Abstract
Background
Cocaine users not seeking treatment have increased regional brain mu-opioid receptor (mOR) binding that correlates with cocaine craving and tendency to relapse. In cocaine-abusing outpatients in treatment, the relationship of mOR binding and treatment outcome is unknown.
Methods
We determined whether regional brain mOR binding before treatment correlates with outcome and compared it to standard clinical predictors of outcome. Twenty-five individuals seeking outpatient treatment for cocaine abuse or dependence (DSM-IV) received up to 12 weeks of cognitive-behavioral therapy and cocaine-abstinence reinforcement whereby each cocaine-free urine was reinforced with vouchers redeemable for goods. Regional brain mOR binding was measured before treatment using positron emission tomography (PET) with [11C] carfentanil (a selective mOR agonist). Main outcome measures were: 1) overall percentage of urines positive for cocaine during first month of treatment, 2) longest duration (weeks) of abstinence from cocaine during treatment, all verified by urine toxicology.
Results
Elevated mOR binding in the medial frontal and middle frontal gyri before treatment correlated with greater cocaine use during treatment. Elevated mOR binding in the anterior cingulate, medial frontal, middle frontal, middle temporal, and sub-lobar insular gyri correlated with shorter duration of cocaine abstinence during treatment. Regional mOR binding contributed significant predictive power for treatment outcome beyond that of standard clinical variables such as baseline drug and alcohol use.
Conclusions
Elevated mOR binding in brain regions associated with reward sensitivity is a significant independent predictor of treatment outcome in cocaine-abusing outpatients, suggesting a key role for the brain endogenous opioid system in cocaine addiction.
Keywords: cocaine, mu-opioid receptor, PET, treatment, addiction, dependence
Introduction
Cocaine addiction is a chronic disease for which there is no broadly effective treatment (1), and few reliable predictors of treatment outcome (2). Reliable long-term predictors of outcome could be used to target treatment resources more effectively.
One potential predictor is brain mu-opioid receptor (mOR) binding. In preclinical studies, intermittent cocaine administration for 2 weeks increases mOR binding in brain regions associated with reward sensitivity (3). In rats, activation of mOR in ventral pallidum or nucleus accumbens reinstates previously extinguished cocaine seeking-behavior (an animal model of relapse), while blockade of mOR reduces reinstatement (4, 5). In non-treatment-seeking human cocaine users on a closed research ward, positron emission tomography (PET) with the mOR agonist [11C]-carfentanil showed that mOR binding was elevated in several brain regions, including frontal and middle temporal cortices, and correlated positively with the severity of self-reported cocaine craving (6, 7). Furthermore, there was a positive association between increased mOR binding in frontal and temporal cortices and shorter interval before resumption of cocaine use after discharge (8).
The present study is the first to determine whether regional brain mOR binding prior to treatment is a reliable independent predictor of treatment outcome in cocaine-abusing outpatients participating in treatment. The primary outcome measures, percentage of cocaine-positive urine samples during the first month of treatment and the longest duration of cocaine abstinence during treatment, were assessed by urine toxicology. Regional brain mOR binding was measured prior to treatment initiation using PET with [11C]-carfentanil.
Methods and Materials
Subjects and Setting
Participants were 25 adult treatment seekers with current cocaine abuse or dependence (DSM-IV criteria) recruited from the community to an outpatient treatment program at the National Institute on Drug Abuse (NIDA) Intramural Research Program (IRP) in Baltimore, Maryland. Screening included medical, psychiatric, and drug-use histories, physical examination, urine and blood tests, Addiction Severity Index (ASI) (9), Shipley Institute of Living Scale (10), and the Diagnostic Interview Schedule (DIS-IV) (11). Eligibility criteria included: at least one positive urine test for cocaine during screening; 18 to 50 years old; seeking treatment for cocaine dependence or abuse. Exclusion criteria included: use of opioids more than 3 times within the past three months; current dependence on or abuse of psychoactive substances except for cocaine or tobacco (DSM-IV criteria); current primary DSM-IV Axis 1 psychiatric disorder (other than substance-use disorder, adjustment disorder, phobia, or posttraumatic stress disorder); any medical condition that would preclude safe participation; history of adverse reactions to opioids; Shipley vocabulary score below 18 (6th-grade reading level); cognitive impairment; abnormal magnetic resonance imaging (MRI) brain scan; clinically significant CNS disease; HIV infection; seizure disorder or history of head injury with loss of consciousness for more than three minutes; claustrophobia; treatment within the past three months with antidepressants, neuroleptics, sedative-hypnotics, isoniazid, glucocorticoids, or psychostimulants; currently pregnant or nursing; blood oxygen saturation (by pulse oximetry) < 95% on room air.
The study was approved by the institutional review boards of the NIDA IRP and the Johns Hopkins Bayview Medical Center. All participants gave written informed consent and were paid for participation.
MRI scan and PET image Acquisition
Brain mOR binding was measured with [11C]-carfentanil, a selective mu-opioid agonist (12). A total of 25 dynamic PET frames were acquired over 90 minutes on a GE Advance PET scanner (GE Medical Systems, Milwaukee, WI) in 3D mode following a bolus injection of [11C]-carfentanil (mean (SD) dose: 19.5 (1.2) mCi; range = 16.3–21.1 mCi; mean (SD) specific activity: 3213 (2351) mCi/µmol; range = 1475–11090 mCi/µmol). Images were reconstructed with the back-projection algorithm with a ramp filter, using the manufacturer’s software, with correction for attenuation, scatter, dead-time, and physical decay to the injection time. Each frame consisted of a 128 × 128 × 35 matrix with voxel size of 2 × 2 × 4.25 mm. Prior to PET scanning, each subject had a standard spoiled-gradient (SPGR) MRI on a 1.5 T Signa Advantage system (GE Medical Systems, Milwaukee, WI).
PET Analysis
The primary measure of mOR availability was [11C]-carfentanil binding potential (BPND) (12). BPND volumes (voxel-by-voxel maps of BPND) were generated with a reference-tissue graphical analysis (RTGA) (13), using occipital lobe as the reference region and setting the brain-blood transport rate constant of the reference region at 0.104 min−1 (14, 15).
The BPND volumes were spatially normalized to a standard brain supplied with SPM2 using BP volume-to-MRI co-registration parameters and MRI-to-standard MRI spatial normalization parameters in one step (16–18). The BPND volumes were smoothed using a 12-mm FWHM Gaussian kernel before submitting to SPM analyses.
Treatment and Outcome Data Collection
Each participant had a PET scan prior to initiation of a 12-week treatment program. PET scans were conducted a mean (standard deviation [SD]) of 5.80 (1.68) days prior to initiation of the treatment program. PET scans were also conducted after 4 and 12 weeks of treatment; only the results of the pre-treatment PET scan are reported here. To increase the likelihood that participants did not use cocaine before the PET scan, they were paid $50 if a urine specimen collected immediately before the scan tested negative for cocaine.
Treatment was a combination of cognitive-behavior therapy (CBT) and abstinence reinforcement. All participants were offered weekly, manual-based individual CBT in which they were taught self-control skills to increase nondrug sources of reinforcement (19), and develop adaptive coping responses to stress (20, 21). Counseling was provided by trained master’s-level social workers or psychologists who had previous experience in addiction treatment. For abstinence reinforcement, 20 participants received vouchers redeemable for goods and services for each urine free of cocaine (22). Six control participants received vouchers on a yoked schedule. For the analyses reported here, these two groups were combined because there was no statistically significant group difference in percentage of cocaine-positive urines during treatment (63.4% (39.6) vs. 81.5% (36.4); t = −0.43, p >0.05, df = 23) or in the longest duration (weeks) of abstinence from cocaine (2.3 (3.4) weeks vs. 1.1 (2.1) weeks; t =0.86, p>0.05, df =23).
Urine specimens were collected under staff observation before each PET scan, three times per week in the clinic (usually Mondays, Wednesdays, and Fridays) during treatment, and at follow-up. Specimens were tested for cocaine (benzoylecgonine equivalents; BZE), opiates (morphine), cannabinoids, and benzodiazepines (oxazepam) by Enzyme Multiplied Immunoassay Technique (EMIT urine test; Syva Corp., Palo Alto, California). Cutoffs were 300 ng/ml for cocaine, opiates, and benzodiazepines, and 50 ng/ml for cannabinoids.
Data Analyses
“Simple regression” analyses in SPM2 were used to test the association between baseline brain mOR binding and treatment outcome, which was operationalized as cocaine use, expressed as: (1) overall percentage of urines positive for cocaine during the first month of treatment, and (2) longest duration (weeks) of abstinence from cocaine during treatment, all verified by urine toxicology. Only the first month of treatment was used to measure outcome due to the high dropout rate during the last 2 months of treatment (only 12 of the 25 participants remained over this period) and consequent limited statistical power over that timeframe. An association was considered significant if a cluster of ≥50 contiguous voxels exceeded an uncorrected peak threshold of P<0.001 (23). This is a widely-accepted approach for controlling the experiment-wide error rate and minimizing type I error (false positive findings) in brain imaging studies that use voxel-based analysis rather than pre-specified regions of interest (24–26). Corrected thresholds of significance sometimes used in other scientific contexts (e.g., FDR or FWE correction) are impractical here because millions of image voxels are being compared in the analyses. (None of the brain imaging associations in this study would have achieved statistical significance using the FDR or FWE correction.) Due to the skewed distribution of the data, data regarding percentage of urines positive for cocaine during the first month of treatment were log-transformed, and data regarding longest duration (weeks) of abstinence from cocaine were cubic-root transformed.
The relative predictive values of mOR binding and participants’ pre-treatment clinical characteristics were evaluated with multiple linear regression analyses in SAS (version 9.1 for Windows, SAS Institute, Cary, NC). First, univariate regression analyses were conducted between clinical characteristics (obtained from ASI and DIS-IV interviews done at screening) and treatment-outcome measures. Clinical predictor variables used in these regression analyses were those suggested as possible predictors by prior studies (2, 8): a cocaine-positive urine sample at the time of first PET scan (i.e., prior to starting treatment); self-reported number of days in the 30 days prior to screening using cocaine, alcohol, or heroin, or having drug or employment problems; amount of money spent on drugs in the 30 days prior to screening; lifetime years of cocaine use; marital status; usual employment pattern in the last 3 years; and years of education completed. Clinical variables that were significant in these univariate regressions were then included with regional brain mOR binding in multiple regressions to assess their relative predictive contribution. In addition, sex and baseline tobacco use status were included as variables in separate sets of multiple regression analyses because prior studies suggest that men and women may differ in regional brain mOR binding (27, 28) and in mOR binding changes in response to pain (29) and because some (but not all) animal studies suggest that nicotine may alter brain mOR binding (30). The entry criterion was SPM threshold (t) value <0.005 for regions that achieved statistical significance in both analyses, and (t) value <0.001 for regions that achieved statistical significance in one of the two analyses. These analyses used mean mOR binding in the most significant cluster in the voxel-based SPM analysis.
To obtain unbiased estimators of the robustness of these results and to test whether they are resistant to outlier effects, we conducted Huber robust regression analyses (31). These analyses included a change point of 1.0 as an outlier value. To assess the potential contribution of pre-treatment differences in substance use to the association between mOR binding and our primary outcome measures, we evaluated in SPM the relationship between pre-treatment mOR binding and pre-treatment drug-use characteristics (cocaine use, obtained by EMIT urine test, and alcohol use and tobacco smoker and nicotine dependence status, obtained from screening interviews).
Results
Forty-five participants enrolled in the study. Of these, two were disqualified because of MRI findings, one could not tolerate MRI scanning, one had a urine sample positive for opiates at the first PET scan, and six did not appear for their first PET scan, leaving 35 participants with PET scans. Of these 35, nine never started outpatient treatment, and one started treatment but never provided a urine specimen. The 25 participants who started treatment and provided at least one urine specimen (only 1 participant provided only one urine specimen) are included in these analyses. The 12 participants who completed the study did not differ significantly from the 13 who did not complete in any pre-treatment demographic or drug use characteristics (Table 1). Participants who completed the study did not differ significantly from those who did not complete in terms of providing a cocaine-negative urine specimen during the initial assessment (4 completers versus 6 non-completers). There was no significant difference between study completers and non-completers in the relationship of mOR binding to treatment outcome, nor did completer status (when included as a covariate in multiple regression analyses) have a significant influence on the relationship between pre-treatment regional mu-opioid receptor binding levels and treatment outcome measures. No participant was clinically depressed at the time of admission, as expected from the study eligibility criteria. There were no significant differences in demographic and drug use characteristics between the 25 participants included in the analyses and the 20 who were not (data not shown).
Table 1.
Pretreatment Characteristics of 25 Cocaine-Abusing Outpatients (mean ± SD)
| Age (years) | 39.7±5.0 |
| Gender (Female) | 31% |
| Race (Non-Caucasian) | 65% |
| Cocaine Use, Lifetime (years) | 9.5±7.5 |
| Days of Cocaine Use* | 19.9±7.7 |
| Heroin Use, Lifetime (years) | 0.1±0.4 |
| Days of Heroin Use* | 0.1±0.3 |
| Employment Income* ($) | 1235±1283 |
| Money spent on drugs* ($) | 1372±1848 |
| Days with Drug Problems* | 19.9±11.1 |
| Usual employment pattern, last 3 years time, 4% In controlled environment |
65% Full time, 19% Unemployed, 12% Part |
| Marital status 46% Never Married, 4% Widowed |
15% Married, 12% Separated, 23% Divorced, |
in the 30 days prior to screening for study enrollment.
Seventeen participants tested positive for cocaine at their first PET scan. An SPM t-test showed no differences in regional mOR binding between participants whose urine specimens were positive for cocaine at the first PET scan (a measure of cocaine use within the prior 2–3 days) and participants with cocaine-free urine specimens (data not shown).
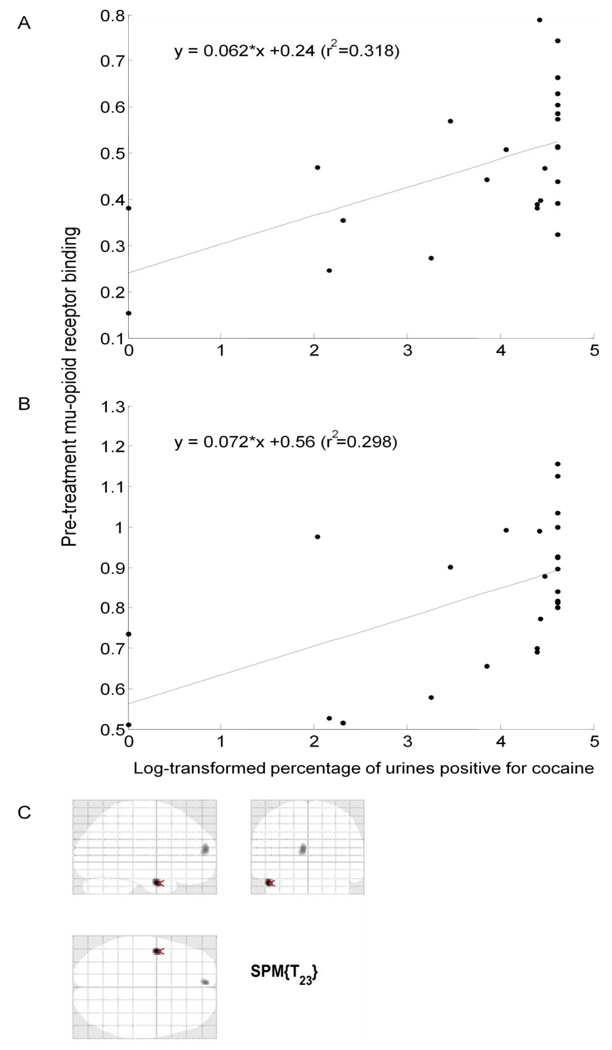
Regional brain mOR binding before treatment was significantly associated with subsequent degree of cocaine use during the first month of treatment, as reflected in the percentage of cocaine-positive urine specimens (mean (SD) = 68% (39), median = 83%). Regional mOR binding in the right middle frontal gyrus (Figure 1a, Table 2a) and left medial frontal gyrus (Figure 1b, Table 2b) was positively correlated with the percentage of cocaine-positive urine specimens during the first month of treatment. Pre-treatment cocaine use (as reflected in a cocaine-positive urine sample at the first PET scan) was also associated with a greater overall percentage of urine specimens positive for cocaine during treatment (78% vs. 46%) (t = 2.89, df = 23, p< 0.01). Therefore, the result of the pre-PET urine cocaine test was included as a covariate in multiple regression analyses evaluating the relationship between mOR binding and cocaine use during treatment. No other non-PET clinical variable (see 'Methods' section) evaluated in univariate regression analyses was significantly associated with this outcome measure (data not shown). Multiple-regression analyses showed that mOR binding in the right middle frontal gyrus (t = 2.94, p<0.01, standardized coefficient beta = 0.53) was a stronger predictor of treatment outcome than was cocaine use at pre-treatment PET (t = 0.63, p>0.05, standardized coefficient beta = 0.11). This relationship was also true for mOR binding in the left medial frontal gyrus (t = 2.73, p = 0.01, standardized coefficient beta = 0.52; vs. t = 0.39, p>0.05, standardized coefficient beta = 0.07). Adding sex as a variable in the multiple regression analyses did not significantly alter these relationships (data not shown).
Figure 1.
Association of regional mOR binding before treatment and overall percentage of urine specimens positive for cocaine during the first month of treatment. Pre-treatment mOR binding in the (A) right middle frontal gyrus, and (B) left medial frontal gyrus shows a significant positive correlation with percentage of urine specimens positive for cocaine. See Table 2 for statistics. Lines are least squares best fit to data points. Display threshold for mOR binding is p<0.005 (uncorrected); cluster size k>50.
Table 2.
Association of Pre-Treatment Brain Regional Mu-Opioid Receptor Binding and log-transformed percentage of urine specimens positive for cocaine during the first month of treatment (n=25)
| Brain | Side of Brain | SPM Result (voxel-level) | Correlation Using Mean Value from the Cluster | ||||
|---|---|---|---|---|---|---|---|
| Region | MNIa (x,y,z) | Max-z | T-value (df=23) | kb | r2 | p-value | |
| (A) Middle Frontal Gyrus Right | 32, 18, 32 | 3.09 | 3.49 | 63 | 0.32 | <0.005 | |
| (B) Medial Frontal Gyrus Left | −16, 52, 4 | 2.85 | 3.15 | 140 | 0.30 | <0.005 | |
SPM, statistical parametric mapping; MNI, Montreal Neurological Institute
Location of peak expressed as x,y,z coordinates in MNI space.
Cluster size k represents number of voxels (2 × 2 × 2 mm3) at a height threshold of p = .005, uncorrected. A correlation was considered significant if a cluster of ≥50 contiguous voxels exceeded an uncorrected peak threshold of P<0.005 (23).
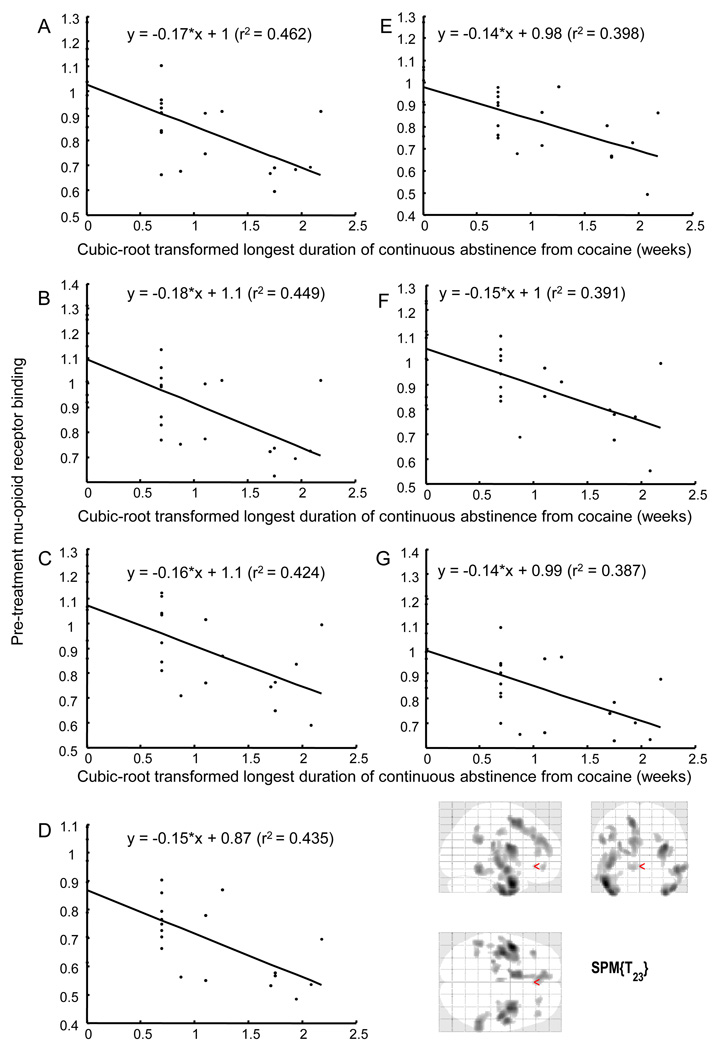
Regional mOR binding in the anterior cingulate, medial frontal, middle frontal, middle temporal, and sub-lobar insular gyri before treatment negatively correlated with the longest duration of abstinence from cocaine achieved during treatment (Figure 2, Table 3) (mean (SD) = 2.0 (3.1) weeks, median = 0.33 weeks). Multiple-regression analyses showed that mOR binding in the left middle temporal gyrus extending to the sub-lobar insular gyrus (t = −4.23, p<0.005, standardized coefficient beta = −0.66) was a stronger predictor of this outcome measure than was cocaine use at pre-treatment PET (t = −0.93, p>0.05, standardized coefficient, beta = −0.14). This relationship was also true for mOR binding in the right middle temporal gyrus (t = −4.08, p<0.001, standardized coefficient beta = −0.65; vs. t =−0.85, p>0.05, standardized coefficient, beta = −0.13), mOR binding in the left medial frontal gyrus (t = −3.76, p<0.005, standardized coefficient beta = −0.63; vs. t =−0.40, p>0.05, standardized coefficient, beta = −0.07), mOR binding in the right middle temporal gyrus (t = −4.06, p<0.001, standardized coefficient beta = − 0.64; vs. t = −1.08, standardized coefficient beta = −0.17), mOR binding in the left middle frontal gyrus (t = −3.57, p<0.005, standardized coefficient beta = −0.61; vs. t =−0.54, p>0.05, standardized coefficient, beta = −0.09), and mOR binding in the anterior cingulate gyrus (t = − 3.54, p<0.005, standardized coefficient beta = −0.60; vs. t =−0.65, p>0.05, standardized coefficient, beta = −0.11). Huber robust regression analyses revealed the same results as the regression analyses described above assessing the relationships between pre-treatment mOR binding and cocaine-use outcome measures. This equivalence suggests that our findings are robust and resistant to outlier effects. Including sex as a variable in the multiple regression analyses did not significantly alter these relationships (data not shown).
Figure 2.
Association of regional mOR binding before treatment and duration (weeks) of longest abstinence from cocaine achieved during treatment. Pre-treatment mOR binding in the (A) left middle temporal gyrus extending to the left sub-lobar insular gyrus, (B) right middle temporal gyrus, (C) left medial frontal gyrus, (D) right middle temporal gyrus, (E) left middle frontal gyrus, (F) left anterior cingulate gyrus, and (G) right middle frontal gyrus shows a significant negative correlation with the duration of longest abstinence achieved during treatment. See Table 3 for statistics. Lines are least squares best fit to data points. Display threshold for mOR binding is p<0.001 (uncorrected); cluster size k>50.
Table 3.
Association of Pre-Treatment Brain Regional Mu-Opioid Receptor Binding and Cubic-Root Transformed Longest Duration of Abstinence (Weeks) from Cocaine Achieved during Treatment (n=25)
| Brain | Side of Brain | SPM Result (voxel-level) | Correlation Using Mean Value from the Cluster | ||||
|---|---|---|---|---|---|---|---|
| Region | MNIa (x,y,z) | Max-z | T-value (df=23) | kb | r2 | p-value | |
| (A) Middle Temporal Gyrus Left extending to Sub-lobar Insula Left |
−48, 2, −30 | 4.23 | 5.28 | 1832 | 0.46 | <0.001 | |
| (B) Middle Temporal Gyrus Right | 36, 2, −42 | 3.92 | 4.75 | 830 | 0.45 | <0.001 | |
| (C) Medial Frontal Gyrus Left | −10, 6, 54 | 3.78 | 4.51 | 900 | 0.42 | <0.001 | |
| (D) Middle Temporal Gyrus Right | 54, −44, −12 | 3.68 | 4.36 | 329 | 0.44 | <0.001 | |
| (E) Middle Frontal Gyrus Left | −40, 32, 34 | 3.52 | 4.1 | 91 | 0.40 | <0.001 | |
| (F) Anterior Cingulate Gyrus Left | −4, −14, 40 | 3.38 | 3.9 | 50 | 0.39 | <0.001 | |
| (G) Middle Frontal Gyrus Right | 44, 40, 14 | 3.33 | 3.83 | 68 | 0.39 | <0.001 | |
SPM, statistical parametric mapping; MNI, Montreal Neurological Institute
Location of peak expressed as x,y,z coordinates in MNI space.
Cluster size k represents number of voxels (2 × 2 × 2 mm3) at a height threshold of p = .001, uncorrected. A correlation was considered significant if a cluster of ≥50 contiguous voxels exceeded an uncorrected peak threshold of P<0.001 (23).
SPM analyses showed that the relationship between pre-treatment mOR binding and the primary outcome measures was not explained by differences in pre-treatment use of nicotine or alcohol. An SPM t-test showed no differences in regional mOR binding between participants who were tobacco smokers vs. nonsmokers at study entry. Univariate regression analyses showed no significant association of any outcome measure with pre-treatment alcohol use or nicotine dependence status. Moreover, including pretreatment cigarette smoking, alcohol use, or nicotine dependence status as covariates in multiple regression analyses did not significantly alter the relationship between regional mOR binding and cocaine-use outcome measures (data not shown). Furthermore, a SPM “simple regression” analysis showed no association between amount of pre-treatment alcohol use and regional mOR binding.
Discussion
The present study found that elevated regional brain mOR binding in the anterior cingulate, medial frontal, middle frontal, middle temporal, and sub-lobar insular gyri before treatment was associated with a shorter duration of cocaine abstinence achieved during treatment among adult, cocaine-abusing or -dependent outpatients. In addition, elevated mOR binding in the medial and middle frontal gyri before treatment correlated with greater cocaine use during the 1st month of outpatient treatment. Because of stringent subject eligibility criteria, the results are unlikely to be affected by common clinical confounds such as depression or co-morbid substance use disorders, nor are they likely due to differences in alcohol or tobacco use. Regional mOR binding contributed significant predictive power beyond that provided by standard clinical variables such as drug and alcohol use. All significant R2 values were between 0.30 to 0.46. R2 values rarely exceeded 0.10 in previous published studies of predictors of cocaine treatment outcome (32, 33). Furthermore, Huber robust regression analyses revealed that the findings were robust and resistant to outlier effects.
The present findings have important clinical implications because they demonstrate, for the first time, the existence of biomarkers significantly associated with response to outpatient treatment for cocaine-use disorders. These results complement our previous findings in a non-treatment residential sample that increased mOR binding in these same brain regions correlates with self-reported cocaine craving during monitored abstinence on a residential research ward (7) and with time to relapse to cocaine use after release from monitored abstinence into the community (8).
Our findings are relevant to randomized clinical trials showing an important role for brain mOR activation in psychostimulant abuse. These trials showed that buprenorphine, a partial mOR agonist, reduces cocaine use in outpatients concurrently dependent on cocaine and opiates (34), and that naltrexone, a mOR antagonist, reduces amphetamine use in amphetamine-dependent outpatients (35).
Our findings might apply to individuals with substance-use disorders other than cocaine. The brain regions with elevated mOR binding in this study are neuroanatomically connected with the mesolimbic dopamine system, a distributed neural network implicated in the reinforcing effects of abused drugs. The medial frontal and anterior cingulate cortices have been shown in a rat model of relapse, the reinstatement model, to play a critical role in several types of relapse behaviors, including drug-, cue-, and stress-induced reinstatement of cocaine, opiate, and alcohol seeking (36). Reinstatement of cocaine-seeking behavior in rats is reduced following repeated treatment with the mOR antagonist naltrexone (37), supporting a role for mOR activation in relapse. In cocaine abusers undergoing fMRI scans, these cortical brain regions show increased activity associated with cocaine craving in response to cocaine-associated cues (38, 39). Moreover, activation of the insular cortex is critically involved in drug craving. Patients with lesions to the right anterior insula or left or right dorsal posterior insula show disruption of preexisting nicotine addiction (40). Further, brain imaging studies demonstrate increased activity in the insula associated with heightened drug craving (41). Lastly, in treatment-seeking methamphetamine users, lower activation in the middle temporal cortex during a decision-making task reliably predicted greater susceptibility to relapse to methamphetamine use (42). Collectively, these findings implicate brain-reward circuitry including the brain regions mentioned above in critical aspects of drug-seeking and drug-craving behaviors in individuals with substance-use disorders.
Conclusions
We demonstrated that elevated pre-treatment regional brain mOR binding in the anterior cingulate, medial frontal, middle frontal, middle temporal, and sub-lobar insular gyri before treatment was associated with shorter duration of cocaine abstinence during treatment, suggesting an important role for the brain endogenous opioid system in cocaine addiction. Our findings suggest that PET imaging of regional mOR binding before treatment may be a useful tool for identifying those patients most likely to need more intensive treatment. This would benefit patients themselves and the drug abuse treatment system by allowing more efficient allocation of scarce treatment resources.
Acknowledgements
This study was supported in part by the Intramural Research Program of the NIH, NIDA and in part by NIH grant R01 DA-09479 to JJF. Yu Kyeong Kim, MD assisted in preliminary statistical analyses in a subset of participants. Robert Dannals, PhD, oversaw the preparation of C-11 carfentanil of the PET scans.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knapp WP, Soares BG, Farrel M, Lima MS. Psychosocial interventions for cocaine and psychostimulant amphetamines related disorders. Cochrane Database Syst Rev. 2007;3 doi: 10.1002/14651858.CD003023.pub2. CD003023. [DOI] [PubMed] [Google Scholar]
- 2.Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33:191–206. doi: 10.1080/00952990701199416. [DOI] [PubMed] [Google Scholar]
- 3.Unterwald EM. Regulation of opioid receptors by cocaine. Ann N Y Acad Sci. 2001;937:74–92. doi: 10.1111/j.1749-6632.2001.tb03559.x. [DOI] [PubMed] [Google Scholar]
- 4.Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J Neurosci. 2005;25:4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmons D, Self DW. Role of mu-and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nature Med. 1996;2:1225–1229. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
- 7.Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, et al. Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry. 2005;57:1573–1582. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino ML, et al. Brain mu-opioid receptor binding: relationship to relapse to cocaine use after monitored abstinence. Psychopharmacology (Berl) 2008;200:475–486. doi: 10.1007/s00213-008-1225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLellan AT, Luborsky L, Cacciola J, Griffith J, Evans F, Barr HL, et al. New data from the Addiction Severity Index. Reliability and validity in three centers. J Nerv Ment Dis. 1985;173:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Zachary RA. Shipley Institute of Living Scale. Revised Manual. Los Angeles, CA: Western Psychological Services; 1986. [Google Scholar]
- 11.Robins LN, Cottler LB, Bucholz KK, Compton WMI. The Diagnostic Interview Schedule, version IV. St. Louis, MO: Washington University; 1995. [Google Scholar]
- 12.Frost JJ, Wagner HN, Jr, Dannals RF, Ravert HT, Links JM, Wilson AA, et al. Imaging opiate receptors in the human brain by positron tomography. J Comput Assist Tomogr. 1985;9:231–236. doi: 10.1097/00004728-198503000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Guardia J, Catafau AM, Batlle F, Martin JC, Segura L, Gonzalvo B, et al. Striatal dopaminergic D(2) receptor density measured by [(123)I]iodobenzamide SPECT in the prediction of treatment outcome of alcohol-dependent patients. Am J Psychiatry. 2000;157:127–129. doi: 10.1176/ajp.157.1.127. [DOI] [PubMed] [Google Scholar]
- 15.Endres CJ, Bencherif B, Hilton J, Madar I, Frost JJ. Quantification of brain mu-opioid receptors with [11C] carfentanil: reference-tissue methods. Nucl Med Biol. 2003;30:177–186. doi: 10.1016/s0969-8051(02)00411-0. [DOI] [PubMed] [Google Scholar]
- 16.Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging. 1997;16:187–198. doi: 10.1109/42.563664. [DOI] [PubMed] [Google Scholar]
- 17.Ashburner J, Friston K, et al. Spatial normalization using basis functions. In: Frackowiak R, Friston K, Frith C, editors. Human Brain Function. 2nd ed. Academic Press; 2003. pp. 655–672. [Google Scholar]
- 18.Ashburner J, Friston K, et al. Rigid body registration. In: Frackowiak R, Friston K, Frith C, et al., editors. Human Brain Function. 2nd ed. Academic Press; pp. 635–654. [Google Scholar]
- 19.Brown RA, Lewinsohn PM. A psychoeducational approach to the treatment of depression: comparison of group, individual, and minimal contact procedures. J Consult Clin Psychol. 1984;52:774–783. doi: 10.1037//0022-006x.52.5.774. [DOI] [PubMed] [Google Scholar]
- 20.Folkman S, Lazarus RS, Gruen RJ, DeLongis A. Appraisal, coping, health status, and psychological symptoms. J Pers Soc Psychol. 1986;50:571–579. doi: 10.1037//0022-3514.50.3.571. [DOI] [PubMed] [Google Scholar]
- 21.Marlatt GA, Curry S, Gordon JR. A longitudinal analysis of unaided smoking cessation. J Consult Clin Psychol. 1988;56:715–720. doi: 10.1037//0022-006x.56.5.715. [DOI] [PubMed] [Google Scholar]
- 22.Epstein DH, Hawkins WE, Covi L, Umbricht A, Preston KL. Cognitive-behavioral therapy plus contingency management for cocaine use: findings during treatment and across 12-month follow-up. Psychol Addict Behav. 2003;17:73–82. doi: 10.1037/0893-164X.17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poline JB, Worsley KJ, Holmes AP, Frackowiak RS, Friston KJ. Estimating smoothness in statistical parametric maps: variability of p values. J Comput Assist Tomogr. 1995;19:788–796. doi: 10.1097/00004728-199509000-00017. [DOI] [PubMed] [Google Scholar]
- 24.Yasuno F, Ota M, Ando K, Ando T, Maeda J, Ichimiya T, et al. Role of ventral striatal dopamine D1 receptor in cigarette craving. Biol Psychiatry. 2007;61:1252–1259. doi: 10.1016/j.biopsych.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. A positron emission tomography study of memories of childhood abuse in borderline personality disorder. Biol Psychiatry. 2004;55:759–765. doi: 10.1016/j.biopsych.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Wong DF, Kuwabara H, Schretlen DJ, Bonson KR, Zhou Y, Nandi A, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology. 2006;31:2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 27.Zubieta JK, Dannals RF, Frost JJ. Gender and age influences on human brain mu-opioid receptor binding measured by PET. Am J Psychiatry. 1999;156:842–848. doi: 10.1176/ajp.156.6.842. [DOI] [PubMed] [Google Scholar]
- 28.Gabilondo A, Meana J, Garcia-Sevilla J. Increased density of μ-opioid receptors in the postmortem brain of suicide victims. Brain Res. 1995;682:245–250. doi: 10.1016/0006-8993(95)00333-l. [DOI] [PubMed] [Google Scholar]
- 29.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berrendero F, Robledo P, Trigo JM, Martín-García E, Maldonado R. Neurobiological mechanisms involved in nicotine dependence and reward: Participation of the endogenous opioid system. Neurosci Biobehav Rev. 2010 doi: 10.1016/j.neubiorev.2010.02.006. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rousseeuw RJ, Leroy AM. Robust Regression and Outlier Detection. New York, NY: Wiley; 1987. [Google Scholar]
- 32.Ahmadi J, Kampman K, Dackis C. Outcome predictors in cocaine dependence treatment trials. Am J Addict. 2006;15:434–439. doi: 10.1080/10550490600998476. [DOI] [PubMed] [Google Scholar]
- 33.Reiber C, Ramirez A, Parent D, Rawson RA. Predicting treatment success at multiple timepoints in diverse patient populations of cocaine-dependent individuals. Drug Alcohol Depend. 2002;68:35–48. doi: 10.1016/s0376-8716(02)00103-5. [DOI] [PubMed] [Google Scholar]
- 34.Montoya ID, Gorelick DA, Preston KL, Schroeder JR, Umbricht A, Cheskin LJ, et al. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jayaram-Lindstrom N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165:1442–1448. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- 36.Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: An update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Gerrits MA, Kuzmin AV, van Ree JM. Reinstatement of cocaine-seeking behavior in rats is attenuated following repeated treatment with the opioid receptor antagonist naltrexone. Eur Neuropsychopharmacol. 2005;15:297–303. doi: 10.1016/j.euroneuro.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 39.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]