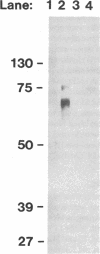
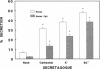
Abstract
Secretory proteins are targeted into either constitutive (secreted upon synthesis) or regulated (stored in vesicles and released in response to a secretagogue) pathways. To investigate mechanisms of protein targeting into catecholamine storage vesicles (CSV), we stably expressed human chromogranin A (CgA), the major soluble protein in human CSV, in the rat pheochromocytoma PC-12 cell line. Chromaffin cell secretagogues (0.1 mM nicotinic cholinergic agonist, 55 mM K+, or 2 mM Ba++) caused cosecretion of human CgA and catecholamines from human CgA-expressing cells. Sucrose gradients colocalized human CgA and catecholamines to subcellular particles of the same buoyant density. Chimeric proteins, in which human CgA (either full-length [457 amino acids] or truncated [amino-terminal 226 amino acids]) was fused in-frame to the ordinarily nonsecreted protein chloramphenicol acetyltransferase (CAT), were expressed transiently in PC-12 cells. Both constructs directed CAT activity into regulated secretory vesicles, as judged by secretagogue-stimulated release. These data demonstrate that human CgA expressed in PC-12 cells is targeted to regulated secretory vesicles. In addition, human CgA can divert an ordinarily non-secreted protein into the regulated secretory pathway, consistent with the operation of a dominant targeting signal for the regulated pathway within the peptide sequence of CgA.
Full text
PDF


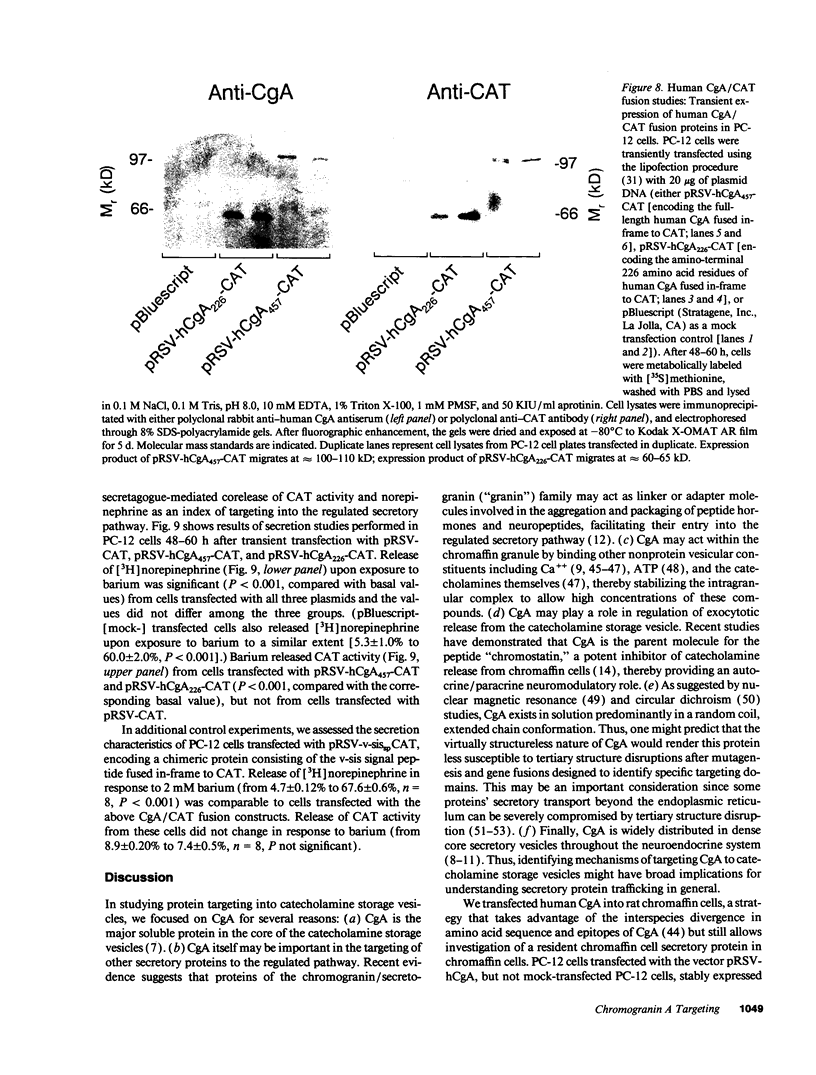
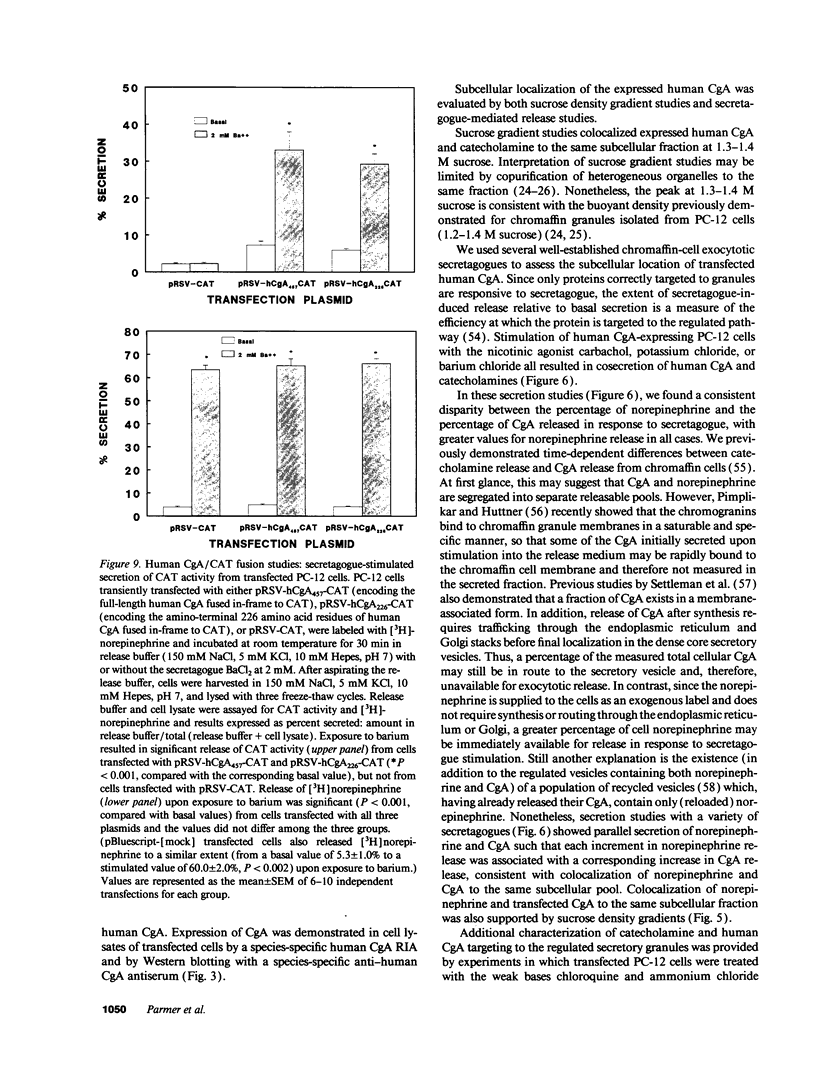
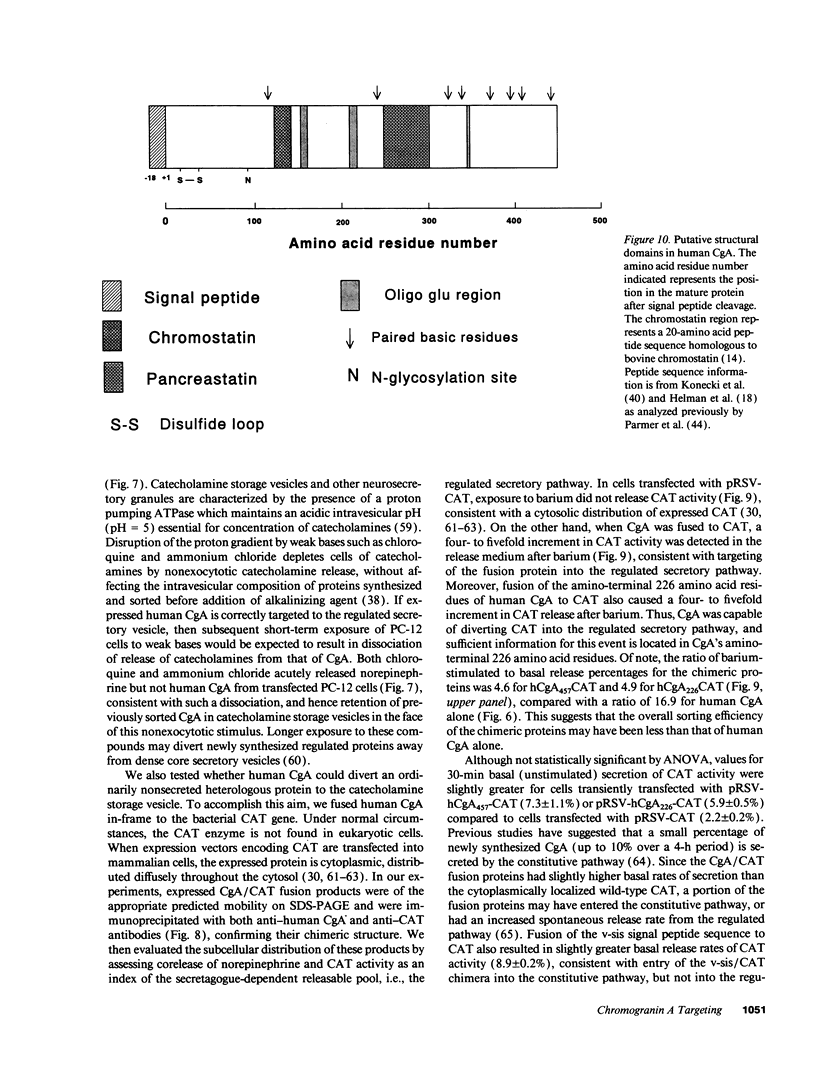



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedum U. M., Baeuerle P. A., Konecki D. S., Frank R., Powell J., Mallet J., Huttner W. B. The primary structure of bovine chromogranin A: a representative of a class of acidic secretory proteins common to a variety of peptidergic cells. EMBO J. 1986 Jul;5(7):1495–1502. doi: 10.1002/j.1460-2075.1986.tb04388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedum U. M., Lamouroux A., Konecki D. S., Rosa P., Hille A., Baeuerle P. A., Frank R., Lottspeich F., Mallet J., Huttner W. B. The primary structure of human secretogranin I (chromogranin B): comparison with chromogranin A reveals homologous terminal domains and a large intervening variable region. EMBO J. 1987 May;6(5):1203–1211. doi: 10.1002/j.1460-2075.1987.tb02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brion C., Miller S. G., Moore H. P. Regulated and constitutive secretion. Differential effects of protein synthesis arrest on transport of glycosaminoglycan chains to the two secretory pathways. J Biol Chem. 1992 Jan 25;267(3):1477–1483. [PubMed] [Google Scholar]
- Chanat E., Huttner W. B. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991 Dec;115(6):1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidgey M. A., Harrison T. M. Renin is sorted to the regulated secretory pathway in transfected PC12 cells by a mechanism which does not require expression of the pro-peptide. Eur J Biochem. 1990 May 31;190(1):139–144. doi: 10.1111/j.1432-1033.1990.tb15556.x. [DOI] [PubMed] [Google Scholar]
- Chu W. N., Baxter J. D., Reudelhuber T. L. A targeting sequence for dense secretory granules resides in the active renin protein moiety of human preprorenin. Mol Endocrinol. 1990 Dec;4(12):1905–1913. doi: 10.1210/mend-4-12-1905. [DOI] [PubMed] [Google Scholar]
- Chung K. N., Walter P., Aponte G. W., Moore H. P. Molecular sorting in the secretory pathway. Science. 1989 Jan 13;243(4888):192–197. doi: 10.1126/science.2911732. [DOI] [PubMed] [Google Scholar]
- Cohn D. V., Elting J. J., Frick M., Elde R. Selective localization of the parathyroid secretory protein-I/adrenal medulla chromogranin A protein family in a wide variety of endocrine cells of the rat. Endocrinology. 1984 Jun;114(6):1963–1974. doi: 10.1210/endo-114-6-1963. [DOI] [PubMed] [Google Scholar]
- Corcoran J. J., Kirshner N. Synthesis of chromogranin A, dopamine beta-hydroxylase, and chromaffin vesicles. Am J Physiol. 1990 Jul;259(1 Pt 1):C161–C168. doi: 10.1152/ajpcell.1990.259.1.C161. [DOI] [PubMed] [Google Scholar]
- Daniels A. J., Williams R. J., Wright P. E. The character of the stored molecules in chromaffin granules of the adrenal medulla: a nuclear magnetic resonance study. Neuroscience. 1978;3(6):573–585. doi: 10.1016/0306-4522(78)90022-2. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar M. G. Progress in unraveling pathways of Golgi traffic. Annu Rev Cell Biol. 1985;1:447–488. doi: 10.1146/annurev.cb.01.110185.002311. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo E., Rill A., Bader M. F., Aunis D. Chromostatin, a 20-amino acid peptide derived from chromogranin A, inhibits chromaffin cell secretion. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1426–1430. doi: 10.1073/pnas.88.4.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. G., Keller G. A., Subramani S. Identification of a peroxisomal targeting signal at the carboxy terminus of firefly luciferase. J Cell Biol. 1987 Dec;105(6 Pt 2):2923–2931. doi: 10.1083/jcb.105.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Subramani S. Identification of peroxisomal targeting signals located at the carboxy terminus of four peroxisomal proteins. J Cell Biol. 1988 Sep;107(3):897–905. doi: 10.1083/jcb.107.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Biosynthesis of the v-sis gene product: signal sequence cleavage, glycosylation, and proteolytic processing. Mol Cell Biol. 1986 Apr;6(4):1343–1348. doi: 10.1128/mcb.6.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannink M., Donoghue D. J. Requirement for a signal sequence in biological expression of the v-sis oncogene. Science. 1984 Dec 7;226(4679):1197–1199. doi: 10.1126/science.6095451. [DOI] [PubMed] [Google Scholar]
- Helman L. J., Ahn T. G., Levine M. A., Allison A., Cohen P. S., Cooper M. J., Cohn D. V., Israel M. A. Molecular cloning and primary structure of human chromogranin A (secretory protein I) cDNA. J Biol Chem. 1988 Aug 15;263(23):11559–11563. [PubMed] [Google Scholar]
- Holt C. E., Garlick N., Cornel E. Lipofection of cDNAs in the embryonic vertebrate central nervous system. Neuron. 1990 Feb;4(2):203–214. doi: 10.1016/0896-6273(90)90095-w. [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Gerdes H. H., Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991 Jan;16(1):27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- Iacangelo A., Affolter H. U., Eiden L. E., Herbert E., Grimes M. Bovine chromogranin A sequence and distribution of its messenger RNA in endocrine tissues. Nature. 1986 Sep 4;323(6083):82–86. doi: 10.1038/323082a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Jr Accumulation of biological amines into chromaffin granules: a model for hormone and neurotransmitter transport. Physiol Rev. 1988 Jan;68(1):232–307. doi: 10.1152/physrev.1988.68.1.232. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Konecki D. S., Benedum U. M., Gerdes H. H., Huttner W. B. The primary structure of human chromogranin A and pancreastatin. J Biol Chem. 1987 Dec 15;262(35):17026–17030. [PubMed] [Google Scholar]
- Kreis T. E., Lodish H. F. Oligomerization is essential for transport of vesicular stomatitis viral glycoprotein to the cell surface. Cell. 1986 Sep 12;46(6):929–937. doi: 10.1016/0092-8674(86)90075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laredo J., Wolff V. L., Lovett P. S. Chloramphenicol acetyltransferase specified by cat-86: relationship between the gene and the protein. Gene. 1988 Dec 15;73(1):209–214. doi: 10.1016/0378-1119(88)90327-7. [DOI] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987 Sep;105(3):1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuuchi L., Kelly R. B. Constitutive and basal secretion from the endocrine cell line, AtT-20. J Cell Biol. 1991 Mar;112(5):843–852. doi: 10.1083/jcb.112.5.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattila P., Korpela J., Tenkanen T., Pitkänen K. Fidelity of DNA synthesis by the Thermococcus litoralis DNA polymerase--an extremely heat stable enzyme with proofreading activity. Nucleic Acids Res. 1991 Sep 25;19(18):4967–4973. doi: 10.1093/nar/19.18.4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H. H., Kelly R. B. Re-routing of a secretory protein by fusion with human growth hormone sequences. Nature. 1986 May 22;321(6068):443–446. doi: 10.1038/321443a0. [DOI] [PubMed] [Google Scholar]
- Moore H. P. Factors controlling packaging of peptide hormones into secretory granules. Ann N Y Acad Sci. 1987;493:50–61. doi: 10.1111/j.1749-6632.1987.tb27180.x. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Gumbiner B., Kelly R. B. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. 1983 Mar 31-Apr 6Nature. 302(5907):434–436. doi: 10.1038/302434a0. [DOI] [PubMed] [Google Scholar]
- Muller S. R., Sullivan P. D., Clegg D. O., Feinstein S. C. Efficient transfection and expression of heterologous genes in PC12 cells. DNA Cell Biol. 1990 Apr;9(3):221–229. doi: 10.1089/dna.1990.9.221. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Burton D., Deftos L. J. Immunoreactive human chromogranin A in diverse polypeptide hormone producing human tumors and normal endocrine tissues. J Clin Endocrinol Metab. 1983 Nov;57(5):1084–1086. doi: 10.1210/jcem-57-5-1084. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Frigon R. P., Sokoloff R. L. Human chromogranin A. Purification and characterization from catecholamine storage vesicles of human pheochromocytoma. Hypertension. 1984 Jan-Feb;6(1):2–12. doi: 10.1161/01.hyp.6.1.2. [DOI] [PubMed] [Google Scholar]
- O'Connor D. T., Pandlan M. R., Carlton E., Cervenka J. H., Hslao R. J. Rapid radioimmunoassay of circulating chromogranin A: in vitro stability, exploration of the neuroendocrine character of neoplasia, and assessment of the effects of organ failure. Clin Chem. 1989 Aug;35(8):1631–1637. [PubMed] [Google Scholar]
- O'Connor D. T., Parmer R. J., Deftos L. J. Chromogranin A: studies in the endocrine system. Trans Assoc Am Physicians. 1984;97:242–250. [PubMed] [Google Scholar]
- Oberlechner E., Westhead E., Neuman B., Schmidt W., Fischer-Colbrie R., Weber A., Sperk G., Winkler H. Characterization of catecholamine-storage organelles in transplantable phaeochromocytoma and adrenal glands of rats. J Neurochem. 1982 Mar;38(3):615–624. doi: 10.1111/j.1471-4159.1982.tb08675.x. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Perrelet A., Powell S. K., Quinn D. L., Moore H. P. The trans-most cisternae of the Golgi complex: a compartment for sorting of secretory and plasma membrane proteins. Cell. 1987 Dec 24;51(6):1039–1051. doi: 10.1016/0092-8674(87)90590-3. [DOI] [PubMed] [Google Scholar]
- Parmer R. J., Koop A. H., Handa M. T., O'Connor D. T. Molecular cloning of chromogranin A from rat pheochromocytoma cells. Hypertension. 1989 Oct;14(4):435–444. doi: 10.1161/01.hyp.14.4.435. [DOI] [PubMed] [Google Scholar]
- Parmer R. J., O'Connor D. T. Enkephalins in human phaeochromocytomas: localization in immunoreactive, high molecular weight form to the soluble core of chromaffin granules. J Hypertens. 1988 Mar;6(3):187–198. doi: 10.1097/00004872-198803000-00002. [DOI] [PubMed] [Google Scholar]
- Patzak A., Winkler H. Exocytotic exposure and recycling of membrane antigens of chromaffin granules: ultrastructural evaluation after immunolabeling. J Cell Biol. 1986 Feb;102(2):510–515. doi: 10.1083/jcb.102.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimplikar S. W., Huttner W. B. Chromogranin B (secretogranin I), a secretory protein of the regulated pathway, is also present in a tightly membrane-associated form in PC12 cells. J Biol Chem. 1992 Feb 25;267(6):4110–4118. [PubMed] [Google Scholar]
- Powell S. K., Orci L., Craik C. S., Moore H. P. Efficient targeting to storage granules of human proinsulins with altered propeptide domain. J Cell Biol. 1988 Jun;106(6):1843–1851. doi: 10.1083/jcb.106.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiffen F. U., Gratzl M. Ca2+ binding to chromaffin vesicle matrix proteins: effect of pH, Mg2+, and ionic strength. Biochemistry. 1986 Jul 29;25(15):4402–4406. doi: 10.1021/bi00363a034. [DOI] [PubMed] [Google Scholar]
- Reiffen F. U., Gratzl M. Chromogranins, widespread in endocrine and nervous tissue, bind Ca2+. FEBS Lett. 1986 Jan 20;195(1-2):327–330. doi: 10.1016/0014-5793(86)80187-9. [DOI] [PubMed] [Google Scholar]
- Roda L. G., Nolan J. A., Kim S. U., Hogue-Angeletti R. A. Isolation and characterization of chromaffin granules from a pheochromocytoma (PC 12) cell line. Exp Cell Res. 1980 Jul;128(1):103–109. doi: 10.1016/0014-4827(80)90392-4. [DOI] [PubMed] [Google Scholar]
- Rosa P., Weiss U., Pepperkok R., Ansorge W., Niehrs C., Stelzer E. H., Huttner W. B. An antibody against secretogranin I (chromogranin B) is packaged into secretory granules. J Cell Biol. 1989 Jul;109(1):17–34. doi: 10.1083/jcb.109.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. Protein sorting by selective retention in the endoplasmic reticulum and Golgi stack. Cell. 1987 Aug 14;50(4):521–522. doi: 10.1016/0092-8674(87)90024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabban E. L., Schwartz J., McMahon A. Effect of compounds which disrupt proton gradients on secretion of neurosecretory proteins from PC12 pheochromocytoma cells. Neuroscience. 1990;38(2):561–570. doi: 10.1016/0306-4522(90)90050-e. [DOI] [PubMed] [Google Scholar]
- Sauer M. K., Hannink M., Donoghue D. J. Deletions in the N-terminal coding region of the v-sis gene: determination of the minimal transforming region. J Virol. 1986 Aug;59(2):292–300. doi: 10.1128/jvi.59.2.292-300.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz G. 17th Sir Hans Krebs lecture. Signals guiding proteins to their correct locations in mitochondria. Eur J Biochem. 1987 May 15;165(1):1–6. doi: 10.1111/j.1432-1033.1987.tb11186.x. [DOI] [PubMed] [Google Scholar]
- Schubert D., Klier F. G. Storage and release of acetylcholine by a clonal cell line. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5184–5188. doi: 10.1073/pnas.74.11.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer E. S., Kelly R. B. Selective packaging of human growth hormone into synaptic vesicles in a rat neuronal (PC12) cell line. J Cell Biol. 1985 Aug;101(2):667–676. doi: 10.1083/jcb.101.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer E. S., Paddock S. Localization of human growth hormone to a sub-set of cytoplasmic vesicles in transfected PC12 cells. J Cell Sci. 1990 Jul;96(Pt 3):375–381. doi: 10.1242/jcs.96.3.375. [DOI] [PubMed] [Google Scholar]
- Seethaler G., Chaminade M., Vlasak R., Ericsson M., Griffiths G., Toffoletto O., Rossier J., Stunnenberg H. G., Kreil G. Targeting of frog prodermorphin to the regulated secretory pathway by fusion to proenkephalin. J Cell Biol. 1991 Sep;114(6):1125–1133. doi: 10.1083/jcb.114.6.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settleman J., Nolan J., Angeletti R. H. Chromogranin, an integral membrane protein. J Biol Chem. 1985 Feb 10;260(3):1641–1644. [PubMed] [Google Scholar]
- Sevarino K. A., Stork P. Multiple preprosomatostatin sorting signals mediate secretion via discrete cAMP- and tetradecanoylphorbolacetate-responsive pathways. J Biol Chem. 1991 Oct 5;266(28):18507–18513. [PubMed] [Google Scholar]
- Sevarino K. A., Stork P., Ventimiglia R., Mandel G., Goodman R. H. Amino-terminal sequences of prosomatostatin direct intracellular targeting but not processing specificity. Cell. 1989 Apr 7;57(1):11–19. doi: 10.1016/0092-8674(89)90167-0. [DOI] [PubMed] [Google Scholar]
- Simon J. P., Aunis D. Biochemistry of the chromogranin A protein family. Biochem J. 1989 Aug 15;262(1):1–13. doi: 10.1042/bj2620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. D., Winkler H. A simple method for the isolation of adrenal chromaffin granules on a large scale. Biochem J. 1967 May;103(2):480–482. doi: 10.1042/bj1030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin W. S., Fisher J. M., Scheller R. H. Sorting within the regulated secretory pathway occurs in the trans-Golgi network. J Cell Biol. 1990 Jan;110(1):1–12. doi: 10.1083/jcb.110.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stoller T. J., Shields D. The propeptide of preprosomatostatin mediates intracellular transport and secretion of alpha-globin from mammalian cells. J Cell Biol. 1989 May;108(5):1647–1655. doi: 10.1083/jcb.108.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiyyuddin M. A., Cervenka J. H., Sullivan P. A., Pandian M. R., Parmer R. J., Barbosa J. A., O'Connor D. T. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990 Jan;81(1):185–195. doi: 10.1161/01.cir.81.1.185. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M. Mutations that influence the secretory path in animal cells. Biochem J. 1983 Oct 15;216(1):1–9. doi: 10.1042/bj2160001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatemoto K., Efendić S., Mutt V., Makk G., Feistner G. J., Barchas J. D. Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature. 1986 Dec 4;324(6096):476–478. doi: 10.1038/324476a0. [DOI] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videen J. S., Mezger M. S., Chang Y. M., O'Connor D. T. Calcium and catecholamine interactions with adrenal chromogranins. Comparison of driving forces in binding and aggregation. J Biol Chem. 1992 Feb 15;267(5):3066–3073. [PubMed] [Google Scholar]
- Weiland G. A., Minneman K. P., Molinoff P. B. Thermodynamics of agonist and antagonist interactions with mammalian beta-adrenergic receptors. Mol Pharmacol. 1980 Nov;18(3):341–347. [PubMed] [Google Scholar]
- Wilson B. S., Lloyd R. V. Detection of chromogranin in neuroendocrine cells with a monoclonal antibody. Am J Pathol. 1984 Jun;115(3):458–468. [PMC free article] [PubMed] [Google Scholar]
- Winkler H., Apps D. K., Fischer-Colbrie R. The molecular function of adrenal chromaffin granules: established facts and unresolved topics. Neuroscience. 1986 Jun;18(2):261–290. doi: 10.1016/0306-4522(86)90154-5. [DOI] [PubMed] [Google Scholar]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5(11):1803–1823. doi: 10.1016/0306-4522(80)90031-7. [DOI] [PubMed] [Google Scholar]
- Wiren K. M., Potts J. T., Jr, Kronenberg H. M. Importance of the propeptide sequence of human preproparathyroid hormone for signal sequence function. J Biol Chem. 1988 Dec 25;263(36):19771–19777. [PubMed] [Google Scholar]
- Wu H. J., Rozansky D. J., Parmer R. J., Gill B. M., O'Connor D. T. Structure and function of the chromogranin A gene. Clues to evolution and tissue-specific expression. J Biol Chem. 1991 Jul 15;266(20):13130–13134. [PubMed] [Google Scholar]
- Yoo S. H., Albanesi J. P. Ca2(+)-induced conformational change and aggregation of chromogranin A. J Biol Chem. 1990 Aug 25;265(24):14414–14421. [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]