Abstract
Previous research has indicated that the amygdala is a critical neural substrate of the emotional modulation of attention. However, a recent case-study suggests that the amygdala may not be essential for all types of emotion-attention interactions. In order to test this hypothesis, we assessed the visual-search performance of patients with unilateral amygdala lesions, matched controls, and medication-matched epilepsy patients with intact amygdalae. All participants completed a visual-search task consisting of trials in which (1) an emotional target was embedded amongst neutral distractors, (2) a neutral target was embedded amongst emotional distractors, or (3) a neutral target was embedded amongst neutral distractors. All participant groups, including those with amygdala lesions, detected emotional targets more efficiently than neutral targets. These data indicate that the amygdala is not necessary for emotion-guided visual search and suggest that other mechanisms beyond the amygdala help guide attention toward threatening stimuli.
Introduction
A wealth of data supports the principal link between the amygdala and the processing of emotionally significant stimuli (Bishop, 2008; Zald, 2003). Although the literature on the role of the human amygdala in emotional processing has grown considerably in the last decade, most of the research involves functional neuroimaging, which is by nature correlative. While such studies are invaluable in many respects, additional methods are needed to determine if the amygdala is merely activated during emotional perception, or if this structure is necessary for the emotional modulation of attention to occur.
An empirical task that is often thought to reflect the amygdala’s processing of emotional stimuli involves the enhanced detection of threat stimuli during visual search. As the ability to locate threats quickly is fundamentally useful, the idea that mammals possess an evolved neural system to detect such threats is both intuitive and appealing. Indeed, there is evidence that when searching visual arrays, humans detect some fear-related content faster than competing neutral stimuli (Ohman, Flykt, & Esteves, 2001). A proposed neuroanatomical model for the effect exists: a presumed sub- (and pre-) cortical system, which sacrifices accuracy to gain speed, relays basic visual information directly from the pulvinar to the amygdala (thus bypassing the slower cortical processing of the occipital and ventral temporal visual processing stream), leading to amygdala activation (Morris, Ohman, & Dolan, 1999). The activated amygdala then signals to the system controlling spatial attention that something particularly important - a threat - needs to be located, and ‘attended’ to, in both meanings of the phrase (Ohman, Carlsson, Lundqvist, & Ingvar, 2007).
Recently, Tsuchiya and colleagues (2009) tested the ability to detect fearful faces in a patient with bilateral amygdala lesions caused by Urbach-Wiethe disease. They showed that despite impaired explicit recognition of fear, the search for fearful faces was enhanced in the patient in a manner similar to that seen in control participants. They conclude that the amygdala is not necessary for rapid detection of fearful faces. Although this finding is intriguing, the study has two major limitations. First, the study used emotional faces, whereas the primary literature on emotional enhancement of visual search uses evolutionarily significant stimuli with threatening characteristics such as snakes and spiders. Additionally, the study represents a single case-study, with a participant who has Urbach-Wiethe disease, a hereditary neurodegenerative disorder that can cause functional impairments that extend beyond those typically ascribed to amygdalar function (Thornton, et al., 2008). Given the uncertainty regarding the full extent of brain damage as well as the rarity of this patient population, the generalizability of this study’s conclusions remain unclear.
Here, we present data obtained with a different visual search paradigm, from two groups of patients with unilateral amygdala lesions. We studied a large sample of patients who had undergone resections of the amygdala due to intractable epilepsy. The study also included two samples of control participants, a matched healthy control sample, and a control sample using anti-convulsant medication at the time of study. We implemented a speeded visual search task based directly on the Ohman et al. (2001) paradigm in which participants located a target which is either emotionally neutral or related to fear (spiders), in a matrix of images. Based on the hypothesis that the amygdala plays a pivotal role in the preattentive direction of visual search for fear described above, it would be predicted that the search for fear-related targets would be enhanced in our control populations, but that such enhancement would be absent in patients with amygdala resections. In contrast, if the results of the Tsuchiya et al. case study are generalizable to other amygdala patients and other stimulus sets, patients with amygdala lesions should show a normal pattern of enhanced visual search for the emotional stimuli.
Methods
Participants
Participants in the study belonged to four groups: patients with amygdala lesions, subdivided into a group with right amygdala lesions (‘right resection’, n=16) and left amygdala lesions (‘left resection’, n=8), a control group of patients using anti-convulsant medication to treat epilepsy (‘medicated controls’, n=7), and a control group of healthy participants matched for age, education, and sex (‘healthy controls’, n=16). See Table 1 for patient demographics. Patients were recruited from the Vanderbilt University Medical Center Epilepsy Surgery Program. We selected patients who underwent surgery which involved the selective resection of the amygdala and anterior parts of the hippocampus, or a partial resection of the temporal lobe, including the amygdala (Figure 1). Patients with lesions outside the anterior temporal lobe, psychiatric conditions or neurological conditions other than epilepsy, and with general cognitive impairment (IQ<80) were excluded from the study. Medicated controls were actively taking either Keppra (Levetiracetam) and/or Lamictal (Lamotrigine) at the time of the study. We focused on these two medications as they were the primary maintenance medications being taken by our post-surgical patients. Whenever possible, IQ was taken from the patients medical record; if unavailable, patients completed the Wechsler Adult Intelligent Scale (WAIS-III). IQ was estimated for control participants with the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). All participants gave informed consent to take part in the study. The study was approved by the Vanderbilt University Institutional Review Board.
Table 1.
Participant demographics and lesion volumes.
| Group | Sex (m/f) | Age | Education in Years | IQ | Left Amygdala Volume | Right Amygdala Volume | ||
|---|---|---|---|---|---|---|---|---|
| Mean (SE) (mm3) | Range (mm3) | Mean (SE) (mm3) | Range (mm3) | |||||
| Healthy Controls | 6/10 | 36.8 | 14.5 | 119 | - | - | - | - |
| Medicated Controls | 5/2 | 36.7 | 14.8 | 111 | - | - | - | - |
| Right Resection | 3/12 | 41.8 | 14.6 | 101 | 1637.5 (48.9) | 1345-1955 | 302.9 (111.6) | 0-1281 |
| Left Resection | 5/3 | 34.8 | 13.4 | 94 | 299.5 (151.5) | 0-1121 | 1490.5 (62.9) | 1152-1649 |
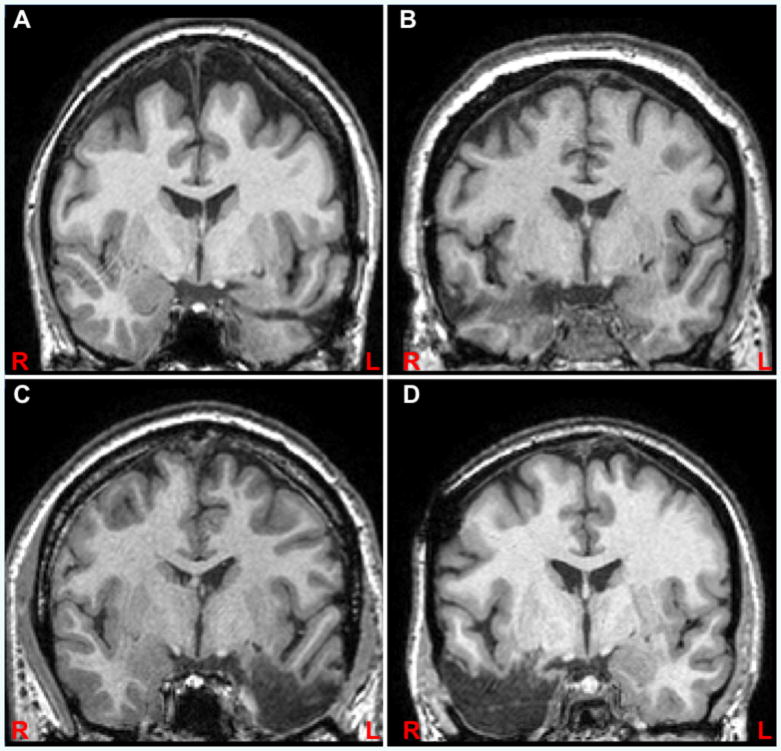
Figure 1.
Examples of participants with left (A) and right (B) selective amygdalo-hippocampal resection and left (C) and right (D) temporal lobe resection shown at y=-4 (MNI) in radiological convention.
Amygdala Volumetric Analysis
High-resolution T1-weighted images (TR=8.969ms; TE=4.6ms; inplane resolution=1mm2; slice thickness=1mm) were acquired on a 3T Philips scanner and used to determine the remaining amygdala volumes in the resection groups (Table 1, Figure 2). The T1-weighted structural image for one participant was acquired on a 1.5T Philips scanner (in-plane resolution=1mm2; slice thickness=1.2mm) because of contra-indications for higher field scanning. Images were normalized to MNI space at 1mm3 resolution using the unified segmentation and normalization procedure in SPM5 (Ashburner & Friston, 2005) as this method has been shown to outperform other techniques for normalizing lesioned brains (Crinion, et al., 2007). Amygdala volumes were traced on the normalized image of each resection participant using FSLView (http://www.fmrib.ox.ac.uk/fsl/fslview/index.html) based on criteria modified from Pruessner et al. (2000) and Honeycutt et al. (1998). The superior border was identified in the coronal plane using a line drawn between the superolateral aspect of the optic tract and the fundus of the circular sulcus of the insula. The posterior, lateral and medial boundaries of the amygdala were defined in the axial plane with reference to the coronal and sagittal planes as necessary. The alveus of the hippocampus (excluding the alveus itself) was used as the posterior border. The lateral boundary was defined as 1mm from the most medial adjacent white matter. In superior slices of the amygdala, the medial border was defined as 1mm from the ambient cistern and the white matter separating the amygdala from the entorhinal cortex served as the medial border in slices inferior to the level of the uncus. The anterior boundary of the amygdala was defined as 1mm from the subarachnoid space in the axial plane or the coronal slice just posterior to the anterior commissure. If the above boundaries were not identifiable on the resected side of an image, voxels in the amygdala were marked with reference to the corresponding non-resected slice excluding one layer of voxels from the resected area in any plane.
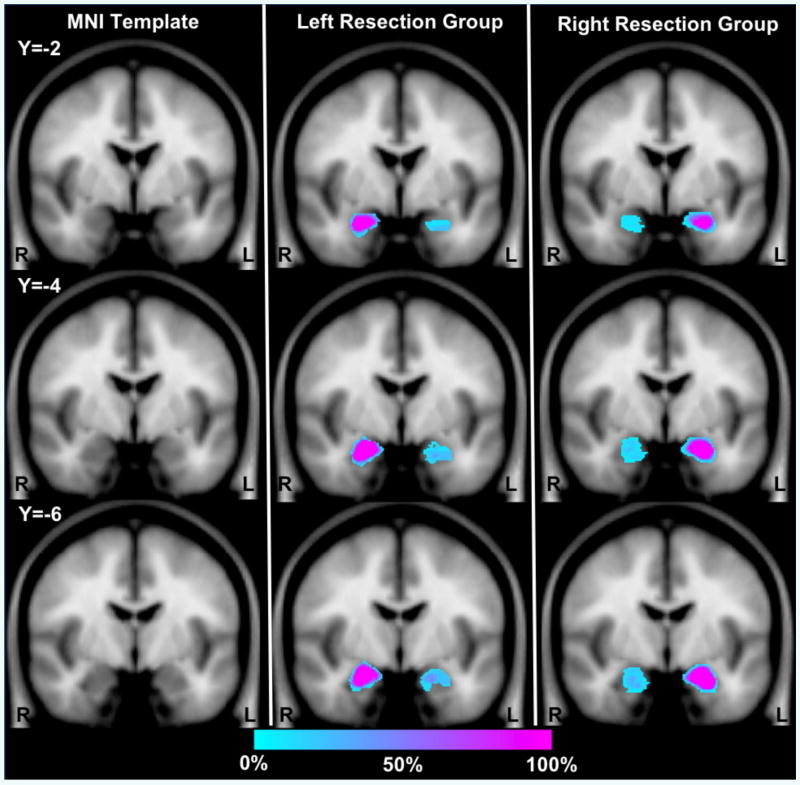
Figure 2.
Amygdala overlap maps in resection groups displayed on three coronal slices from SPM5 MNI template brain. The color of each voxel indicates the percentage of subjects within each group that have a voxel identified as part of the amygdala. Within Blue indicates areas in which few participants have overlapping voxels; Pink indicates areas in which many participants have overlapping voxels. Note the area around the edge of the amygdala in the intact hemisphere shows less than 100% because of individual differences in the boundaries of the amygdala within stereotactic space. In the resected hemisphere, damage to the amygdala is always present but the amount of damage and precise location varies between subjects, leading to the identification of some participants with labeled voxels throughout different regions of the amygdala.
Visual Search Task
Visual search performance was assessed with a task similar to the one described by Ohman and colleagues (2001). During each trial of the task, participants saw a matrix of pictures on a computer screen. The majority of the pictures belonged to one category, and had the function of distractors, while one, the target, belonged to another category. Participants sought out the deviant target, and were asked to press a key as soon as they found it. To confirm a successful search, participants then had to indicate the target’s location within the matrix. The pictures belonged to three categories, spiders (fear), mushrooms (neutral), and sprinklers (neutral). They were combined to produce three experimental conditions: (1) a neutral target among neutral distractors, (e.g., a mushroom among sprinklers - neutral-among-neutrals condition); (2) a neutral target among fear-related distractors, (e.g., a mushroom among spiders - neutral-among-threat condition); and (3) a fear-related target among neutral distractors, (e.g., a spider among sprinklers - threat-among-neutrals). We note that the original Ohman et al. (2001) paradigm consisted only of spiders and mushrooms. However, with only two types of stimuli, it is not possible to determine whether performance in the threat-among-neutrals condition is faster than the neutral-among-threat condition because of enhanced detection of fear in the threat-among neutral-condition, or greater distraction by fear stimuli in the neutral-among-threat condition. By including a third neutral-among-neutral condition (i.e., a neutral baseline condition), it is possible to distinguish between these two possibilities. We selected sprinklers because they are generally perceived as affectively neutral, but share some physical characteristics with spiders (in that they have leg like features). Search difficulty was manipulated by varying the size of the array, which could comprise 4, 9, or 16 pictures. The combination of picture categories and positions were fully counterbalanced. The measure of interest was response time (RT) on correct trials. For each participant, we excluded RTs more than three standard deviations longer than their mean. Additionally, we excluded one participant (a patient with a right amygdala lesion), whose overall RT was more than two standard deviations above the mean (mean RT of over 5s). A post-hoc inclusion of that participant produced results identical to the reported ones in all significant respects.
Results
The visual search performance of the four groups is depicted in Figure 3. The fear target condition had an advantage over the two control conditions for trials with the large matrix of 16 pictures. Most strikingly, this emotional modulation of search performance occurred across all participant groups. Patients with resected amygdalae in either hemisphere still showed an advantage for threat targets.
Figure 3.
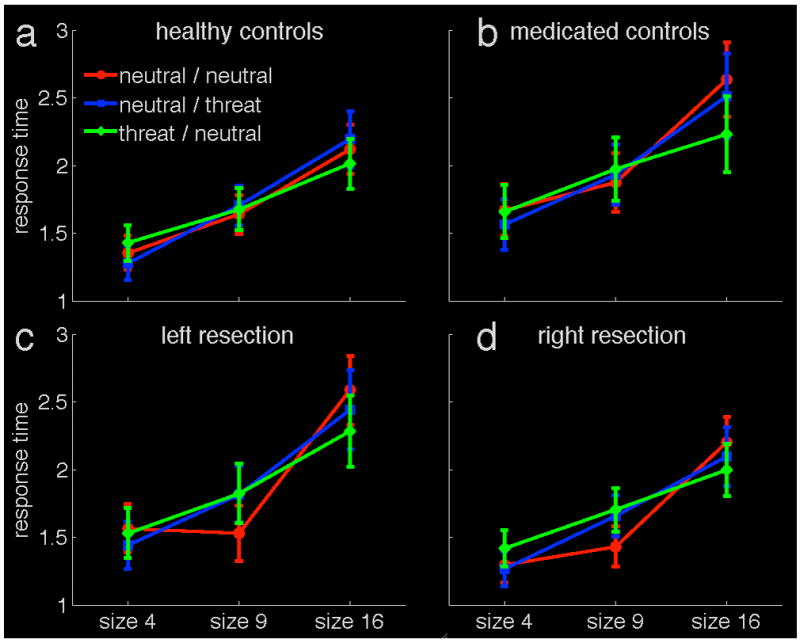
Visual search task performance: in trials with 16 images (‘size 16’), threat-related targets are detected more quickly than other targets. Both control groups and all patients show this pattern. The panels show the four experimental groups: healthy control participants (a), patients using anticonvulsive medication (b), patients with left amygdala resections (c) and patients with right amygdala resections (d). The y-axis displays mean response time on correct trials, in seconds. The varying size of the search image display is shown on the x-axis. The three experimental conditions are: neutral / neutral - neutral targets among neutral distractors; neutral / threat - neutral targets among threat-related distractors; threat / neutral - threat-related targets among neutral distractors. Error bars represent one standard error of the mean.
These data were analyzed using a mixed effects ANOVA, with three factors: Group, Condition, and Size. The between-subjects factor Group had four levels (healthy matched controls, medicated controls, patients with a left amygdala resection, patients with a right amygdala resection). The within-subjects factors were Condition (neutral-among-neutrals, neutral-among-threat, threat-among-neutrals), and Matrix Size (4, 9, or 16 pictures). The analysis demonstrated a significant within subjects effect of Matrix Size (F(2,41)=265.9, p<.0005). In contrast, the analysis produced no main effect of Group (F(3,42)=0.632, p=.598), and the Group factor interacted only with the combination of Condition and Size (F(12,123)=2.24, p=.023).
To understand the observed interaction, we conducted separate tests for each matrix size. Response times differed significantly between conditions for all three matrix sizes (all ps<.0005). In particular, when the matrix consisted of four items, responses in the neutral-among-threat condition were fastest (ps=<.003). For a nine item matrix, the neutral-among-neutrals condition was fastest (ps=<.001). For a matrix with 16 items, the threat-among-neutrals condition was fastest (ps<.0005). This pattern of data indicates that the advantage for fear target searches is present only at the largest matrix size in our data set. We observed no main effects of Group at any matrix size. For Matrix Size 9, there was a trend for an interaction between Group and Condition (F(6,84)=2.4, p=.055), reflecting somewhat faster detection of neutral-among-neutral stimuli relative to other conditions in the resected patients. These results do not lend support to a different pattern of responding to threat for the patient and control groups.
To further explore the performance of patients, we conducted an analysis of the target search slope for each experimental group. The slope is a common measure of efficiency in visual search tasks. It conveys how much additional time is required for a search per additional item with increasing cluster size, and is expressed in milliseconds per item. The mean slope values for each group and condition are illustrated in Figure 4. As expected, the fear target condition showed the flattest slopes for all participants groups (i.e., response times in this condition were less influenced by increases in the matrix size than were response times in the other conditions). Critically, this difference in search slope functions occurred for all participant groups. A mixed-effects ANOVA, with the factors Group and Condition, showed no main effect of Group (F(3,42)=.675, p=.572), nor did the Group factor interact with Condition (F(6,84)=1.059, p=.394). There was a main effect of Condition (F(2,41)=26.3, p<.0005). Planned comparisons showed that the fear target condition was characterized by a flatter slope than either of the control conditions (ps<.0005). This means that the cost of increasing matrix size was smaller for the threat targets than for other targets.
Figure 4.
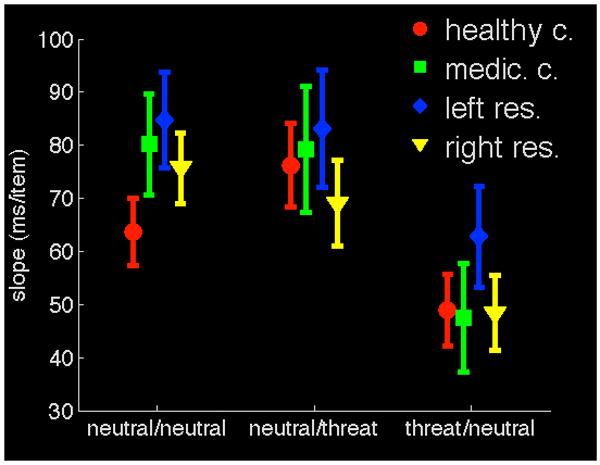
Search slopes are flatter for threat-related targets, and this is true for both control groups and all patients. The search slope is a measure of search performance and conveys how much additional time is needed for each additional image as the image display grows. The y-axis represents time needed per additional display item in milliseconds. The three experimental conditions are on the x-axis: neutral / neutral - neutral targets among neutral distractors; neutral / threat - neutral targets among threat-related distractors; threat / neutral - threat-related targets among neutral distractors. Error bars represent one standard error of the mean.
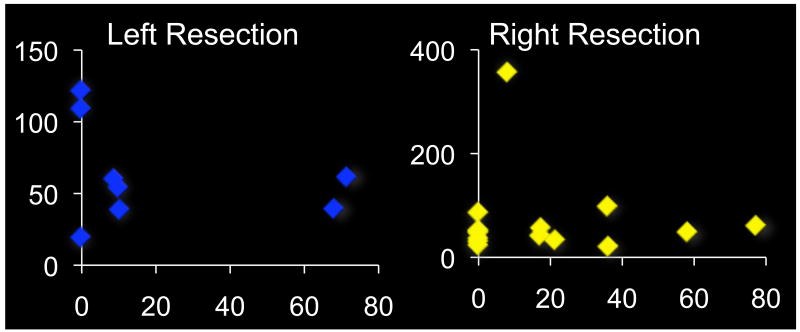
As is often the case with selective temporal resections, the amygdalae were not completely removed in most subjects, with an average of 82.4 % volume loss in the right amygdala (range = 22.7-100%), or 78.9% volume loss in the left amygdala (range = 28.6-100%). While the functional status of this residual tissue is not clear (as many of the inputs and outputs will have been severed during the resection of neighboring tissue), it is possible that some subjects have enough residual tissue to still contribute to an emotional modulation of attention. To test this possibility, we analyzed whether search slopes in the threat target among neutral distractors condition correlated with the degree of amygdala tissue remaining in the resected temporal lobe for each post-surgery group. The residual amygdala volume was not correlated with visual search performance in either patient group (R2 left resection group = 0.08, p=.505; R2 right resection group = 0.003, p =.852, Figure 5), making it unlikely that the preservation of visual search ability for threat is related to the spared portions of the amygdala in subjects with smaller lesions.
Figure 5.
Search slopes for the threat-among-neutrals condition in relation to lesion volume. The x-axes display the slopes in milliseconds per item, while the y-axes display what percentage of the amygdala volume was retained after resections. Panel a) shows patients with lesions of the left amygdala, panel b) of the right amygdala. Note that the x-axis scales are different due to an outlier in the right lesion group. Search slopes for threat targets did not correlate with completeness of amygdala lesions.
Discussion
We investigated the role of the amygdala in the enhancement of visual search for fear-related objects by comparing the performance of healthy matched controls and medicated controls with that of patients with amygdala lesions in either the right or left hemisphere. In accordance with past results, fear-related search showed some advantages over other conditions. This effect is shown by substantially quicker responses for fear-related targets in the large matrix size, and a flatter target search slope for detecting fear relative to neutral stimuli. Contrary to the hypothesis that the amygdala plays a critical role in the enhanced detection of fear stimuli, this advantage was present both for controls and for patients with lesions of either the left or the right amygdala. There is no statistical indication that the advantage is in any way compromised in patients. These data are striking given the frequent assumption that the amygdala is a key structure for a range of interactions between emotion and cognition. Our data speak to the notion that, at least in the case of visual search, the amygdala is not necessary for such interactions to occur. As such, the amygdala does not appear to be a desideratum for the emotional modulation of attention, and its influence on attention may be far more task-specific than has been previously appreciated.
A critical limitation of the current study is that patients with unilateral lesions might be able to rely on their contralateral intact amygdala to perform the task. While this is possible, there is ample support from other lesion studies that functions supported by the amygdala are robustly affected by unilateral lesions. For example, Anderson and Phelps (2001) documented emotional enhancement deficits in left amygdala lesion patients that mirrored that of a patient with bilateral lesions. Another study investigating emotional modulation of visual cortex activation showed that unilateral damage of the amygdala led to ipsilateral deficits which were proportional to the lesioned volume (Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004). Other studies examining startle and autonomic responses similarly indicate that unilateral medial temporal lobe lesions that include the amygdala can profoundly alter responding to emotional stimuli (Adolphs & Tranel, 2004; Buchanan, Tranel, & Adolphs, 2004; Davidson, Fedio, Smith, Aureille, & Martin, 1992; Weike, et al., 2005). Thus, unilateral lesions can lead to deficits which are not compensated by the contralateral structure. When taken together with the case study of Tsuchiya et al. (2009), who showed no impairment in visual search for fearful faces in their patient with bilateral amygdala lesions, it seems unlikely that the present results are simply due to the preserved functioning of the unlesioned amygdala. Rather, these data suggest that the amygdala does not play a critical role in guiding visual search. As such, an as yet undetermined, non-amygdala dependent mechanism appears to be involved in the guidance of attention towards threat stimuli. With normal enhanced detection for spiders (the present study), and fear faces (Tsuchiya, et al., 2009) the preservation of this function appears to be fairly generalizable as it occurs both for facial emotion and emotion-inducing objects.
The present result does not rule out the possibility that the amygdala is essential for other situations in which emotion guides attention, as suggested by other paradigms (Anderson & Phelps, 2001). However, as visual search is an exemplar paradigm for studying spatial attention, the lack of impact of amygdalar damage on this paradigm is striking. When considered anatomically, this may not be completely surprising. The amygdala has only relatively restricted projections to parietal cortex (Amaral & Price, 1984), which is known to be critical for efficient visual search (Corbetta & Shulman, 2002). The far denser projections from the amygdala to the ventral visual stream than to the dorsal visual stream suggests that tasks with high spatial demands, such as visual search, should in fact be far less vulnerable to amygdalar influences than tasks with high object-processing demands. Thus utilization of tasks that rely on visual discrimination could potentially be more sensitive to amygdalar influence than tasks such as visual search that rely on the spatial guidance of attention.
The current data also provide little evidence for the lateralization of amygdalar influences on visual search for emotionally valenced targets. Ohrmann and colleagues (2007) have proposed a greater involvement of the right than left amygdala in visual search for fear stimuli. This conclusion was based on a correlation between the magnitude of right amygdalar blood oxygen level dependent (BOLD) fMRI response to backwards masked fearful faces and detection speed for negative faces in a visual search task. While such a correlation may exist, the fact that in the current study patients with right amygdala lesions show a normal enhancement for detecting threat stimuli suggests that this correlation is not directly driven by the right amygdala. Indeed, it is worth noting that the effect observed by Ohrmann et al. was not stimulus-specific as the response to masked fearful faces additionally correlated with the detection of neutral faces among neutral faces. Additionally, there was no evidence of an overall deficit in search speed arising from right amygdala lesions. If anything, the patients with right amygdala lesions appeared slightly faster than healthy controls and patients with intact medial temporal lobes who were taking anticonvulsants.
A critical question arises from the present results. If the amygdala is not necessary for the preferential localization of threat stimuli, what mechanism is responsible for the enhanced detection of these stimuli when they appear in relatively large matrices? Bar and colleagues (Bar, et al., 2006) have recently proposed the orbitofrontal cortex might play such a role, although direct evidence for this hypothesis remains limited. It is additionally possible that the visual features that help facilitate detection trigger preattentive processes even prior to their projection to the amygdala or orbitofrontal cortex. Such preattentive mechanisms could lead to rapid automatic amygdala activations (Luo, et al., 2010; Pourtois, Spinelli, Seeck, & Vuilleumier, 2010), even if the amygdala itself is not necessary for an initial biasing of spatial attention, and regardless of whether later less automatic activations in the amygdala play a role in more sustained emotional guidance of attention. As such, the present data appear to highlight the classic problem when interpreting correlative data - correlation does not indicate causation.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institutes of Health 5R01MH74567-4 and the Manitoba Health Research Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D. Impaired judgments of sadness but not happiness following bilateral amygdala damage. Journal of Cognitive Neuroscience. 2004;16(3):453–462. doi: 10.1162/089892904322926782. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) Journal of Comparative Neurology. 1984;230(4):465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam K, Ghuman A, Boshyan J, Schmid A, Dale A, et al. Top-down facilitation of visual recognition. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(2):449. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences. 2008;1129:141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Buchanan T, Tranel D, Adolphs R. Anteromedial temporal lobe damage blocks startle modulation by fear and disgust. Behavioral Neuroscience. 2004;118(2):429–437. doi: 10.1037/0735-7044.118.2.429. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crinion J, Ashburner J, Leff A, Brett M, Price C, Friston K. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage. 2007;37(3):866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R, Fedio P, Smith B, Aureille E, Martin A. Lateralized mediation of arousal and habituation: differential bilateral electrodermal activity in unilateral temporal lobectomy patients. Neuropsychologia. 1992;30(12):1053–1063. doi: 10.1016/0028-3932(92)90098-7. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Smith PD, Aylward E, Li Q, Chan M, Barta PE, et al. Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Research: Neuroimaging. 1998;83(2):85–94. doi: 10.1016/s0925-4927(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Luo Q, Holroyd T, Majestic C, Cheng X, Schechter J, Blair R. Emotional Automaticity Is a Matter of Timing. Journal of Neuroscience. 2010;30(17):5825. doi: 10.1523/JNEUROSCI.BC-5668-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman A, Carlsson K, Lundqvist D, Ingvar M. On the unconscious subcortical origin of human fear. Physiology & Behavior. 2007;92(1-2):180–185. doi: 10.1016/j.physbeh.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Ohman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130(3):466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Ohrmann P, Rauch AV, Bauer J, Kugel H, Arolt V, Heindel W, et al. Threat sensitivity as assessed by automatic amygdala response to fearful faces predicts speed of visual search for facial expression. Experimental Brain Research. 2007;183(1):51–59. doi: 10.1007/s00221-007-1022-0. [DOI] [PubMed] [Google Scholar]
- Pourtois G, Spinelli L, Seeck M, Vuilleumier P. Temporal precedence of emotion over attention modulations in the lateral amygdala: Intracranial ERP evidence from a patient with temporal lobe epilepsy. Cognitive, Affective & Behavioral Neuroscience. 2010;10(1):83. doi: 10.3758/CABN.10.1.83. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10(4):433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Thornton HB, Nel D, Thornton D, van Honk J, Baker GA, Stein DJ. The neuropsychiatry and neuropsychology of lipoid proteinosis. Journal of Neuropsychiatry and Clinical Neuroscience. 2008;20(1):86–92. doi: 10.1176/jnp.2008.20.1.86. [DOI] [PubMed] [Google Scholar]
- Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R. Intact rapid detection of fearful faces in the absence of the amygdala. Nature Neuroscience. 2009;12(10):1224–1225. doi: 10.1038/nn.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson M, Armony J, Driver J, Dolan R. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio: Harcourt Assessment; 1999. [Google Scholar]
- Weike A, Hamm A, Schupp H, Runge U, Schroeder H, Kessler C. Fear conditioning following unilateral temporal lobectomy: Dissociation of conditioned startle potentiation and autonomic learning. Journal of Neuroscience. 2005;25(48):11117. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.