mice injected with increasing numbers of HFL HSC.
Engraftment of UCB CD34+ HSC preparations
Consistent with HFL HSC engraftment, NOD-scid/γc−/− mice also harbored higher levels of human CD45+ cells following engraftment with UCB HSC compared to Balb/c-Rag1−/−γc−/− mice (Table 2 and Fig. 1A). In contrast, human cells were not detected in C.B-17-scid/bg mice following inoculation with UCB HSC (Table 2). Subpopulations of engrafted human CD45+ cells detected in NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice reconstituted with UCB HSC paralleled those observed in these same strains of mice reconstituted with HFL HSC. Specifically, NOD-scid/γc−/− mice harbored CD3+ T cells, CD19+ B cells, and CD11c+ DCs (Fig. 2B, top plots), whereas Balb/c-Rag1−/−γc−/− mice harbored B cells and DCs, but insignificant levels of human T cells (Fig. 2B, bottom plots). Human platelets were never observed in NOD-scid/γc−/− mice engrafted with UCB HSC (Fig. 2B, upper right plot). In NOD-scid/γc−/− mice reconstituted with either HFL or UCB HSC, CD34+ cells were detected in the bone marrow at harvest, suggesting a stable, self-renewing pool of hematopoietic stem cells is maintained for at least 15 weeks post-engraftment (data not shown). Tissue immunophenotyping and platelet detection was not performed on Balb/c-Rag1−/−γc−/− mice engrafted with UCB HSC because all mice died prior to harvest, presumably due to infections with ubiquitous pathogens in light of low or declining engraftment levels. Compared to peripheral blood (Fig. 1A and 1B), spleens from HSC-engrafted mice typically harbored higher percentages of human CD45+ cells. Relative levels of various leukocyte subtypes mirrored those observed in peripheral blood, with T cells in Balb/c-Rag1−/−γc−/− (but not NOD-scid/γc−/− mice) making only a minor contribution to the total splenocyte pool (Fig. 4A). The total numbers of human leukocytes contained in the spleen of HFL HSC-reconstituted NOD-scid/γc−/− mice ranged from 108.0 – 143.7 × 106, whereas reconstituted Balb/c-Rag1−/−γc−/− mice harbored ~10-fold fewer cells (Fig. 4A).
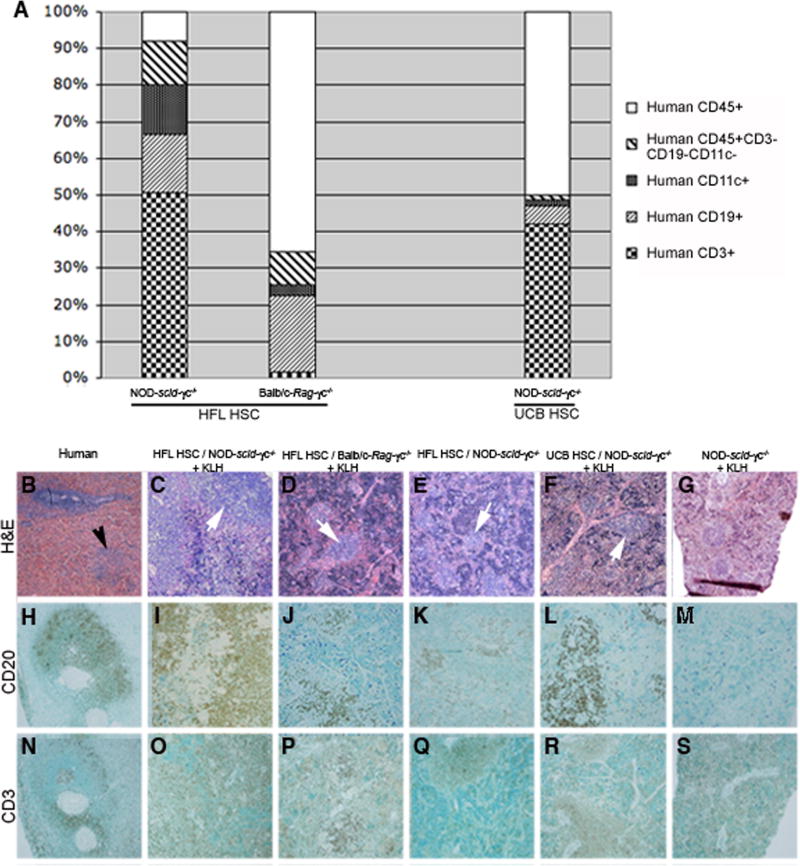
Figure 4. Analysis of human immune cell subsets in spleens of representative NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice receiving HFL or UCB HSC.
At 1 day of age, NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− mice were irradiated and then injected intrahepatically with 4.0 × 104 HFL HSC or 1.0 × 105 UCB HSC or left as non-irradiated, non-transplanted controls. The phenotype of human CD45+ splenocytes (CD3, CD19, or CD11c) from representative HFL HSC- or UCB HSC-engrafted mice was determined by flow cytometry following euthanasia at 15 or 28 weeks of life, respectively (A). Paraffin-embedded sections originating from a human spleen (B, H, N), or spleens from a KLH-immunized, HFL HSC-engrafted NOD-scid/γc−/− mouse (C, I, O), KLH-immunized, HFL HSC-engrafted Balb/c-Rag1−/− γc−/− mouse (D, J, P), non-immunized, HFL HSC-engrafted NOD-scid/γc−/− mouse (E, K, Q), KLH-immunized, UCB HSC-engrafted NOD-scid/γc−/− mouse (F, L, R), and KLH-immunized, non-engrafted NOD-scid/γc−/− mouse (G, M, S) were stained with H&E, CD20, or CD3, respectively (H&E: 4x magnification, CD20 and CD3: 10x magnification). White pulp in human splenic section and white pulp-like regions in engrafted mice are shown in panel B (black arrow) and panel C – F (white arrows), respectively.
Architecture of engrafted human immune cells
Normal spleen consists of red pulp, the site of red blood cell disposal, and white pulp (Fig. 4B, black arrow), which collects antigen from the blood and is comprised of periarteriolar T cells (Fig. 4N) and adjacent B cell regions (Fig. 4H). White pulp-like regions were present in the spleens of HFL HSC-reconstituted, KLH-immunized NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice and immunized UCB HSC-reconstituted NOD-scid/γc−/− mice (white arrows in Fig. 4C, 4D, and 4F, respectively). Of note, spleens from engrafted, non-immunized mice also contained white pulp-like regions, albeit smaller in size (Fig. 4E, white arrow). Spleens from non-engrafted NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice were markedly smaller in size (representative section from a NOD-scid/γc−/− mouse in Fig. 4G) compared to engrafted mice. Further analysis by IHC revealed that splenic lymphoid aggregates contained cells expressing the B cell marker CD20 in HFL HSC-engrafted/non-immunized NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice, HFL HSC-engrafted/KLH-immunized NOD-scid/γc−/− mice, and UCB HSC-engrafted/KLH-immunized NOD-scid/γc−/− mice (Fig. 4I – 4L, respectively) as well as T cells expressing CD3 (Fig. 4O – 4R). Human CD20 and CD3 staining was not apparent in sections originating from a non-engrafted/KLH-immunized NOD-scid/γc−/− mouse (Fig. 4M and 4S, respectively).
Normal thymi are multi-lobular structures with distinct cortical and medullary regions (Fig. 5A, white and red arrows, respectively), that differ in their function, gene expression profiles and morphological appearance. Thymic tissue in HFL HSC-engrafted NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice (Fig. 5B and 5C, white and red arrows, respectively) appeared as disorganized regions of darker medullary-like and lighter cortical-like regions. Thymi were also observed to possess typical thymic multi-lobular organization in HFL and UCB HSC-engrafted NOD-scid/γc−/− mice (Fig. 5B and 5D). Based on flow cytometric staining, thymi from HSC-engrafted mice were shown to contain human CD45+CD3+ T cells (Fig. 5E), the majority of which (56.6%) were CD4+CD8+ double positive cells (Fig. 5F). Single positive CD4+ and CD8+ cells were also present in relatively high numbers (23.5% and 17.7%, respectively, Fig. 5F). Thymi were not detected in non-engrafted NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− mice. Mesenteric (Fig. 5G and 5H) and popliteal (Fig. 5I) lymph nodes were also occasionally detected in HFL HSC-engrafted NOD-scid/γc−/− following immunization with inactivated influenza virus. Both mesenteric and popliteal lymph nodes were shown to contain human leukocytes based on positive CD45+ IHC stains (insets in Fig. 5H and 5I, respectively).
Figure 5. Analysis of thymi and lymph nodes in NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice receiving HFL or UCB HSC.
A – C: Paraffin-embedded, H&E-stained thymic tissue sections from an immunocompetent NOD mouse (A, 4x); NOD-scid/γc−/− (B, 4x) and Balb/c-Rag1−/−γc−/− (C, 10x) mice engrafted 28 weeks earlier with 4.0 × 104 HFL HSC. White and red arrows show medullary-like and cortical-like regions, respectively. D – F: H&E-stained thymic tissue section (D, 4x) and flow cytometric plots of thymic leukocytes showing human CD45+CD3+ cells (E) and CD4+, CD8+ and CD4+CD8+ cells (F) from representative NOD-scid/γc−/− mice injected with 1.0 × 105 UCB HSC and analyzed at 28 weeks of age. G – I: Paraffin-embedded mesenteric (G, H; 4x and 20x magnification, respectively) and popliteal (I, 40x magnification) lymph node sections were harvested at 12 months of age from influenza-immunized NOD-scid/γc−/− mice that received 5.0 × 104 HFL HSC as neonates and stained with either H&E or anti-human CD45 (insets).
Function of engrafted human immune cells
Spleens harvested from HFL HSC-engrafted NOD-scid/γc−/− mice harbored functional T cells and APCs based on high levels of 3H-thymidine incorporation in response to 2 μg/ml PHA, whereas splenocytes from a non-injected littermate control did not exhibit any response to PHA (Fig. 6A). Splenocytes from matched, reconstituted Balb/c-Rag1−/−γc−/− mice did not proliferate in response to PHA (Fig. 6A), consistent with the lack of T cells harbored in the splenic parenchyma following engraftment. Antigen-specific proliferation was not evident in splenocyte cultures from NOD-scid/γc−/− mice immunized and subsequently re-stimulated in vitro with either KLH or influenza antigen (data not shown).
Figure 6. Splenocyte blastogenesis and human-specific immunoglobulin responses in HSC-engrafted, KLH- or influenza-immunized NOD-scid/γc−/− and Balb/c-Rag1−/− γc−/− mice.

At 1 day of age, NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice were irradiated and then injected intrahepatically with 4.0 × 104 HFL HSC (KLH-immunized group), 5.0 × 104 HFL HSC (influenza-immunized group), 1.0 × 105 UCB HSC (KLH-immunized group), or left as non-irradiated, non-transplanted controls. A: At 15 weeks of age, splenocytes were harvested and cultured in the presence or absence of 2 μg/ml phytohemagglutinin (PHA). An 18 hour 3H-thymidine incorporation pulse was determined in triplicate beginning at 48 hours of culture (+/− standard deviation). * indicates HSC-engrafted mice with <1% peripheral blood chimerism. B-D: Mice began a series of 2 intradermal immunization doses with either KLH at 12 weeks (HFL HSC-engrafted) or 25 weeks (UCB HSC-engrafted) or influenza at 21 weeks. Total human IgM, total human IgG, and influenza-specific human (IgM+IgG+IgA) immunoglobulin (B-D, respectively), present in the chimeric mouse plasma, both immediately prior to the first immunization dose (pre) and 2 weeks following the second dose (post), was determined by ELISA. E – F: Flow cytometric plots showing human CD19+ and CD5+ co-expression (F) in the gated human CD45+ population (E) in peripheral blood leukocytes from a representative Balb/c-Rag−/−γc−/− mouse injected at 2 days of age with 1.0 × 105 UCB HSC and analyzed at 11 weeks of age.
To assess B cell function, total plasma human IgM (Fig. 6B) and IgG (Fig. 6C) were measured using a commercial ELISA kit both before and after immunization with KLH or influenza in NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice engrafted with HFL or UCB HSC. Human IgM was present in all engrafted mice prior to immunization, and levels of human IgM increased following KLH exposure to a maximum of 280 μg/ml, yet declined somewhat following influenza vaccination. Low levels of human IgG (<5 μg/ml) were detectable by ELISA from plasma of mice that harbored T cells (i.e. NOD-scid/γc−/− mice) following, but not prior to, immunization with KLH (Fig. 6C). Human anti-KLH specific IgM (~4.0 μg/ml), but not IgG, was detected in the plasma of HFL HSC-engrafted NOD-scid/γc−/− mice immunized with KLH (data not shown). In an older cohort of mice, human IgG was detected by ELISA in both pre- and post-immunization plasma in NOD-scid/γc−/− mice vaccinated with influenza (Fig. 6C). Low levels of influenza-specific human immunoglobulin were detected in several HFL HSC-engrafted NOD-scid/γc−/− mice immunized with influenza and both prior to and following immunization (Fig. 6D). The levels of human antibody contained in convalescent serum from an infected, normal human was at least 1000-fold higher than that harvested from reconstituted NOD-scid/γc−/− mice (data not shown). Furthermore, phenotyping of the human CD45+ population (Fig. 6E) revealed that approximately 50% of CD19+ B cells co-expressed CD5 (Fig. 6F), a B-1 cell marker in mice and possibly in humans. None of these anti-human antibodies stained splenocytes from non-engrafted NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− mice (data not shown).
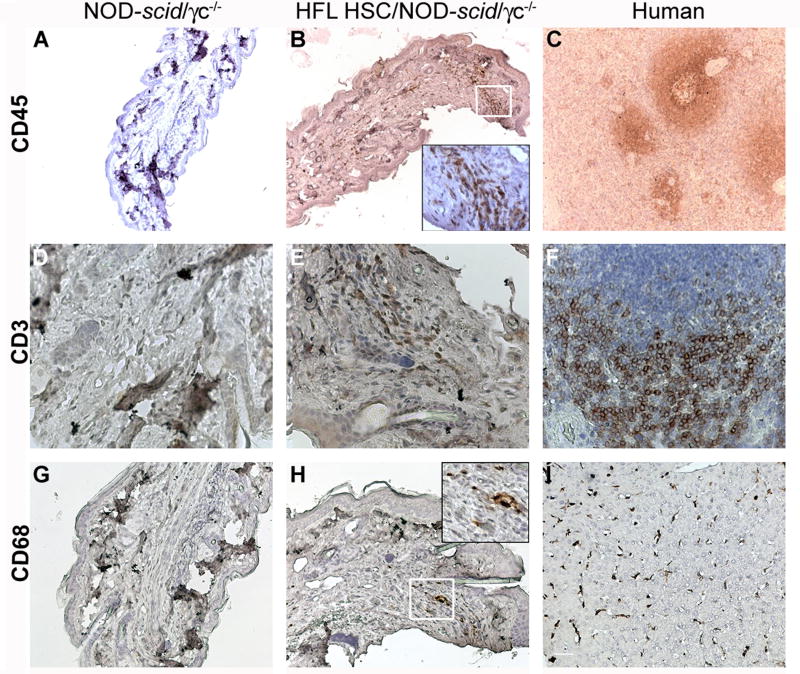
To assess a delayed type hypersensitivity (DTH) response, HFL and UCB HSC-engrafted NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice were challenged with KLH in the footpad and the ear 18 days after their initial KLH exposure. No significant swelling was ever detected in any mouse strain-HSC combination relative to non-engrafted littermate controls (data not shown). However, human CD45+ infiltrate, specifically CD3+ T cells and CD68+ macrophages, was observed by IHC in the footpads of HFL HSC-engrafted NOD-scid/γc−/− mice (Fig. 7B, 7E, and 7H, respectively) but not in a non-engrafted littermate control (Fig. 7A, 7D, and 7G, respectively). Fig. 7C and Fig. 7F demonstrate positive controls showing CD45 and CD3 staining, respectively, in human splenic sections. Similarly, Fig. 7I demonstrates CD68+ macrophage staining in a human liver.
Figure 7. Delayed type hypersensitivity responses in HFL HSC-engrafted, KLH-immunized NOD-scid/γc−/− mice.
At 1 day of age, NOD-scid/γc−/− mice were irradiated and then injected intrahepatically with 4.0 × 104 HFL HSC or left as an non-irradiated, non-transplanted controls. Mice were immunized twice intradermally with KLH then challenged in the footpad and the ear 18 days after their initial KLH exposure. Paraffin-embedded footpad sections originating from a challenged, non-engrafted (A, D, G) or engrafted (B, E, H) KLH-immunized NOD-scid/γc−/− mouse, harvested at 15 weeks of age, or from relevant human sections were stained for human CD45 (A – C; 10x magnification), human CD3 (D-F; 40x magnification), or human CD68 (G-I; 20x magnification). Insets in B and H are 40x magnifications of the respective white-boxed regions.
Engraftment of adult CD34+ HSC preparations
G-CSF-AB-isolex CD34+ cells did not reconstitute Balb/c-Rag1−/−γc−/− or C.B-17-scid/bg mice, whereas low levels of engraftment were observed in all engrafted NOD-scid/γc−/− mice (Table 2), consistent with earlier reports (26). In NOD-scid/γc−/− mice, levels of human CD45+ cells ranged from 9.8 to 18.1% by 6 weeks post-engraftment (Table 2). Human CD19+ and CD11c+ cells were detected in the peripheral blood, spleen, and bone marrow of these mice at 16 weeks post-engraftment. CD3+ cells were never observed (data not shown).
Peripheral blood human CD45+ cells were observed following engraftment of C.B-17-scid/bg with G-CSF-AB-column CD34+ preparations, with engraftment levels ranging from 2.0 to 60.8% when 1.0 × 106 cells were injected. No reconstitution was observed following injection of 0.5 × 106 G-CSF-AB-column HSC (Table 2). Mice with high levels of human CD45+ cells in the circulation developed severe dyspnea and lethargy by 6 weeks following engraftment, requiring early euthanasia due to humane concerns. Human CD4+ and CD8+ T cells (up to 20% and 10% of total leukocytes, respectively), as well as CD11c+ DCs (up to 7.5%), were detected in the peripheral blood and spleen of surviving mice, and lower numbers were noted in the bone marrow at 12 weeks post-engraftment (data not shown). Other leukocyte populations, including CD19+ B cells and CD56+ NKs, were not detected. Due to the lethality caused by neonatal engraftment of G-CSF-AB-column HSC inocula, experimentation was not performed in either NOD-scid/γc−/− or Balb/c-Rag1−/−γc−/− mice. We believe that engraftment with cells from G-CSF-AB likely resulted from transfer of mature leukocytes present in this preparation rather than by de novo differentiation of HSC, since none of the other HSC sources successfully engrafted scid/bg mice.
Discussion
In this study, we compared the engraftment of human CD34+ HSC in NOD-scid/γc−/−, Balb/c-Rag1−/−γc−/−, and C.B-17-scid/bg mice. To allow direct comparisons in these 3 mouse strains, a number of critical parameters were held constant throughout the study, including an established conditioning regime for each mouse strain, an intrahepatic injection site, age at the time of engraftment, and an identical number of input cells from each HSC source in a given experimental cohort of mice. Furthermore, on many occasions, cells from identical aliquots of HFL, UCB, or G-CSF-AB HSC were injected on the same day into mice of the 3 different strains. We discovered that mice of the NOD-scid/γc−/− strain, followed by those of the Balb/c-Rag1−/−γc−/− strain, were the most receptive for all HSC sources analyzed. The engraftment kinetics and breadth of developing immune cell types were similar when NOD-scid/γc−/− mice were reconstituted with either HFL or UCB HSC. HFL and UCB HSC-engrafted NOD-scid/γc−/− mice harbored T cells, B cells, NK cells, and DCs in multiple compartments and demonstrated human immune cell viability and immunological competence based on 1) splenocyte proliferation in response to PHA; 2) splenic and secondary node lymphoid enlargement in association with human immune cell engraftment; 3) human IgM and low levels of IgG antibody production following immunization with T-dependent antigens; and 4) human macrophage and T cell recruitment in response to a DTH challenge.
Previous reports have described administration of human HSC to mouse neonates via several routes, including the facial vein (18), intraperitoneally (27), and directly to the liver (19). We found that direct injection of between 1.5 – 5.0 × 104 HFL HSC and 1.0 × 105 UCB HSC into the neonatal liver gave consistent engraftment if injected into 1–2 day old neonates, and in our hands, was technically simpler than the facial vein injection method described by Ishikawa and colleagues (18). The survival rate of injected mice was ~ 90% using these methods, such that 113 of a total of 123 injected mice reached 6–10 weeks of age (ie. age of first blood draw) through the course of our studies. Typically, engraftment success rates of greater than 60% were achieved in NOD-scid/γc−/− mice engrafted with HFL or UBC HSC. Engraftment has been previously shown to vary depending on the age of Balb/c-Rag1−/−γc−/− mice injected intraperitoneally with HFL HSC, with younger neonates (1 day of age) more supportive of engraftment than older neonates (1 and 2 weeks of age) (27). Furthermore, it is likely that a higher proportion of HSC would take up residence in the liver following direct intrahepatic injection compared to intraperitoneal delivery, circumventing the need for cell homing to the liver where hematopoiesis primarily occurs during the first several weeks of mouse postnatal development. Accordingly, direct delivery of greater numbers of HSC to the liver would lower the cell dose required to achieve optimal engraftment. For these reasons, intrahepatic injection of HSC into young neonates was held constant within our experimental design.
Our direct comparisons between various lines of mice and those of Takenaka and colleagues (28) reveal that HSC engraftment is dramatically affected by the background strain of mouse. A polymorphism in the Sirpa gene in the NOD background, but not in the Balb/c or C.B-17 backgrounds, may contribute to the increased survival and subsequent engraftment of transplanted HSC in NOD-scid/γc−/− mice by rendering the SIRP-α receptor on mouse macrophages cross-reactive with human CD47 expressed on HSC (28). Appropriate binding of CD47 to SIRP-α prevents HSC phagocytosis by macrophages. In contrast, minimal or nonexistent engagement of Balb/c SIRP-α with human CD47 may result in the targeted destruction of the HSC compartment in Balb/c-Rag1−/−γc−/− mice, yielding lower engraftment levels than observed in NOD-scid/γc−/− mice. Because T cell engraftment in NOD-scid/γc−/− mice was positively correlated with the number of HFL HSC transplanted, failure to generate a reproducible T cell compartment in Balb/c-Rag1−/−γc−/− mice may be due to limited HSC survival. Though the C.B-17 background of scid/bg mice used in this report is largely genetically identical to the Balb/c background, reconstitution was not apparent in neonates in spite of defects in NK cell function conferred by the beige mutation. The γc mutation, in contrast, renders mice deficient in NK cells, as well as causing deficiencies in T and B cell development and function, providing an absolutely critical environment for human HSC development in Balb/c-Rag1−/− or NOD-scid mice.
In congruence with other reports, engraftment of NOD-scid/γc−/− mice with human UCB HSC supported T and B cell reconstitution (17,18,29), while engraftment with purified human G-CSF-AB HSC supports only B cells (26). In contrast, we found that Balb/c-Rag1−/−γc−/− mice supported B cell and DC development, but negligible T cell development, following engraftment with either HFL or UCB HSC. Similarly, Gimeno et al. showed that engraftment of 1 × 106 HFL HSC into Balb/c-Rag1−/−γc−/− mice resulted primarily in B cell development, although lower numbers of T cells were also detected (27). It appears that engraftment of HFL HSC provides no obvious advantages over UCB in either the Balb/c-Rag1−/−γc−/− or NOD-scid/γc−/− strain except for the possibility of co-engrafting autologous thymic tissue to mediate positive clonal selection of developing T cells on a human thymus. In vivo T cell responses have been reported in the presence of autologous human fetal thymic tissue, likely due to the requirement of positive selection on autologous human HLA class I-expressing stromal cells (1). Similarly, a recent paper by Tonomura et al (30) showed that co-engraftment of fetal thymus and liver with HFL HSC produced chimeric NOD-scid mice capable of demonstrating T-dependent antibody responses to KLH. Very recently, Giassi and colleagues reported human immunoglobulin responses to T-dependent antigens following of UCB-reconstitution of TNF-α-treated NOD-scid/γc−/− mice, which were enhanced in the presence of the B-cell cytokine BLyS (31). Though subclass typing was not done to rule out the possibility that our detected IgG is of subclass IgG3, which develops independently of T cell help, IgG was detected only in mice harboring T cells following immunization with KLH, suggesting that appropriate antigen-specific T cell-B cell interactions are likely occurring. Furthermore, low levels of human immunoglobulin were detected in both pre- and post-immunization plasma in those HFL HSC-engrafted NOD-scid/γc−/− mice immunized with influenza antigen. This latter group of mice were 5 months of age at the time of pre-immunization bleed and subsequent primary vaccination, compared to only 3 months of age for the KLH-immunized HFL HSC-engrafted mice, suggesting that successful class switching to IgG occurred as human immune responses matured and/or mice were exposed to more antigens throughout life. Reconstituted mice in our study do not likely possess a human thymic stromal microenvironment, however positive selection may be occurring on other human cells transferred in the inoculum, including DCs or other T cells (32). Co-engraftment of autologous thymic tissue or bone marrow stromal cells with HFL HSC may enable more robust antigen-specific T-dependent responses than our current system supports.
Approximately half of human B cells in the peripheral blood and tissues of HSC reconstituted mice expressed the cell surface protein CD5, possibly suggesting they are B-1 cells that respond mainly to bacterial lipopolysaccharide and phosphorylcholine (33–35) rather than conventional B-2 cells that mediate antigen-specific adaptive immunity. It is well established that CD5+ B cells represent B-1 cells in mice (36) and are produced mainly in the fetal liver during development (37). It is currently uncertain whether the similar B-1 and B-2 cell lineages are present in humans (37). However if indeed human B-1 cells develop as described in mice, reconstitution of the neonatal mouse liver with HSC may account for the large proportion of B cells detected in HFL HSC-engrafted mice being of this subset. In mice, CD5+ B-1 cells (33,34) produce mainly natural IgM antibodies without the requirement for T cell help. The relatively high levels of this B cell subset may explain why IgM, rather than IgG, is the predominant immunoglobulin class in our reconstituted mice. Alternatively, or perhaps in addition, class-switching is dysfunctional due to inadequate interactions between T cells and conventional B-2 cells. B-1 cells in naïve normal (immunocompetent) mice are known to produce natural IgM antibodies that react with influenza A and B strains (38,39), and perhaps the influenza hemagglutinin molecule in particular (40,41), providing a possible explanation for the presence of influenza-reactive antibodies in our mice prior to immunization. Higher levels of IgM antibody were apparent in pre-immunization plasma compared to post-immunization plasma, harvested at 5 and 12 months of age, respectively, in influenza-immunized NOD-scid/γc−/− mice, mimicking the typical decline of natural IgM throughout life. Analysis of cytokines within the B cell microenvironment may provide additional insight to explain the suboptimal immunoglobulin diversity observed in our mice.
Interestingly, mice from all 3 strains analyzed occasionally became ill during adult life and required premature euthanasia due to humane reasons if they failed to reconstitute or had very low engraftment levels (<1%). Infections (typically dermatitis) or a hunched and dehydrated appearance became predictive indicators of poor engraftment efficiency. This observation may be a consequence of a small (or steadily declining) human immune cell compartment that cannot (or can no longer) support an irradiation-compromised host innate immune system in the defense of ubiquitous pathogens. Alternatively, mice could have become clinically ill due to irradiation-induced anemia or thrombocytopenia. These mice were not used in the functional analysis experiments, rather were euthanized on humane grounds when clinical disease became severe.
We noted white pulp-like regions in the spleens of all engrafted NOD-scid/γc−/− mice and KLH-vaccinated Balb/c-Rag1−/−γc−/− mice at 15 weeks post-engraftment. In KLH-immunized NOD-scid/γc−/− mice, these follicle-like collections were remarkably larger. The presence of white pulp-like regions in non-immunized mice may arise spontaneously or be attributed to environmental antigen exposure. Within observed white pulp-like regions, distinct T and B cell zones were observed, but not well-organized. This lack of appropriate B and T cell compartmentalization may provide another explanation for the inadequate adaptive immune responses noted in HSC-engrafted mice.
HFL and UCB could be highly enriched for CD34+ cells using commercial anti-human CD34 columns in a single step; in contrast, adult G-CSF-mobilized blood preparations contained many mature leukocytes when processed using the same method, likely reflecting their predominance in a developmentally more mature sample prior to enrichment. These lin+ cells, and notably xenoreactive memory T cells, were presumably responsible for the graft-versus-host syndrome evident in many mice by 6–8 weeks when engrafted as irradiated neonates, as previously reported following engraftment of PBMC in adult scid mice (42,43). Interestingly, column-enriched G-CSF-AB HSC did not cause disease when transplanted into irradiated, adult C.B-17-scid/bg and NOD-scid/γc−/− mice. Instead, mice remained healthy and could be used to investigate macrophage expansion, homing, and tissue injury to allogeneic tissues (44). This suggests that it is imperative that HSC preparations be CD3+ T cell-deficient to prevent graft-versus-host symptoms following neonatal mouse engraftment.
The individual contributions of CD34-lin- and CD34+lin- progenitors, as well as more mature CD34-lin+ cells, to the multi-lineage hematolymphoid reconstitution of either NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice is unknown. However, the phenotype of specific leukocyte subsets observed in the peripheral blood of engrafted mice frequently correlated with the specific phenotypes of inocula mature contaminants. For example, both strains consistently harbored B cells, and all HSC preparations contained CD34-CD19+ population. Therefore, B cells detected in the chimeric mice could have originated from the de novo differentiation of true CD34+ hematopoietic stem cells or the expansion and homing of pre-existing mature CD19+ B cells. Interestingly, neonatal NOD-scid/γc−/− mice injected with Isolex-purified adult HSC, which lacked CD3+ T cells, never harbored T cells. In contrast, engraftment of HFL or UCB HSC preparations, which usually contained low levels of T cells, routinely produced mice harboring T cells, suggesting that a naïve T cells may be required for successful T cell development in our system. Consistent with mice inoculated with Isolex-purified, mice receiving HFL HSC from a preparation which lacked T cells, harbored B cells and DCs, but never showed any T cell engraftment. Lineage depletion prior to engraftment would allow further characterization of the developmental origin of each immune cell identified in vivo. Furthermore, studies citing HSC differentiation as the source for hematolymphoid development must be carefully evaluated.
Optimizing mouse models of human hematopoietic stem cell engraftment to further support a more complete and functional human immune system is paramount to a comprehensive understanding of human disease pathogenesis and progression. To our knowledge, our findings offer the first reported systematic comparison of neonatal hematopoietic reconstitution using four types of HSC preparations in 3 commonly-used strains of immunodeficient mice. We demonstrate successful multi-lineage hematolymphoid reconstitution of NOD-scid/γc−/− by intrahepatic injection of HFL and UCB HSC and demonstrate that this strain is more receptive to engraftment than age-matched Balb/c-Rag1−/−γc−/− mice. Immunologic function was confirmed based on proliferation of splenocytes in response to PHA and the presence of multiple human immunoglobulin isotypes following immunization with T-dependent antigens. In all cases, responses were less robust in Balb/c-Rag1−/−γc−/− mice likely owing to the limited T cell development observed in this strain.
Acknowledgments
We thank Dr. Leonard Shultz, Jackson Laboratories, and Dr. Drew Pardoll, Johns Hopkins University for the donations of breeding pairs of NOD-scid/γc−/− and Balb/c-Rag1−/−γc−/− mice, respectively; Drs. Li Wen and Sara Rockwell for the donations of control NOD and Balb/c mice, respectively; Dr. Bradford Poulos of the Human Fetal Tissue Repository, Albert Einstein College of Medicine, for human fetal liver tissue; Dr. Diane Krause and Wendy Haskell at the Yale Center of Excellence in Molecular Hematology (NIH No. DK0724429) for G-CSF-AB-isolex cells; Labor and Birth Yale–New Haven Hospital staff and Ann Marie Franco for UCB cells; Paul Bonjiorni for assistance with irradiation; and Lisa Gras, Louise Benson and Michelle Benevento for assistance with mice. Helpful discussions with Drs. Elizabeth Eynon, Anthony Rongvaux, Tim Willinger, and Diane Krause are also gratefully acknowledged. This study was supported by the Section of Comparative Medicine and NIH Grant P01-HL070295. MJH is the recipient of a Research Scholar Award from the American Gastroenterology Association.
Abbreviations
- BSA
bovine serum albumin
- DTH
delayed type hypersensitivity
- FCS
fetal calf serum
- G-CSF-AB
granulocyte-colony stimulating factor-mobilized adult blood
- γc
gamma chain
- HBSS
Hank’s Balanced Salt Solution
- HFL
human fetal liver
- hFTR
Human Fetal Tissue Repository
- HRP
horse radish peroxidase
- HSC
hematopoietic stem cell
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- iPSC
inducible pluripotent stem cells
- KLH
keyhole limpet hemocyanin
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- PBS-T
phosphate buffered saline/0.05% Tween
- PHA
phytohemagglutinin
- TBS-T
tris-buffered saline/0.05% Tween
- UCB
umbilical cord blood
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006 Nov;12(11):1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 2.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007 Feb;7(2):118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 3.Zollner TM, Podda M, Pien C, Elliott PJ, Kaufmann R, Boehncke WH. Proteasome inhibition reduces superantigen-mediated T cell activation and the severity of psoriasis in a SCID-hu model. J Clin Invest. 2002 Mar;109(5):671–679. doi: 10.1172/JCI12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pober JS, Bothwell AL, Lorber MI, McNiff JM, Schechner JS, Tellides G. Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Semin Immunopathol. 2003 Sep;25(2):167–80. doi: 10.1007/s00281-003-0135-1. [DOI] [PubMed] [Google Scholar]
- 5.Banuelos SJ, Shultz LD, Greiner DL, Burzenski LM, Gott B, Lyons BL, et al. Rejection of human islets and human HLA-A2.1 transgenic mouse islets by alloreactive human lymphocytes in immunodeficient NOD-scid and NOD-Rag1(null)Prf1(null) mice. Clin Immunol. 2004 Sep;112(3):273–283. doi: 10.1016/j.clim.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 6.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241(4873):1632–9. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 7.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172(4):1055–63. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kyoizumi S, Baum CM, Kaneshima H, McCune JM, Yee EJ, Namikawa R. Implantation and maintenance of functional human bone marrow in SCID-hu mice. Blood. 1992;79(7):1704–11. [PubMed] [Google Scholar]
- 9.Carballido JM, Namikawa R, Carballido-Perrig N, Antonenko S, Roncarolo MG, de Vries JE. Generation of primary antigen-specific human T- and B-cell responses in immunocompetent SCID-hu mice. Nat Med. 2000;6(1):103–6. doi: 10.1038/71434. [DOI] [PubMed] [Google Scholar]
- 10.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 11.Hesselton RM, Greiner DL, Mordes JP, Rajan TV, Sullivan JL, Shultz LD. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. J Infect Dis. 1995 Oct;172(4):974–982. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 12.Tereb DA, Kirkiles-Smith NC, Kim RW, Wang Y, Rudic RD, Schechner JS, et al. Human T cells infiltrate and injure pig coronary artery grafts with activated but not quiescent endothelium in immunodeficient mouse hosts. Transplantation. 2001 Jun 15;71(11):1622–1630. doi: 10.1097/00007890-200106150-00023. [DOI] [PubMed] [Google Scholar]
- 13.Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992 Feb 28;255(5048):1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 14.Pflumio F, Izac B, Katz A, Shultz LD, Vainchenker W, Coulombel L. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996 Nov 15;88(10):3731–3740. [PubMed] [Google Scholar]
- 15.Goldman JP, Blundell MP, Lopes L, Kinnon C, Di Santo JP, Thrasher AJ. Enhanced human cell engraftment in mice deficient in RAG1 and the common cytokine receptor gamma chain. Br J Haematol. 1998 Nov;103(2):335–42. doi: 10.1046/j.1365-2141.1998.00980.x. [DOI] [PubMed] [Google Scholar]
- 16.Mazurier F, Fontanellas A, Salesse S, Taine L, Landriau S, Moreau-Gaudry F, et al. A novel immunodeficient mouse model--RAG1 × common cytokine receptor gamma chain double mutants--requiring exogenous cytokine administration for human hematopoietic stem cell engraftment. J Interferon Cytokine Res. 1999 May;19(5):533–41. doi: 10.1089/107999099313983. [DOI] [PubMed] [Google Scholar]
- 17.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002 Nov 1;100(9):3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005 Sep 1;106(5):1565–73. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004 Apr 2;304(5667):104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 20.Rajesh D, Chinnasamy N, Mitalipov SM, Wolf DP, Slukvin I, Thomson JA, et al. Differential requirements for hematopoietic commitment between human and rhesus embryonic stem cells. Stem Cells. 2007 Feb;25(2):490–499. doi: 10.1634/stemcells.2006-0277. [DOI] [PubMed] [Google Scholar]
- 21.Trivedi P, Hematti P. Simultaneous generation of CD34+ primitive hematopoietic cells and CD73+ mesenchymal stem cells from human embryonic stem cells cocultured with murine OP9 stromal cells. Exp Hematol. 2007 Jan;35(1):146–154. doi: 10.1016/j.exphem.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007 Nov 30;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Akahira-Azuma M, Szczepanik M, Tsuji RF, Campos RA, Itakura A, Mobini N, et al. Early delayed-type hypersensitivity eosinophil infiltrates depend on T helper 2 cytokines and interferon-gamma via CXCR3 chemokines. Immunology. 2004 Mar;111(3):306–317. doi: 10.1111/j.0019-2805.2004.01818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JS, Curtsinger J, Berthold M, Malvey K, Bliss RL, Le CT, et al. Diminished neo-antigen response to keyhole limpet hemocyanin (KLH) vaccines in patients after treatment with chemotherapy or hematopoietic cell transplantation. Clin Immunol. 2005 Nov;117(2):144–151. doi: 10.1016/j.clim.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Murray AG, Petzelbauer P, Hughes CC, Costa J, Askenase P, Pober JS. Human T-cell-mediated destruction of allogeneic dermal microvessels in a severe combined immunodeficient mouse. Proc Natl Acad Sci USA. 1994 Sep 13;91(19):9146–9150. doi: 10.1073/pnas.91.19.9146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005 May 15;174(10):6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 27.Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL, et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG1−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 2004 Dec 15;104(13):3886–93. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- 28.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat Immunol. 2007 Dec;8(12):1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 29.Yahata T, Ando K, Nakamura Y, Ueyama Y, Shimamura K, Tamaoki N, et al. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice. J Immunol. 2002 Jul 1;169(1):204–209. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- 30.Tonomura N, Habiro K, Shimizu A, Sykes M, Yang YG. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008 Apr 15;111(8):4293–4296. doi: 10.1182/blood-2007-11-121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giassi LJ, Pearson T, Shultz LD, Laning J, Biber K, Kraus M, et al. Expanded CD34+ human umbilical cord blood cells generate multiple lymphohematopoietic lineages in NOD-scid IL2rgamma(null) mice. Exp Biol Med (Maywood) 2008 Aug;233(8):997–1012. doi: 10.3181/0802-RM-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Sofi MH, Rietdijk S, Wang N, Terhorst C, Chang CH. The SLAM-associated protein signaling pathway is required for development of CD4+ T cells selected by homotypic thymocyte interaction. Immunity. 2007 Nov;27(5):763–774. doi: 10.1016/j.immuni.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987 Apr 3;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 34.Hardy RR, Hayakawa K, Shimizu M, Yamasaki K, Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987 Apr 3;236(4797):81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura M, Burastero SE, Ueki Y, Larrick JW, Notkins AL, Casali P. Probing the normal and autoimmune B cell repertoire with Epstein-Barr virus. Frequency of B cells producing monoreactive high affinity autoantibodies in patients with Hashimoto’s disease and systemic lupus erythematosus. J Immunol. 1988 Dec 15;141(12):4165–4172. [PubMed] [Google Scholar]
- 36.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 37.Dorshkind K, Montecino-Rodriguez E. Fetal B-cell lymphopoiesis and the emergence of B-1-cell potential. Nat Rev Immunol. 2007 Mar;7(3):213–219. doi: 10.1038/nri2019. [DOI] [PubMed] [Google Scholar]
- 38.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: a key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005 Mar;26(4):347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 39.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, Chen J. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med. 2000 Jul 17;192(2):271–280. doi: 10.1084/jem.192.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke SH, Huppi K, Ruezinsky D, Staudt L, Gerhard W, Weigert M. Inter- and intraclonal diversity in the antibody response to influenza hemagglutinin. J Exp Med. 1985 Apr 1;161(4):687–704. doi: 10.1084/jem.161.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baccala R, Quang TV, Gilbert M, Ternynck T, Avrameas S. Two murine natural polyreactive autoantibodies are encoded by nonmutated germ-line genes. Proc Natl Acad Sci USA. 1989 Jun;86(12):4624–4628. doi: 10.1073/pnas.86.12.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tary-Lehmann M, Lehmann PV, Schols D, Roncarolo MG, Saxon A. Anti-SCID mouse reactivity shapes the human CD4+ T cell repertoire in hu-PBL-SCID chimeras. J Exp Med. 1994 Nov 1;180(5):1817–1827. doi: 10.1084/jem.180.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greiner DL, Shultz LD. NOD Mice and Related Strains. In: Leiter E, Atkinson MJ, editors. Research Applications in Diabetes, AIDS, Cancer and Other Diseases Austin. Landes Bioscience; 1998. pp. 173–203. [Google Scholar]
- 44.Kirkiles-Smith NC, Harding MJ, Shepherd BR, Fader SA, Yi T, Wang Y, et al. Development of a humanized mouse model to study the role of macrophages in allograft injury. Transplantation. 2009 Jan 27;87(2):189–197. doi: 10.1097/TP.0b013e318192e05d. [DOI] [PMC free article] [PubMed] [Google Scholar]