Abstract
Background
Despite the remarkable activity of artemisinin and its derivatives their monotherapy has been associated with high rates of recrudescence. The temporary growth arrest of ring stage parasites (dormancy) following exposure to artemisinin drugs provides a plausible explanation for this phenomenon.
Methods
Ring stage parasites of several P. falciparum lines were exposed to different doses of dihydroartemisinin (DHA) alone or in combination with mefloquine (MQ). For each regime the proportion of parasites recovering was determined daily for 20 days.
Results
Parasite development was abruptly arrested following a single exposure to DHA, with some parasites being dormant for up to 20 days. Approximately 50% of dormant parasites recovered to resume growth within the first 9 days. The overall proportion of parasites recovering was dose dependant with recovery rates ranging from 0.044% to 1.313%. Repeated treatment with DHA, or DHA in combination with MQ, led to a delay in recovery and a ~10 fold reduction in total recovery. Strains with different genetic backgrounds appear to vary in their capacity to recover.
Conclusions
These results imply that artemisinin-induced growth arrest occurs readily in laboratory treated parasites, and may be a key factor in treatment failure of P. falciparum malaria.
Keywords: Plasmodium falciparum, Artemisinin, Treatment-failure, Dormancy, Recovery-rates, Recovery-duration
Introduction
Parasite resistance to anti-malarial drugs is widespread and treatment of falciparum malaria presently relies on a limited number of drugs. Artemisinin and its derivatives are the most potent and rapidly acting of the antimalarial drugs and are the first line treatment in more than 100 malaria endemic countries [1, 2]. Despite the remarkable activity of the artemisinin drugs, 3–50% of patients fail treatment if artemisinins are given as a mono-therapy to non-immune patients [3]. Prolonging the treatment regimen from <5 days to 5–10 days has been shown to reduce recrudescence rates [4]. Importantly, retreatment of recrudescence with the same artemisinin compound is equally effective as the initial treatment [5], suggesting that the failures are not due to the development of parasite resistance. To date it is not clear why such a potent drug is associated with a high rate of recrudescence. To overcome the problem artemisinin drugs are only recommended in combination with other anti-malarials.
Artemisinins are metabolized rapidly in vivo and eliminated within hours [5]. It is hypothesized that recrudescence occurs because the short half-life of artemisinin results in plasma drug concentrations not remaining above the minimum inhibitory concentration (MIC) for long enough periods to kill all parasites [6, 7]. To support this hypothesis Bwijo et al. [8] showed that exposure to artemisinin for 3 hours daily in vitro did not completely eradicate the parasites; this could only be achieved if the dosing regimen was extended to a threshold concentration of 3×10−8 M twice daily for at least 5 days, or by using artemisinin in combination with mefloquine at levels above the respective MICs. These in vitro studies closely approximate the observations from a series of clinical studies in Thailand after treatment with artesunate and artemether. In these studies a decrease in recrudescence was directly correlated with drug concentration and dosing frequency [9]. However, this hypothesis does not explain the frequency of recrudescence after 7 days of treatment with a drug which can produce reductions in asexual parasite biomass of up to 10,000 fold per cycle [6].
An alternative novel mechanism has been proposed [10, 11] in which parasites are able to tolerate artemisinin treatment by entering a temporarily growth-arrested state (dormancy) similar to that described for the persistence of bacteria [12, 13]. It has been shown that development of P. falciparum ring-stage parasites is interrupted rapidly after exposure to artesunate or dihydroartemisinin (DHA), but that these parasites recover to resume normal growth [10, 11]. To understand how parasite dormancy may impact on artemisinin treatment failure, it is critical to know how long parasites remain dormant after artemisinin exposure and what proportion of dormant parasites recover, as well as the dynamics of the recovery. Equally important is whether the companion drugs used in artemisinin combination therapy (ACT) formulations are effective against the dormant parasites. This knowledge could significantly improve the understanding of the parasite response to artemisinin drugs and will enable us to evaluate current ACT combinations and develop further rationale combination dosing regimens. Moreover, dormancy may facilitate the development of resistance to artemisinin drugs and a better understanding of dormancy may help to reveal artemisinin resistance mechanisms.
In this paper, we report investigations of the duration of dormancy and recovery rates in vitro by exposing synchronous ring stage parasites of different P. falciparum strains to a range of concentrations of DHA. Repeated treatment and combination treatment with mefloquine was also carried out to assess the receptiveness of dormant parasites to subsequent drug exposure.
Materials and methods
Parasite cultivation
Plasmodium falciparum strains W2, D6, HB3, S55 and PH1 were cultivated using standard techniques with a haematocrit of 3%. The culture medium consisted of standard RPMI1640 supplemented with 10% human plasma [14]. Prior to the start of each experiment parasites were synchronized at ring stage using two rounds of 5% sorbitol [15].
Drug dilutions
A series of dilutions of DHA (DK Pharma, Vietnam) and mefloquine (Walter Reed Army Institute, USA) in 100% methanol was prepared and used as stock solutions. The working concentration for mefloquine was 250 ng/ml, while DHA ranged from 20 to 500 ng/ml (~7×10−8 M to 2×10−6 M).
Experimental design
Monitoring recrudescence in bulk cultures
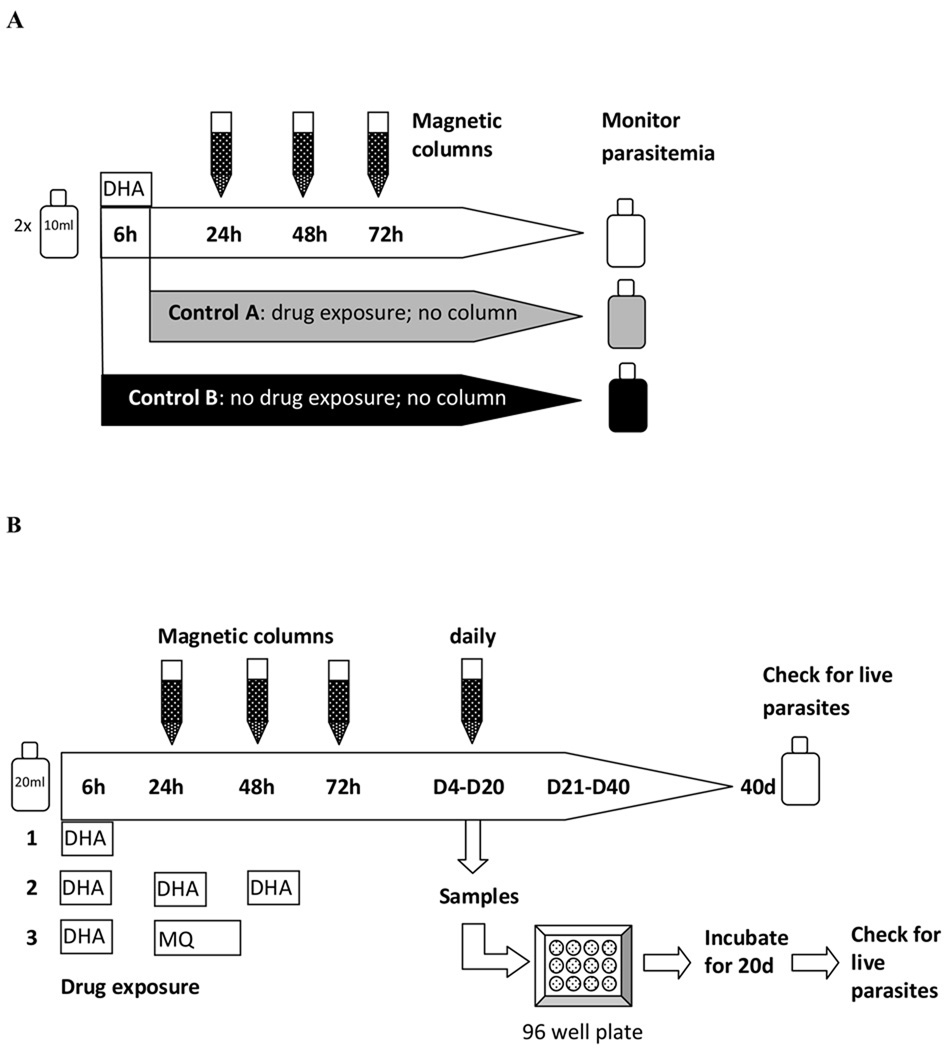
Two 10 ml cultures of ring stage parasites (W2, HB3 and D6) with an initial parasitemia of 2% were each exposed to DHA (200 ng/ml; ~7×10−7 M) for 6 hours, after which the DHA was removed by washing the culture with fresh medium. After treatment one 10 ml culture (Control A) was maintained and monitored until the parasitemia reached ~10%, while the other was passed through a magnetic column (MACS, 25 CS Separation columns) on 3 consecutive days, starting 24 hours after treatment, before being maintained by standard culture techniques until ~10% parasitemia was reached (Fig 1A). The magnetic columns remove mature parasites (late trophozoites and schizonts) [16, 17], thereby ensuring that parasites unaffected by the drug were removed and did not contribute to the estimated recovery of parasites. For each use of the column cultures were resuspended in 2 ml cold RPMI and passed through the column three times, after which the column was washed with 30 ml RPMI. All flow through was spun down, resuspended in 10 ml culture medium and returned to the incubator. Sham-treated parasites (without drug and magnetic column treatment) were used as a negative control (Fig 1A; Control B). Thin and thick blood films were made daily, stained with Giemsa stain and examined under light microscopy to determine the parasitemia.
Figure 1.
Experimental design to A) monitor recovery in bulk cultures; B) to determine rates and duration of recovery of parasites following treatment with single or multiple doses of DHA or DHA in combination with mefloquine.
Determining rates & duration of recovery
20 ml of synchronous ring stage parasites with an initial parasitemia of 2% were exposed to a single dose of DHA, or multiple doses of DHA on 3 consecutive days, or a single dose of DHA followed by a 24 hour exposure to mefloquine on the following day (Fig 1B). All DHA exposures were for 6 hours. Following drug treatment parasites were washed and culture medium was replaced. Cultures were passed through a magnetic column 24 hours after the first treatment and then every 24 hours for up to 20 consecutive days as described above. The daily use of the columns removed parasites that had previously recovered.
From Day 4 to Day 20, samples were taken before the cultures were passed through the column and plated into 96-well microculture plates (Microtest™, Falcon) at three dilutions (32 wells at each dilution). Dilutions were adjusted against RBC counts obtained at regular intervals throughout the experiment using a haemacytometer. Plates were gassed and maintained at 37°C for 19–21 days (culture medium was replaced every four days and RBCs added after 10 days), after which Giemsa-stained thick blood films were made for each well and examined under light microscopy. Sham-treated parasite cultures ran parallel to all experiments as negative controls.
At the completion of the 17–20 days of plating, each bulk culture from which the plates were made was maintained for a further 20 days after which slides were made and examined for the presence of growing parasites.
Evaluation of Recovery Rates
The number of parasite positive wells in each plate at each concentration was used as the basis to calculate the parasite recovery rate. It was assumed for plate i that the number of positive wells at concentration j followed the binomial distribution ~Bi(pi,j, 32) where pi,j = 1 − e−rici,j,ci,j is the expected number of parasites per well and ri is the proportion of parasites that recover to resume growth within the plate. The variable ri was assumed to be the sum of the daily recovery rates potentially contributing to the growth seen within a well. The recovery days which could contribute to each plate varied between plates and experiments and were calculated assuming 1) all parasites growing in a well recovered after passing through the last column before plating, 2) growing parasites have an average replication rate of 5 every 48 hours and 3) it takes 12 days for one parasite within a well to replicate to sufficient numbers to be visible by thick film. The proportion of parasites recovering on each day (or group of several days) was specified by a random effects model and fit using MCMC implemented by the WinBUGS software (Version 1.4, Imperial College & MRC, UK). The calculated overall recovery rate was the recovery from Day 4 onwards when all parasites unaffected by the drug had been removed.
Definitions
Overall recovery rate - the proportion of treated parasites recovering and returning to normal growth after drug exposure.
Daily recovery rate - the percentage of treated parasites that recovered on a certain day (or over several days).
Time to recovery (duration) - the number of days between the first day of treatment and the day when a defined proportion of dormant parasites started to grow.
Results
Detection of recovery
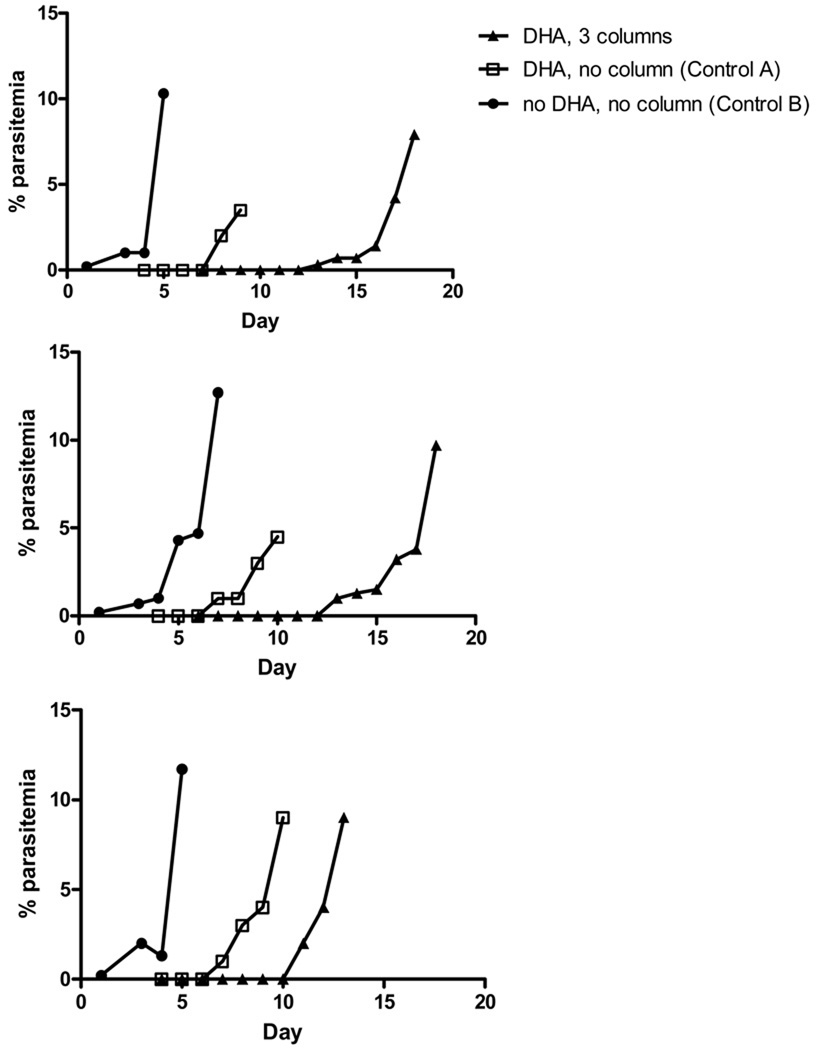
Following a single exposure to 200 ng/ml DHA morphologically abnormal rings and no mature parasite forms were observed in the Control A culture for 3 days. In all three strains (HB3, W2 and D6) tested, developing parasites were first sighted on thick films on Day 4 and reached >10% parasitemia by Day 11 (Fig 2). In contrast, the time to reach >10% parasitemia in each culture that passed through the magnetic column for the first 3 days after treatment was 19 days for W2 and HB3 and 14 days for D6 (Fig 2). This delay in recovery indicated that there was a small number of parasites that was either unaffected by the drug or had become dormant but recovered in the first 3 days and had been removed by the columns.
Figure 2.
Effect of 200 ng/ml DHA on bulk cultures of three parasite strains (W2, HB3 and D6) with and without the use of a magnetic column on Days 1, 2 and 3. The starting parasitemias were 0.20% and 2% for control and DHA treated samples, respectively.
Recovery Rates and duration after single exposure to DHA
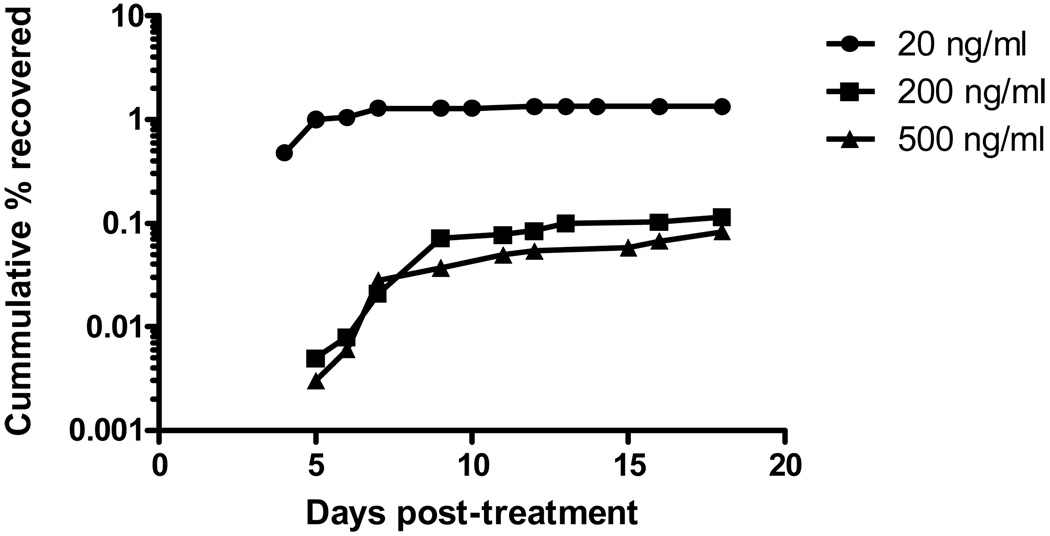
P. falciparum W2 parasites were exposed to 3 different concentrations of DHA: 20 ng/ml, 200 ng/ml and 500 ng/ml. The proportion of parasites that recovered was dose-dependent (Table 1). 50% of parasites recovered by Day 9 after treatment with 200 and 500 ng/ml DHA and by Day 5 for 20 ng/ml (Fig 3).
Table 1.
|
P. falciparum strain |
Concentration (ng/ml) |
Estimated median % recovered (2.5th & 97.5th percentile) |
Presence of growing parasites in bulk culture 14 days after last plate |
|
|---|---|---|---|---|
| DHA | MQ | |||
| 20 | - | 1.313 (0.898 – 1.940) | − | |
| 200 | - | 0.124 (0.104 – 0.155) | + | |
| W2 | 500 | - | 0.083 (0.068 – 0.096) | + |
| 3×200 | - | 0.011 (0.009 – 0.014) | − | |
| 200 | 250 | 0.014 (0.011 – 0.016) | − | |
| D6 | 200 | - | 0.144 (0.118 – 0.175) | − |
| PH1 | 200 | - | 0.044 (0.036 – 0.056) | − |
| HB3 | 200 | - | 0.071 (0.057 – 0.090) | − |
| S55 | 200 | - | 0.145 (0.116 – 0.185) | − |
Figure 3.
Cumulative recovery rates for W2 at three DHA concentrations.
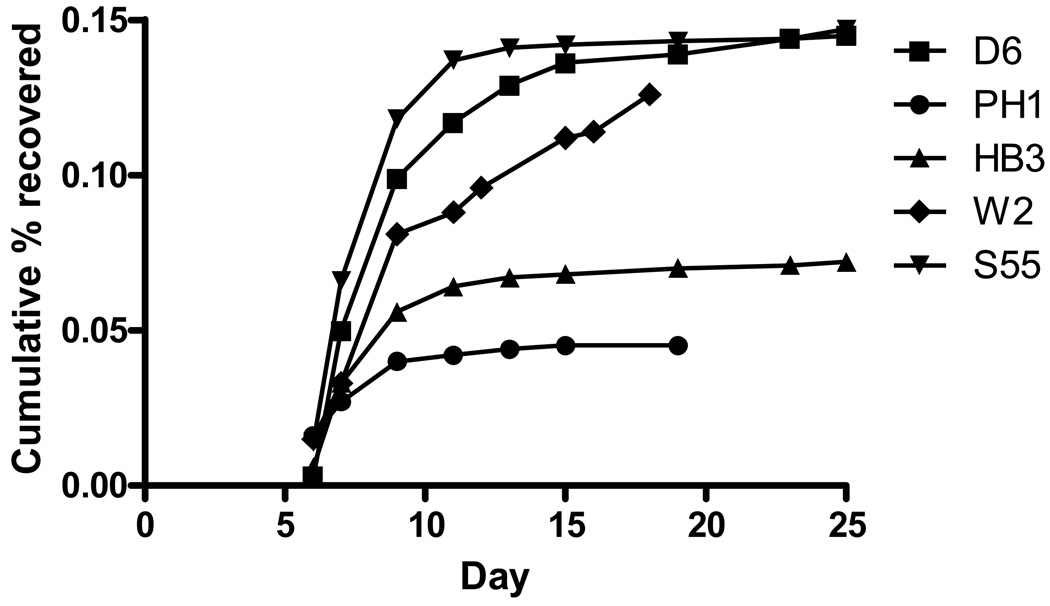
Five parasite strains with different genetic backgrounds were exposed to a single dose (200 ng/ml) of DHA. All strains recovered following treatment. W2, D6 and S55 had similar overall recovery rates (0.124%–0.145%), while HB3 and PH1 had lower overall recovery rates (Table 1). The timing and dynamics of recovery appeared fairly consistent between strains with the majority of parasites recovered by Day 15 (Fig 4). With the exception of W2, no growing parasites were detected in the bulk culture after 40 days (20 days of daily column followed by 20 days culture), indicating no parasite recovery from dormancy between Day 20 and Day 40. This result also demonstrated that the daily columns successfully removed parasites that recovered and resumed normal growth between Days 4 and 20. Several repeat experiments were conducted using W2 exposed to 200 ng/ml and the presence of growing parasites at the end of the experiment was a common occurrence (data not shown). This suggests that some W2 parasites may recover from dormancy after Day 20.
Figure 4.
Cumulative recovery rates for 5 parasite strains each treated with 200 ng/ml DHA for 6 hours on Day 1.
Recovery Rates and duration after multiple drug exposures
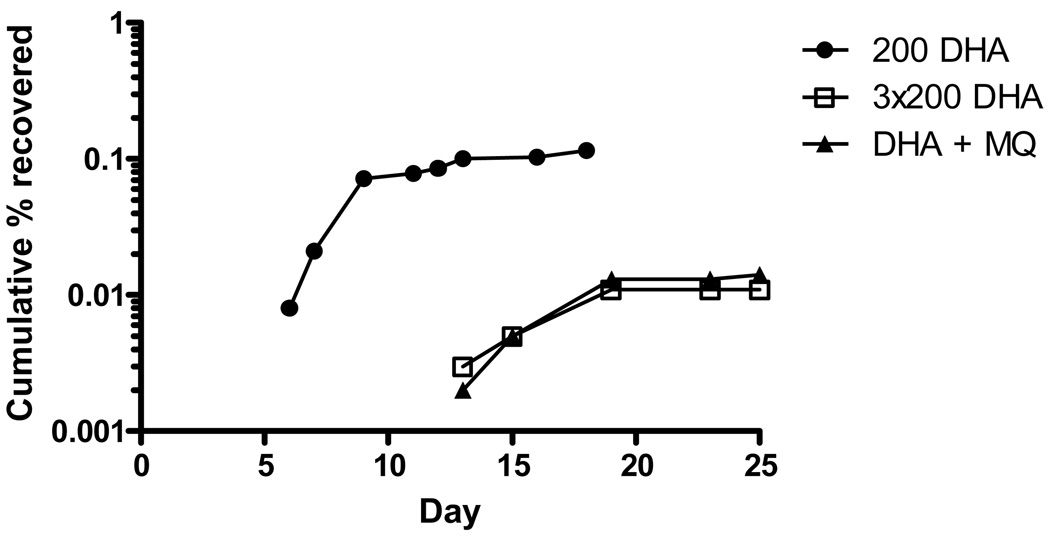
Recurrence of parasites was detected after repeated exposure to 200 ng/ml DHA on 3 consecutive days. The overall recovery rate (0.011%) was 10-fold lower than that following a single dose (Table 1) and the recovery occurred much later (Fig 5).
Figure 5.
Parasite recovery after repeated treatment with DHA and combination treatment with DHA and mefloquine (MQ).
An almost identical pattern was observed when DHA exposure was followed by mefloquine exposure. The overall recovery rate was 0.014% (Table 1) with delayed recurrence compared to single-dose DHA exposure (Fig 5).
Discussion
Recrudescence following artemisinin treatment is not uncommon, but the underlying mechanism is unclear. The recent report about the development of resistance to artemisinin [18] is alarming but can’t explain the common occurrence of recrudescence; clinical experience has shown that retreatment after recrudescence is equally effective as the initial treatment [5]. The occurrence of dormancy provides a plausible explanation for the high rate of recrudescence following treatment with the artemisinin class of drugs. Previous studies suggest the dormancy phenomenon is specific to artemisinin derivatives since it could not be induced by other anti-malarials such as quinine [10].
In this report we characterize parasite recovery in a variety of P. falciparum strains in vitro following a range of different DHA exposures. We considered only ring stage parasites since previous reports showed that only the ring stages arrested the development following treatment [10, 19] . Most concentrations of DHA used in our experiments (200–500 ng/ml) were more than 100 fold higher than the reported IC90 for these parasites in a standard 3 day IC90 assay, and were within the range of peak plasma DHA concentrations in humans treated with artemisinin derivatives [20]. Parasite growth was arrested at ring stage after exposure to DHA, but recovery was always observed. Overall recovery rates ranged from 0.044% to 1.313%, with parasites recovering continuously over the experimental period. These findings not only provide further evidence for the “dormancy” theory but also, for the first time, quantify the proportion of parasites recovering and estimate the duration that they remain dormant.
Magnetic columns were used in the experiments to remove growing parasites so that we could monitor parasite recovery over an extended time period. This was essential in assessing how long dormant parasites survive and recover. Without the columns the bulk culture would have been overgrown by the first brood of recovered parasites, prohibiting the detection of later parasite recovery. Two sets of results from our study demonstrate the use of the column was effective in achieving this goal. Firstly, bulk cultures which were DHA treated and passed through the magnetic column for 3 days recovered much later than the same parasites that were not passed through the column. This effect is likely due to the removal of parasites which may not have been affected by the treatment and parasites which recovered during the first 3 days following treatment. Secondly, the majority of drug treated bulk cultures did not show recurrent parasitemia after passing through the magnetic column daily for 20 days. Had the column not been effective at removing growing parasites, the parasites which recovered during the experiment (as evidenced by parasite positive plates taken from the bulk culture) would have multiplied in the culture producing a positive parasitemia at the end of the experiment. While it is unlikely that the column is able to completely remove large numbers of growing parasites [16, 17], in our experiments the proportion of growing parasites was expected to be low due to the high drug concentrations used and the gradual recovery of the parasites.
The total recovery rates calculated ranged from 0.001% to 1.313% of the treated parasites with the rates clearly being dose dependant. Higher DHA doses yielded a lower recovery rate, in line with the results from a previous in vitro study [21]. Under the same treatment dose (200 ng/ml), the overall recovery rates between 3 parasite strains with different geographical backgrounds (W2, D6 and S55) were remarkably similar, while two other strains showed rates of approximately one half (HB3) and one third (PH1). This may reflect the intrinsic tolerability of the parasites to DHA.
Our results show that parasites recover from dormancy and resume growth from 4 to 25 days after a single exposure to DHA. Our experimental design did not allow us to assess recovery during the first three days after treatment, so recovery may commence earlier than 4 days. While the majority of parasites appeared to have completed their recovery during the experimental period, we repeatedly saw parasite-positive cultures at the end of experiments in the W2 strain treated with 200 ng/ml and 500 ng/ml DHA. Since this feature was observed multiple times, but not observed at 20 ng/ml, we hypothesize that the recovery of dormant parasites was still continuing at the end of the experimental period. This is also supported by the failure of the cumulative recovery curves to reach a plateau or asymptote prior to the end of the experiment. Based on the declining trend of the recovery rate over time, we expect the rate of parasite recovery after the experimental period to be very low, however only a few parasite recovering will be sufficient to generate a positive bulk culture. The timing of recovery observed in our experiments agrees with field data where recrudescence appeared from less than 14 and up to 28 to 42 days after mono-therapy with artemisinin [7, 22] or artesunate [22–24].
Repeated DHA treatment on 3 consecutive days reduced the proportion of parasites recovering by 10 fold and delayed, but did not prevent parasites recurring. We hypothesize that continuation of repeated treatment over a longer time period would result in a further reduction of the recovery rate. Our results provide a plausible explanation for the outcome of clinical studies undertaken in Vietnam where recrudescence rates were highest in patients that received artemisinin treatment for 5 or less days compared to those receiving treatment for 5–10 days [4]. Our findings also closely reflect clinical studies undertaken in Thailand using artesunate and artemether which showed a decrease in recrudescence in direct correlation with concentration and dosing frequency [9].
It has been suggested for some time that antimalarial drugs should no longer be used alone to protect them from the emergence of resistance [25]. The combination of artesunate with mefloquine has been shown to be effective, even in an area of Thailand with the most mefloquine resistant parasites in the world [26, 27]. Cure rates were reported to remain well above 90% at day 42 even after 13 years of continuous mefloquine-artesunate deployment on the northwestern border of Thailand [28]. It has also been shown that mefloquine reduces recrudescence in vitro when used in combination with artemisinin [8]. The effectiveness of the combination has been attributed to the long elimination half-life of mefloquine which slowly eliminates the residual, artesunate-affected parasites [25].
In this study we exposed W2 parasites to a single dose of 200 ng/ml DHA for 6h followed by a single dose of 250 ng/ml mefloquine for 24h, with an interval of 18h between treatments. We showed that the proportion of parasites recovering was decreased by 10 fold as compared to DHA 200 ng/ml alone and that parasite recovery was also delayed. Interestingly, this delay and decrease in recovery was comparable to that after repeated DHA treatment. A longer exposure to mefloquine may have greater effect on the dormant parasites as the drug has a long half life in vivo. Hypothetically, these findings could have been caused by mefloquine (or the subsequent DHA dose) killing parasites that may have continued growing after the first treatment as well as dormant parasites recovering early after treatment. However, given daily use of the magnetic column to remove growing parasites a more likely explanation is that parasites that become dormant after one DHA treatment were still receptive to other drugs. This implies that dormant parasites still maintain a basic metabolism, which warrants further investigation.
The reduction and delay we observed in recovery after combination treatment with MQ agrees with the results of a field study undertaken in Ecuadorian children where recrudescence after artesunate mono-therapy occurred at higher rates and earlier than in the patient groups that received artesunate in combination with mefloquine [29]. Another study in Vietnam demonstrated that there is no effect of different dosing times of mefloquine within the first 24 hours after an initial single dose of artesunate [30].
Our findings reinforce the use of ACT to reduce the risk of recrudescence. Moreover, our results provide valuable information for dosing and drug combination regimens in the treatment of P. falciparum malaria. It may prove to be useful to adjust dosing according to the timing of parasite recovery.
In summary, the dormancy-recovery phenomenon provides a good explanation for the observed high rate of recrudescence following artemisinin mono-therapy, especially after short drug exposures. Although it does not directly provide explanations for the slow parasite clearance times emerging in Southeast Asia [18] dormancy may facilitate the development of artemisinin resistance as persister cells of various microbes have been associated with the emergence of resistant mutants [31]. Since dormancy appears to be a mechanism used by P. falciparum parasites to survive treatment with artemisinin, the possible link between dormancy and the development of resistance requires urgent attention. Since the success of current ACT treatment regimes hinges on the artemisinin component of the combination it is imperative that we confirm dormancy in vivo and find a molecular marker to assist with elucidating its impact on treatment efficacy and resistance.
Acknowledgements
The authors would like to thank the Australian Red Cross Blood Service in Brisbane for providing human erythrocytes and serum used for in vitro cultivation of P. falciparum.
Financial support: This work was supported by the National Institutes of Health grant # RO1-AI058973. M. L. Gatton is supported by NHMRC CDA.
Footnotes
Part of the information was presented at the “American Society of Tropical Medicine and Hygiene, Annual meeting”: 18–22 Nov 2009, Washington DC.
The authors do not have a commercial or other association that might pose a conflict of interest.
The opinions expressed herein are those of the authors and do not necessarily reflect those of the Defence Health Service or any extant policy of Department of Defence, Australia.
References
- 1.White NJ, Nosten F, Looareesuwan S, et al. Averting a malaria disaster. The Lancet. 1999;353:1965–1967. doi: 10.1016/s0140-6736(98)07367-x. [DOI] [PubMed] [Google Scholar]
- 2.WHO. World malaria report 2008. Geneva: World Health Organization; WHO/HTM/GMP/2008-1.
- 3.Meshnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiol Rev. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen DS, Dao BH, Nguyen PD, et al. Treatment of malaria in Vietnam with oral artemisinin. Am J Trop Med Hyg. 1993;48:398–402. [PubMed] [Google Scholar]
- 5.De Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- 6.White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–1422. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giao PT, Binh TQ, Kager PA, et al. Artemsinin for treatment of uncomplicated falciparum malaria: Is there a place for monotherapy? Am J Trop Med Hyg. 2001;65:690–695. doi: 10.4269/ajtmh.2001.65.690. [DOI] [PubMed] [Google Scholar]
- 8.Bwijo B, Hassan Alin M, Wernsdorfer W, Björkman A. Efficacy of artemisinin and mefloquine combinations against Plasmodium falciparum. In vitro simulation of in vivo pharmacokinetics. Trop Med Int Health. 1997;2:461–467. [PubMed] [Google Scholar]
- 9.Looareesuwan S. Overview of clinical studies on artemisinin derivatives in Thailand. Trans R Soc Trop Med Hyg. 1994;88 Suppl 1:9–11. doi: 10.1016/0035-9203(94)90462-6. [DOI] [PubMed] [Google Scholar]
- 10.Kyle DE, Webster HK. Postantibiotic effect of quinine and dihydroartemisinin on Plasmodium falciparum in vitro: implications for a mechanism of recrudescence. XIVth International Congress for Tropical Medicine and Malaria; Nagasaki, Japan. 1996. (abstract) [Google Scholar]
- 11.Hoshen MB, Na-Bangchang K, Stein WD, Ginsburg H. Mathematical modelling of the chemotherapy of Plasmodium falciparum malaria with artesunate: postulation of 'dormancy', a partial cytostatic effect of the drug, and its implication for treatment regimens. Parasitology. 2000;121(Pt 3):237–246. doi: 10.1017/s0031182099006332. [DOI] [PubMed] [Google Scholar]
- 12.Bigger JW. Treatment of staphylococcal infections with penicillin. Lancet. 1944:497–500. [Google Scholar]
- 13.Gomez JE, Mc Kinney JD. M. tuberculosis persistence, latency and drug tolerance. Tuberculosis (Edinb) 2004;84:29–44. doi: 10.1016/j.tube.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 15.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 16.Ribaut C, Berry A, Chevalley S, et al. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malaria J. 2008;7 doi: 10.1186/1475-2875-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlemann AC, Staalsoe T, Klinkert MQ, Hviid L. Analysis of Plasmodium falciparum-infected red blood cells. MACS & More. 2000;4:7–8. [Google Scholar]
- 18.Dondorp AM, Nosten F, Yi PDD, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Witkowski B, Lelievre J, Lopez Barragan MJ, et al. Increased tolerance to artemisinin in Plasmodium falciparum is mediated by a quiescence mechanism. Antimicrob Agents Chemother. 2010;54:1872–1877. doi: 10.1128/AAC.01636-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White NJ. Qinghaosu (Artemisinin): The price of success. Science. 2008;320:330–334. doi: 10.1126/science.1155165. [DOI] [PubMed] [Google Scholar]
- 21.Bwijo B, Hassan Alin M, Abbas N, Eriksson Ö, Björkman A. Repetitive dosing of artemisinin and quinine against Plasmodium falciparum in vitro: a simulation of the in vivo pharmacokinetics. Acta Trop. 1997;65:11–22. doi: 10.1016/s0001-706x(96)00565-7. [DOI] [PubMed] [Google Scholar]
- 22.Hassan Alin M, Ashton M, Kihamia CM, Mtey GJB, Björkman A. Multiple dose pharmacokinetics of oral artemisinin and comparison of its efficacy with that of oral artesunate in falciparum malaria patients. Trans R Soc Trop Med Hyg. 1996;90:61–65. doi: 10.1016/s0035-9203(96)90480-0. [DOI] [PubMed] [Google Scholar]
- 23.Menard D, Matsika-Claquin MD, Djalle D, et al. Association of failures of seven-day courses of artesunate in a non-immune population in Bangui, Central African Republic with decreased sensitivity of Plasmodium falciparum. Am J Trop Med Hyg. 2005;73:616–621. [PubMed] [Google Scholar]
- 24.Borrmann S, Adegnika AA, Missinou MA, et al. Short-course artesunate treatment of uncomplicated Plasmodium falciparum malaria in Gabon. Antimicrob Agents Chemother. 2003;47:901–904. doi: 10.1128/AAC.47.3.901-904.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White NJ. Delaying antimalarial drug resistance with combination chemotherapy. Parassitologia. 1999;41:301–308. [PubMed] [Google Scholar]
- 26.Looareessuwan S, Kyle DE, Viravan C, et al. Treatment of patients with recrudescent falciparum malaria with a sequential combination of artesunate and mefloquine. Am J Trop Med Hyg. 1992;47:794–799. doi: 10.4269/ajtmh.1992.47.794. [DOI] [PubMed] [Google Scholar]
- 27.Nosten F, Van Vugt M, Luxemburger C, et al. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet. 2000;356:297–301. doi: 10.1016/s0140-6736(00)02505-8. [DOI] [PubMed] [Google Scholar]
- 28.Carrara VI, Zwang J, Ashley EA, et al. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of deployment. PloS ONE. 2009;4:e4551. doi: 10.1371/journal.pone.0004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez LEA, Jurado MH, Cambon N. Randomised efficacy and safety study of two 3-day artesunate rectal capsule/mefloquine regimens versus artesunate alone for uncomplicated malaria in Ecuadorian children. Acta Trop. 2003;89:47–53. doi: 10.1016/j.actatropica.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Hung LE, De Vries PJ, Binh TQ, et al. Artesunate with mefloquine at various intervals for non-severe Plasmodium falciparum malaria. Am J Trop Med Hyg. 2004;71:160–166. [PubMed] [Google Scholar]
- 31.Lewis K. Persister cells, dormancy and infectious disease. Nature Reviews Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]