Abstract
Dimethandrolone (DMA: 7α,11β-dimethyl-19-nortestosterone) and 11β-methyl-19-nortestosterone (MNT) are potent androgens in development for hormonal therapy in men. As 5α-reduced androgens, such as 5α-dihydrotestosterone (DHT), may raise the risk of benign prostate hyperplasia, accelerate the development of prostate carcinoma, and increase male pattern baldness and acne, we investigated the role of 5α-reduction in the androgenic activity of DMA and MNT. The authentic 5α-reduced metabolites, 5α-dihydroDMA (5α-DHDMA) and 5α-dihydroMNT (5α-DHMNT), were prepared by chemical synthesis and compared in vitro and in vivo to the parent compounds. Both 5α-reduced androgens bound with high affinity to the rat androgen receptor (AR) and were potent inducers of transactivation of 3XHRE-LUC in CV-1 cells cotransfected with a human AR expression plasmid. To examine in vivo androgenic (stimulation of ventral prostate [VP] and seminal vesicle [SV] weights) and anabolic (stimulation of levator ani [LA] muscle weights) activity, 22 day-old castrate male rats were treated sc for 7 days with various doses of DMA, 5α-DHDMA, or testosterone (T) or MNT, 5α-DHMNT, or T and necropsied on day 8. 5α-DHDMA was at least 3-fold more potent than T in stimulating growth of the VP but only 30-40% as potent as DMA. 5α-DHMNT was 4- to 8-fold more potent than T, whereas MNT was approximately equipotent to T. To assess the possible role of 5α-reduction in VP and SV growth, castrate immature rats were treated with maximally effective doses of T, DHT, DMA, MNT, or the related 19-norandrogen, 7α-methyl-19-nortestosterone (MENT), or vehicle, with or without dutasteride (DUT), an inhibitor of 5α-reductases types 1 and 2. In rats treated with T + DUT, serum T was significantly higher (P<0.05) than in rats treated with T alone, and serum DHT was decreased (P<0.001) to levels observed in castrate vehicle-treated rats. DUT significantly reduced both VP and SV weights in T-treated rats, whereas there was no significant effect of DUT on weights of these accessory sex glands in rats treated with DMA, MNT, DHT, or MENT. These results indicate that inhibition of 5α-reductase activity in vivo does not affect the androgenic potency of DMA, MNT, or MENT.
Keywords: Synthetic androgens, hormonal therapy for men, 5α-reductase, dutasteride, accessory sex glands, androgen receptor
1. Introduction
Testosterone (T) is the principal androgen secreted by Leydig cells. It exerts both androgenic effects involving growth stimulation and functional maintenance of the male reproductive tract and anabolic effects involving growth stimulation of nonreproductive organs such as muscle, kidney, liver, and submaxillary salivary glands [1]. T levels may decline in aging men resulting in clinical symptoms similar to those observed in hypogonadal men [2,3]. Current androgen replacement therapies involving the use of T have been shown to alleviate symptoms. When natural T is administered orally, however, only a small amount reaches the circulation due to absorption from the gastrointestinal tract into the portal blood and degradation by the liver (first-pass effect) [2,3]. The biological actions of T depend in part on its metabolism. In the male accessory sex organs and skin, T is converted by 5α-reductases to 5α-dihydrotestosterone (DHT), whereas in brain, adipose tissue, and gonads, T is converted to estradiol by aromatase cytochrome P450 [4].
Alternative androgens are being developed for hormonal therapy which may demonstrate greater potency, duration of action, and oral efficacy than T. Dimethandrolone (DMA: 7α,11β-dimethyl-19-nortestosterone) and the 11β-monomethylated analogue of DMA (11β-methyl-19-nortestosterone, MNT) are potent orally active synthetic 19-norandrogens. We are currently assessing the 17β-undecanoic acid ester of DMA (DMAU) and the 17β-dodecylcarbonate ester of MNT (MNTDC) as potential agents for androgen therapy [5]. Cleavage of the 17β-ester bond in the circulation liberates the biologically active androgens. As a result of their potency and oral bioavailability, DMAU/DMA and MNTDC/MNT have the potential advantage that they could be used at relatively low doses.
5α-reduced androgens such as DHT may raise the risk of benign prostate hyperplasia (BPH), accelerate the development of prostate carcinoma, and increase male pattern baldness and acne. Therefore, it was desirable to investigate whether DMA and MNT are 5α-reduced in vivo, potentially resulting in adverse effects similar to those of DHT. Authentic 5α-dihydroDMA (5α-DHDMA) and 5α-dihydroMNT (5α-DHMNT) were prepared by chemical synthesis and their activities compared in vitro (androgen receptor [AR] binding and transactivation in CV-1 cells mediated by AR) and in vivo (stimulation of ventral prostate [VP], seminal vesicles [SV], and levator ani [LA] muscle weights) with those of the parent compounds. The potential 5α-reduction in vivo of DMA and MNT was assessed indirectly as there are currently no assays to measure 5α-DHDMA or 5α-DHMNT in serum. Rats were treated with maximal stimulatory doses of various androgens in the absence or presence of the dual 5α-reductase inhibitor, dutasteride (DUT), at a dose which completely blocked conversion of T to DHT, but did not cause overt signs of toxicity. The effect of these treatments on androgenic endpoints was evaluated. The related 19-norandrogen, 7α-methyl-19-nortestosterone (MENT) was also tested as MENT had been shown previously not to undergo 5α-reduction in vivo or by rat liver, prostate, and epididymis in vitro [6,7]. The results of these experiments indicated that DUT treatment, which completely inhibited the conversion of T to DHT and significantly decreased androgenic effects in T-treated animals, did not alter the stimulatory effect of DMA, MNT, or MENT on growth of the VP and SV. Thus, inhibition of 5α-reductase activity in vivo does not affect the androgenic potency of DMA, MNT, or MENT.
2. Materials and Methods
2.1. Chemicals
T and DHT were purchased from Steraloids (Newport, RI). DMA, 5α-DHDMA, MNT, 5α-DHMNT, MENT, and 5α-dihydroMENT (5α-DHMENT) were synthesized by the Southwest Foundation for Biomedical Research (San Antonio, TX) under contract NO1-HD-6-3255 and were >98% to 100% pure by HPLC and NMR. The dual 5α-reductase inhibitor, dutasteride (17β-N-2,5-bis-[trifluoromethyl]-phenylcarbamoyl-4-aza-5a-androstan-1-en-3-one), was from AK Scientific, Inc. (Mountain View, CA) and was >98% pure by HPLC.
2.2. Animals and androgenic assays
Immature male Sprague-Dawley CD rats (Crl:CD(SD)) were purchased from Charles River Laboratories (Kingston, NY) and castrated at 22 days of age. To determine the potency of the 5α-reduced derivatives of DMA and MNT compared to the parent compounds and to T, which is the standard in the sc androgenic assay, rats (8/group) were injected sc daily, starting on the day of castration (day 22) and continuing for 7 additional days, with either vehicle (10% EtOH/sesame oil) or various doses of T, DHT, DMA, 5α-DHDMA, MNT, or 5α-DHMNT. Twenty-four hours after the final dose, the animals were euthanized, and body weights were obtained. The VP, SV, and LA muscle were excised, trimmed, blotted, and weighed to the nearest 0.1 mg [8].
To determine the effect of DUT in the androgenic assay, rats (n = 10/group) were initially treated sc with vehicle, T (0.23 mg/day), or T and 0.1, 1.0, or 10.0 mg/day of DUT orally for 8 days. Thereafter, rats (10/group) were injected sc with either vehicle or maximal stimulatory doses of T, DHT, DMA, MNT, or MENT for 8 consecutive days. Concurrent with the sc injections, vehicle or DUT was administered orally at 1.0 mg/day which was determined to be the optimal dose (see Section 3.3). Two hours after the final dose, the rats were euthanized, blood was collected for measurement of serum androgen levels, and body weights were obtained. The VP and SV were excised, trimmed, blotted, and weighed.
2.3. Radioimmunoassays (RIA’s)
Serum levels of DMA and MNT and their putative immunoreactive metabolites were determined using specific RIA’s developed at Bioqual, Inc. [5,9]. The limits of detection were 125 pg/ml (5 μl serum) for DMA and 2.37 ng/ml (0.5 μl serum) for MNT. MENT was measured by RIA [10] at the Population Council, courtesy of Dr. N. Kumar. The limit of detection was 130 pg/ml. T and DHT were also measured by RIA using kits from DPC (Coat-a-Count, Los Angeles, CA) and DSL (DSL-9600 ACTIVE® DHT Coated-Tube RIA, Webster, TX), respectively. The limit of detection in the T assay was 60 or 70 pg/ml (EC90) and in the DHT assay, 25 pg/ml (lowest standard).
2.4. Androgen receptor binding and transactivation
Binding to androgen receptors (AR) was performed as described previously [11]. Briefly, purified rat AR ligand binding domain (ARLBD; ~ 200 fmol/tube), purchased from Invitrogen (Carlsbad, CA), was incubated in microfuge tubes with 5 nM 17α-methyl-[3H]R1881 (PerkinElmer, Boston, MA) in 50 mM Tris, pH 7.5, 0.8 M NaCl, 10% glycerol, 0.1% BSA, 0.002% NP-40 in the absence or presence of 5 μM R1881 to measure total and nonspecific binding, respectively. Unlabeled competitors were added at final concentrations from 0.1 to 500 nM. After an overnight incubation at 2-6°C, [3H]R1881-ARLBD complexes were separated from unbound radioligand by addition of 100 μl 50% hydroxylapatite and centrifugation. Precipitates were washed 3 times, and bound radioactivity was extracted with 200 μl 95% ethanol and counted in a LKB Wallac Rackbeta scintillation counter. R1881 was the standard. DUT did not bind to the ARLBD within the limits of the concentrations tested (IC50 > 500 nM).
CV-1 cells (ATCC, Manassas, VA) were used to assess transcriptional activity of the various androgens. Cells were cotransfected with 3XHRE-LUC (containing 3 copies of a hormone response element fused to the firefly luciferase gene) and a human AR expression plasmid (gifts of Dr. D. Robins, University of Michigan, Ann Arbor, MI), treated for 20 h with vehicle (100% ethanol) or various concentrations of androgens (range 10−12 to 10−5 M), and harvested. Luciferase activity was measured and normalized as specified previously [5].
2.5. Statistical analysis
Data are expressed as the mean ± SE. Statistical analyses were performed using SigmaStat for Windows (version 3.50, SPSS Inc., Chicago, IL) or Microsoft Excel (Microsoft Corp.). EC50’s in transactivation assays or IC50’s in competitive binding assays for the ARLBD were determined using GraphPad PRISM™, version 4.0 (GraphPad Software, San Diego, CA,) and compared statistically by the nonparametric Kruskal-Wallis one way ANOVA on ranks, as the data did not pass normality, followed by Dunn’s all pairwise multiple comparison test. Effects of various doses of DMA, 5α-DHDMA, MNT, 5α-DHMNT, and T on VP, SV, and LA muscle weights in the androgenic assays were compared by one way ANOVA followed by the Holm-Sidak multiple comparison test. If the raw data did not meet the assumptions of normal distribution and homogeneity of variance, they were log10-transformed before analysis or compared using Kruskal-Wallis one way ANOVA on Ranks followed by the Student-Newman-Keuls multiple comparison test. Potency ratios were estimated using an NIH program based on the Prophet System [12]. Serum levels of DHT in rats treated with T alone or T with various doses of DUT were compared by Kruskal-Wallis one way ANOVA on ranks followed by Dunn’s all pairwise multiple comparison test. VP and SV weights and serum hormone levels in rats treated with androgens with or without DUT were compared by two-tailed Student’s t tests. P< 0.05 was considered statistically significant.
3. Results
3.1. Binding of DMA, MNT, MENT, and their 5α-reduced derivatives to AR and transactivation mediated by AR
Table 1 summarizes the in vitro data comparing the IC50’s and relative binding affinities (RBA’s) for the ARLBD and potency in transactivation assays (EC50’s) of T, DHT, DMA, MNT, the related 19-norandrogen MENT, and those of the authentic 5α-reduced derivatives of the synthetic androgens obtained by chemical synthesis. When the IC50’s of all androgens were compared by ANOVA, binding affinities of T and DHT were significantly lower (P<0.05) than those of 5α-DHDMA and MNT, but there were no other significant differences. The only significant difference (P<0.05) among the androgens in the EC50’s for transactivation of 3XHRE-LUC in CV-1 cells was between T and DMA.
Table 1.
AR Binding and Transactivation
| Androgen | Binding to ARLBD | Transactivation in CV-1 Cells |
|
|---|---|---|---|
| IC50 (nM)1 | RBA (%)2 | EC50 (nM)1 | |
| R1881 | 4.4 ± 0.4 (12) | 1003 | 0.39 ± 0.15 (7) |
| T | 27.2 ± 3.1a (4) | 22 | 16.8 ± 6.1a (9) |
| DHT | 21.4 ± 3.4a (14) | 33 | 6.5 ± 2.5a,b (10) |
| DMA | 9.5 ± 1.8ab (5) | 106 | 1.5 ± 0.6b (7) |
| 5α-DHDMA | 5.5 ± 1.1b (4) | 105 | 5.9 ± 2.6 a,b (7) |
| MNT | 5.9 ± 0.6b (6) | 105 | 1.9 ± 0.7a,b (7) |
| 5α-DHMNT | 7.9 ± 1.1a,b (5) | 86 | 5.5 ± 4.1a,b (5) |
| MENT | 9.3 ± 0.5a,b (5) | 72 | 3.1 ± 1.0 a,b (3) |
| 5α-DHMENT | 17.6 ± 0.3a,b (3) | 26 | 2.2 ± 1.9 a,b (4) |
Values represent the mean ± SE the number of determinations indicated in parentheses.
The relative binding affinity (RBA) % = IC50 R1881/IC50 test compound X 100.
The RBA for R1881, the reference standard, is arbitrarily defined as 100%.
Means with different letters are significantly different (P<0.05).
Means with different letters are significantly different (P<0.05).
3.2. Androgenic activity of DMA, MNT, and their 5α-reduced derivatives in the castrate immature rat
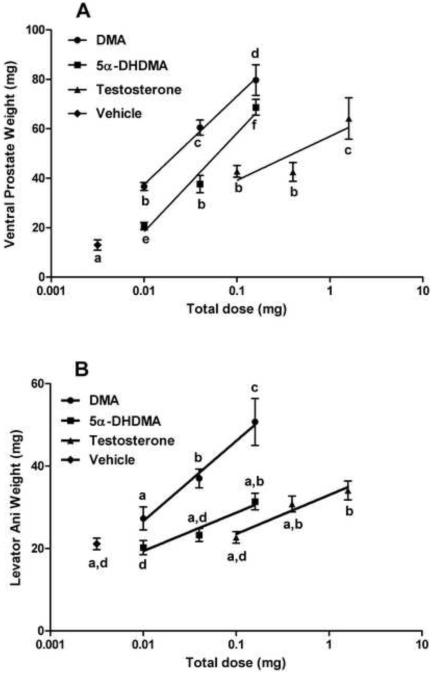
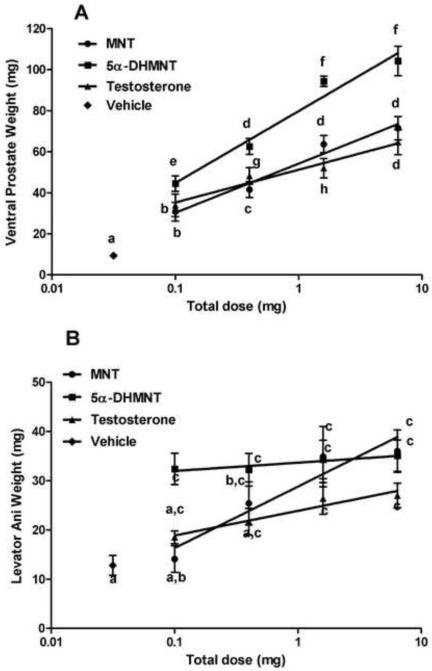
Two androgenic bioassays were performed to compare the relative potencies of DMA and MNT with those of 5α-DHDMA and 5α-DHMNT. T served as the standard in both assays, and all compounds were administered sc. DMA was about 3 times as potent as 5α-DHDMA and at least 9 times as potent as T in stimulating VP weight (Fig. 1a) and had similar relative effects on SV weights (data not shown). Anabolic activity of DMA (stimulation of LA muscle weight) was also greater than that of 5α-DHDMA or T (Fig. 1b). In contrast, MNT was equipotent to T in stimulating VP weight, whereas 5α-DHMNT had 4- to 8-fold greater androgenic activity than MNT or T (Fig. 2a). Relative effects of MNT and 5α-DHMNT on SV weights, in comparison to T, were comparable to the effects on the VP (data not shown). In this assay, MNT, 5α-DHMNT, and T stimulated growth of the LA muscle 2- to 3-fold at the highest dose (6.4 mg/rat, Fig. 2b) compared to vehicle, but only MNT showed a dose-response. For comparison, DHT was 2 to 3 times as potent as T in stimulating VP weights (data not shown).
Fig. 1.
Effect of 5α-DHDMA compared to DMA and T after 7 days in the sc androgenic assay. 5α-DHDMA was less potent than DMA and more potent than T in increasing ventral prostate weight (A). DMA was more potent than 5α-DHDMA and T in increasing levator ani muscle weight (B). Groups with different letters are significantly different (P<0.05).
Fig. 2.
Effect of 5α-DHMNT compared to MNT and T after 7 days in the sc androgenic assay. 5α-DHMNT was more potent than MNT and T in increasing ventral prostate weight (A). MNT, 5α-DHMNT and T increased levator ani muscle weight compared to vehicle, but there was no significant difference among them at the highest doses (B). Groups with different letters are significantly different (P<0.05).
3.3 Effect of dutasteride on androgenic activity of T, DMA, MNT, MENT, and DHT
To evaluate the possible role of 5α-reduction in the androgenic activity of DMA or MNT in vivo, we measured VP and SV weights in castrate immature rats treated with maximally effective doses of these synthetic androgens or T, in the presence or absence of DUT. A preliminary experiment was carried out to determine a dose of DUT which was effective in blocking conversion of T to DHT, but did not result in overt signs of toxicity. Rats were treated sc with 0.23 mg T for 8 days with or without various doses of DUT (see Section 2.2.), or vehicle, and killed approximately 2 h after the final dose for blood collection and determination of accessory sex gland weights. VP and SV weights were significantly decreased by DUT at all doses in T-treated rats. There was a dose-dependent decrease in serum levels of DHT with increasing doses of DUT: serum DHT levels were 125 ± 20, 1,247 ± 106, 606 ± 111, 235 ± 36, and 56 ± 4 pg/ml in groups (n=10 rats/group) treated with vehicle, T alone, T + DUT at 0.1 mg/rat, T + DUT at 1.0 mg/rat, and T + DUT at 10 mg/rat, respectively. The levels of DHT in rats treated with either 1.0 or 10 mg/day DUT were not significantly different (P>0.05) from those of castrate vehicle-treated rats. As DUT at 10 mg/day showed evidence of toxicity, judged by suppression of body weights, the dose of 1.0 mg/day was determined to be optimal for subsequent experiments.
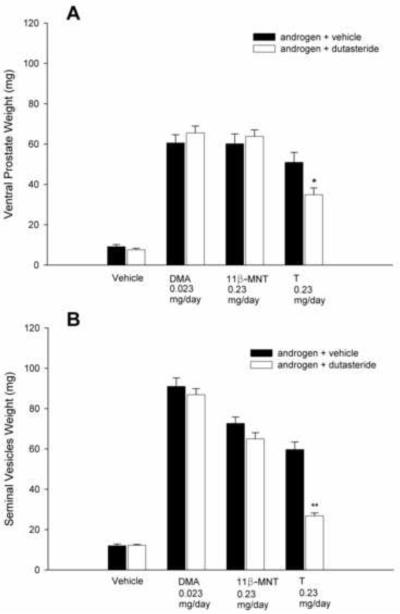
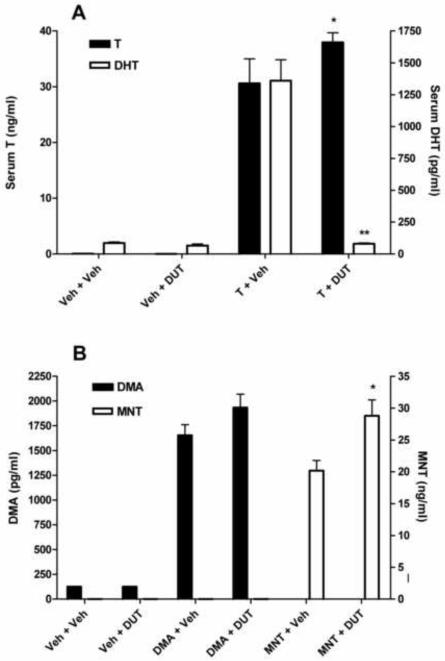
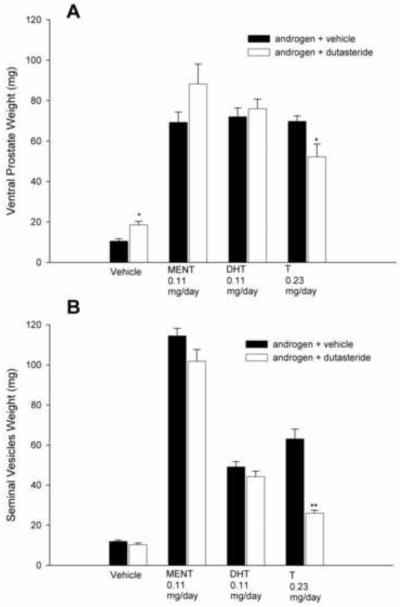
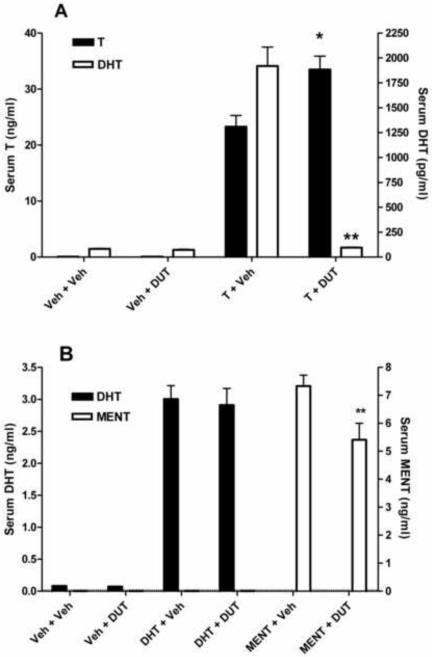
In rats treated with T and DUT, the androgenic activity of T (stimulation of VP and SV weights) was significantly blunted (P<0.05, Fig. 3). Serum levels of T were significantly elevated by DUT treatment, whereas DHT was suppressed to levels observed in castrate vehicle-treated rats (Fig. 4A). As the rats in this study received a maximally effective dose of T and blood was collected for serum androgen measurements 2 h after dosing, pharmacological levels of T and DHT were produced by T treatment alone. In contrast, DUT administered concurrently with DMA or MNT did not affect VP or SV weights (Fig. 3). Serum levels of DMA were not significantly different (P>0.05) in the presence or absence of DUT, but MNT levels were higher (P<0.05) in rats treated with MNT and DUT than in rats treated with MNT and vehicle (Fig. 4B). In a second experiment, rats were treated with T, DHT, or MENT, with or without 1.0 mg DUT/day for 8 days. As expected, VP and SV weights were significantly reduced in T-treated rats (Fig. 5), serum T levels were increased, and serum DHT levels were decreased to baseline (Fig. 6A). Accessory sex gland weights were comparable (P>0.05) in rats treated with DHT or MENT in the presence or absence of DUT (Fig. 5B). Serum DHT levels in DHT + DUT-treated rats were not significantly different (P>0.05) from those in DHT + vehicle-treated rats, whereas circulating MENT was significantly decreased (P<0.05) following DUT treatment (Fig. 6B).
Fig. 3.
Effect of dutasteride on accessory sex gland weights after 8 days in the sc androgenic assay. Dutasteride significantly suppressed T-stimulated ventral prostate (*P<0.05) and seminal vesicle (**P<0.001) weights. There was no significant effect of dutasteride on accessory sex gland weights in rats treated with vehicle, DMA, or MNT.
Fig. 4.
Effect of dutasteride on serum androgen levels in the rats from Fig. 3. (A) In rats treated with T + dutasteride (DUT), there was a significant decrease in serum DHT levels (**P<0.001) and a significant increase in serum T levels (*P<0.05) compared to rats treated with T + vehicle (Veh). (B) There was a slight, but not significant (P=0.12), increase in serum DMA levels in rats treated with DMA + DUT compared to rats treated with DMA + Veh. In rats treated with MNT + DUT, serum levels of MNT were significantly elevated (*P<0.01) compared to rats treated with MNT + Veh.
Fig. 5.
Effect of dutasteride on accessory sex gland weights after 8 days in the sc androgenic assay. As in the experiment shown in Fig. 3, dutasteride significantly suppressed T-stimulated ventral prostate (*P<0.05) (A) and seminal vesicle (**P<0.001) (B) weights. There was no significant effect of dutasteride on accessory sex gland weights in rats treated with MENT or DHT. VP, but not SV, weights were increased in rats treated with vehicle + DUT (*P<0.05).
Fig. 6.
Effect of dutasteride on serum androgen levels in the rats from Fig. 5. (A) In rats treated with T + DUT, there was a significant decrease in serum DHT levels (**P<0.001) and a significant increase in serum T levels (*P<0.01) compared to rats treated with T + vehicle, confirming the results in Fig. 4A. (B) There was no significant difference in serum DHT levels (P=0.78) between rats treated with DHT + DUT or DHT + Veh. In rats treated with MENT + DUT, serum levels of MENT were significantly lower (**P<0.05) than in rats treated with MENT + Veh.
4. Discussion
The two synthetic androgens described here, DMA and MNT (and their 17β-esters, DMAU and MNTDC), are in development for hormonal therapy in men as alternatives to T. Both are orally active and equipotent to, or more potent than, T when administered sc as long-acting injectibles. The 17β-esters of the synthetic androgens demonstrated prolonged duration of androgenic action: after a single sc injection (1.2 mg/rat) of DMAU or MNTDC to castrate 22 day-old rats, VP and SV growth was maintained above castrate levels for about 21 and 14 weeks, respectively [5 and unpublished observations]. In castrate adult rats which received a single sc injection of DMAU or MNTDC (12 mg/kg), circulating LH was suppressed for at least 18 and 10 weeks, respectively, corresponding to sustained serum levels of DMA or MNT [5 and unpublished observations]. Oral administration of DMAU or MNTDC was also effective in reducing LH secretion with daily dosing, and serum androgen levels remained elevated for the duration of dosing. Other desirable properties for an androgen targeted for hormonal therapy in men include: beneficial effects on bone mineral density (BMD) and body composition (increased muscle mass and decreased fat mass); minimal or no hepatotoxicity; lack of enhancement of alopecia, acne, and prostate hyperplasia. In the first case, we have recently demonstrated that DMAU and MNTDC maintain BMD, increase muscle mass, and decrease fat mass in castrate adult male rats injected sc with these androgens over a 4-month period [9].
By analogy to the metabolism of T, DMA and MNT could be converted to 5α-DHDMA and 5α-DHMNT, respectively, by 5α-reductase, or to their corresponding aromatic A-ring estrogenic derivatives by aromatase. We showed previously that DMA and MNT were not aromatized to 7α,11β-dimethylestradiol and 11β-methylestradiol, respectively, by recombinant human aromatase in vitro under conditions which allowed complete conversion of T to estradiol [13]. It was important to know also whether DMA and MNT undergo 5α-reduction in vivo as the 5α-reduced metabolites of these synthetic androgens might have undesirable effects similar to those of DHT. Because there is no assay currently available to measure these potential metabolites directly in serum, we assessed the effect of blocking 5α-reductase activity on the androgenic potency of DMA and MNT. The reduced ability of exogenous T in the presence of a 5α-reductase inhibitor to stimulate VP and SV growth demonstrates the importance of DHT in prostatic androgen signaling in the intact animal or in castrate animals treated with T [14-16]. Likewise, blockade of 5α-reductase might affect the androgenic potency of DMA and/or MNT. Clearly, 5α-DHDMA and 5α-DHMNT, obtained by chemical synthesis, are potent androgens: compared with the sc reference standard T, the sc androgenic potency ratios (based on VP weights) for DMA and 5α-DHDMA were approximately 9 and 3, respectively, and for MNT and 5α-DHMNT, approximately 1 and 4 to 8, respectively. For comparison, DHT was 2 to 3 times as potent as T in this assay. However, as 5α-DHDMA is less potent than DMA, inhibiting its formation by DUT might have resulted in increased weights of the VP and SV. On the other hand, blocking formation by DUT of 5α-DHMNT, which like DHT, is more potent than the parent compound, might have led to decreased VP and SV weights. As demonstrated here, weights of these accessory sex glands were significantly greater in rats treated with T and vehicle than in rats treated with T and DUT. However, stimulation of VP and SV growth by DMA and MNT, as well as by MENT, was similar in the presence or absence of DUT, implying the synthetic androgens do not need to undergo 5α-reduction to manifest full androgenic potency. Sundaram et al. [1] reported previously that MENT was not 5α-reduced by rat liver or prostate homogenates, an observation attributed to steric hindrance from the 7α-methyl group. Likewise, the presence of a 7α-methyl group in DMA may affect its ability to be 5α-reduced in vivo.
In rats treated with T + DUT, serum levels of DHT were reduced to those of castrate rats, and serum levels of T were significantly elevated, indicating the efficacy of the DUT treatment in blocking 5α-reductase activity. In rats which received MNT + DUT, serum MNT was significantly higher than in rats treated with MNT + vehicle despite the lack of an effect of MNT on accessory sex gland weights. This result suggests that metabolism of MNT was inhibited by DUT, but clearly the blockade of 5α-reductase did not result in reduced androgenic activity. In contrast, there was no significant difference in serum DMA levels in the presence or absence of DUT in DMA-treated rats. Rats treated with MENT and DUT had significantly lower levels of MENT than rats treated with MENT and vehicle. In this case, inhibiting 5α-reductase activity may have allowed metabolism of MENT by other pathways. Further investigation of these alternatives will depend on development of methods for identification and quantification of the various metabolites of the synthetic androgens.
Whereas the data from the in vitro analyses indicate that DMA, MNT, and MENT and their 5α-reduced derivatives bind with high affinity to the ARLBD and are efficacious in stimulating transcription mediated by AR in CV-1 cells, they do not predict the relative potencies of these androgens in vivo. The pathways by which these synthetic androgens are metabolized are largely unknown, and differences between the in vitro and in vivo results may be due to differences in metabolism, both in cells and in the intact animals, as well as to other factors (e.g. serum binding) which occur in vivo, but not in vitro. For example, DMA was more potent in the androgenic assay (Fig. 1) than 5α-DHDMA and slightly more potent in the transactivation assay (Table 1), but both bound equally well to the ARLBD. On the other hand, 5α-DHMNT was more potent than MNT in the androgenic assay (Fig. 2) but slightly less potent in the transactivation and ARLBD binding assays. DHT was more potent than T in the androgenic assay, in agreement with the results of others [see 17], and showed a slightly, but not significantly, higher affinity for the ARLBD than T. DHT has been reported to bind to AR in rat ventral prostate cytosol with higher affinity than T [18], but we are not aware that binding of these androgens to purified recombinant AR has been examined by others.
In conclusion, the present results confirm that DMA/DMAU and MNT/MNTDC are promising candidates for hormonal therapy and/or contraception in men. They do not need to undergo 5α-reduction in vivo to exert their full androgenic effects, which may be advantageous given the potential undesirable effects of DHT on prostatic epithelial cell turnover, prostate gland growth, and other endpoints. Clinically, these synthetic androgen esters could be developed either for oral administration or as long-acting injectibles. Pursuit of DMAU and MNTDC for clinical applications has been bolstered by favorable results from safety studies recently performed in adult male cynomolgus monkeys and rats (unpublished observations).
Acknowledgments
We would like to thank Dr. Richard Blye, formerly of the Contraception and Reproductive Health Branch of NICHD, for his input into the design of these studies and for his continued support and Dr. Narender Kumar of The Population Council for assaying selected serum samples for MENT. This work was supported by contract NIH NO1-HD-2-3338 awarded to BIOQUAL, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A preliminary version of this work was presented at the 89th Annual Meeting of the Endocrine Society, June 2007.
References
- 1.Sundaram K, Kumar N, Monder C, Bardin CW. Different patterns of metabolism determine the relative anabolic activity of 19-norandrogens. J. Steroid Biochem. Mol. Biol. 1995;53:253–257. doi: 10.1016/0960-0760(95)00056-6. [DOI] [PubMed] [Google Scholar]
- 2.Byrne MM, Nieschlag E. Testosterone replacement therapy in male hypogonadism. J Endocrinol. Invest. 2003;26:481–489. doi: 10.1007/BF03345206. [DOI] [PubMed] [Google Scholar]
- 3.Oettel M. The endocrine pharmacology of testosterone therapy in men. Naturwissenschaften. 2004;91:66–76. doi: 10.1007/s00114-003-0494-4. [DOI] [PubMed] [Google Scholar]
- 4.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, Amarneh B, Ito Y, Fisher CR, Michael MD, Mendelson CR, Bulun SE. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrine Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 5.Attardi BJ, Hild SA, Reel JR. Dimethandrolone undecanoate: a new potent orally active androgen with progestational activity. Endocrinology. 2006;14:3016–3026. doi: 10.1210/en.2005-1524. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal AK, Monder C. In vitro metabolism of 7α-methyl-19-nortestosterone by rat liver, prostate and epididymis. Endocrinology. 1988;123:2187–2193. doi: 10.1210/endo-123-5-2187. [DOI] [PubMed] [Google Scholar]
- 7.Kumar N, Didolkar AK, Monder C, Bardin CW, Sundaram K. The biological activity of 7α-methyl-19-nortestosterone is not amplified in male reproductive tract as is that of testosterone. Endocrinology. 1992;130:3677–3683. doi: 10.1210/endo.130.6.1597164. [DOI] [PubMed] [Google Scholar]
- 8.Hershberger LG, Shipley EG, Meyer RK. Myotrophic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method. Proc. Soc. Exp. Biol. Med. 1953;83:175–180. doi: 10.3181/00379727-83-20301. [DOI] [PubMed] [Google Scholar]
- 9.Attardi B, Marck BT, Matsumoto AM, Koduri S, Hild SA. Long-term effects of dimethandrolone 17β-undecanoate (DMAU) and 11β-methyl-19-nortestosterone 17β-dodecylcarbonate (11β-MNTDC) on body composition, bone mineral density, serum gonadotropins, and androgenic/anabolic activity in castrate male rats. doi: 10.2164/jandrol.110.010371. submitted. [DOI] [PubMed] [Google Scholar]
- 10.Kumar N, Didolkar AK, Ladd A, Thau R, Monder C, Bardin CW, Sundaram K. Radioimmunoassay of 7α-methyl-19-nortestosterone and investigation of its pharmacokinetics in animals. J. Steroid Biochem. 1990;37:587–592. doi: 10.1016/0960-0760(90)90405-a. [DOI] [PubMed] [Google Scholar]
- 11.Attardi BJ, Burgenson J, Hild SA, Reel JR, Blye RP. CDB-4124 and its putative monodemethylated metabolite, CDB-4453, are potent antiprogestins with reduced antiglucocorticoid activity: in vitro comparison to mifepristone and CDB-2914. Mol. Cell. Endocrinol. 2002;188:111–123. doi: 10.1016/s0303-7207(01)00743-2. [DOI] [PubMed] [Google Scholar]
- 12.Raub WF. The PROPHET system and resource sharing. Fed. Proc. 1974;33:2390–2392. [PubMed] [Google Scholar]
- 13.Attardi BJ, Pham TC, Radler LC, Burgenson J, Hild SA, Reel JR. Dimethandrolone (7alpha, 11beta-dimethyl-19-nortestosterone and 11beta-methyl-19-nortestosterone are not converted to aromatic A-ring products in the presence of recombinant human aromatase. J. Steroid Biochem. Mol. Biol. 2008;110:214–222. doi: 10.1016/j.jsbmb.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, Russell D, Tindall D. Dihydrotestosterone and the prostate: The scientific rationale for 5α-reductase inhibitors in the treatment of benign prostatic hyperplasia. J. Urology. 2004;172:1399–1403. doi: 10.1097/01.ju.0000139539.94828.29. [DOI] [PubMed] [Google Scholar]
- 15.Bramson HN, Hermann D, Batchelor KW, Lee FW, James MK, Frye SV. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J. Pharmacol. Exp. Ther. 1997;282:1496–1452. [PubMed] [Google Scholar]
- 16.Carson C, III, Rittmaster R R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61:2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 17.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of the androgen receptor. Chem. Rev. 2005;105:3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Christiansen RG, Bell MR, D’Ambra TE, Mallamo JP, Herrmann JL, Ackerman JH, Opalka CJ, Kullnig RK, Winneker RC, Snyder BW, Batzold FH, Schane HP. Antiandrogenic steroidal sulfonylpyrazoles. J. Med. Chem. 1990;33:2094–2100. doi: 10.1021/jm00170a008. [DOI] [PubMed] [Google Scholar]