Abstract
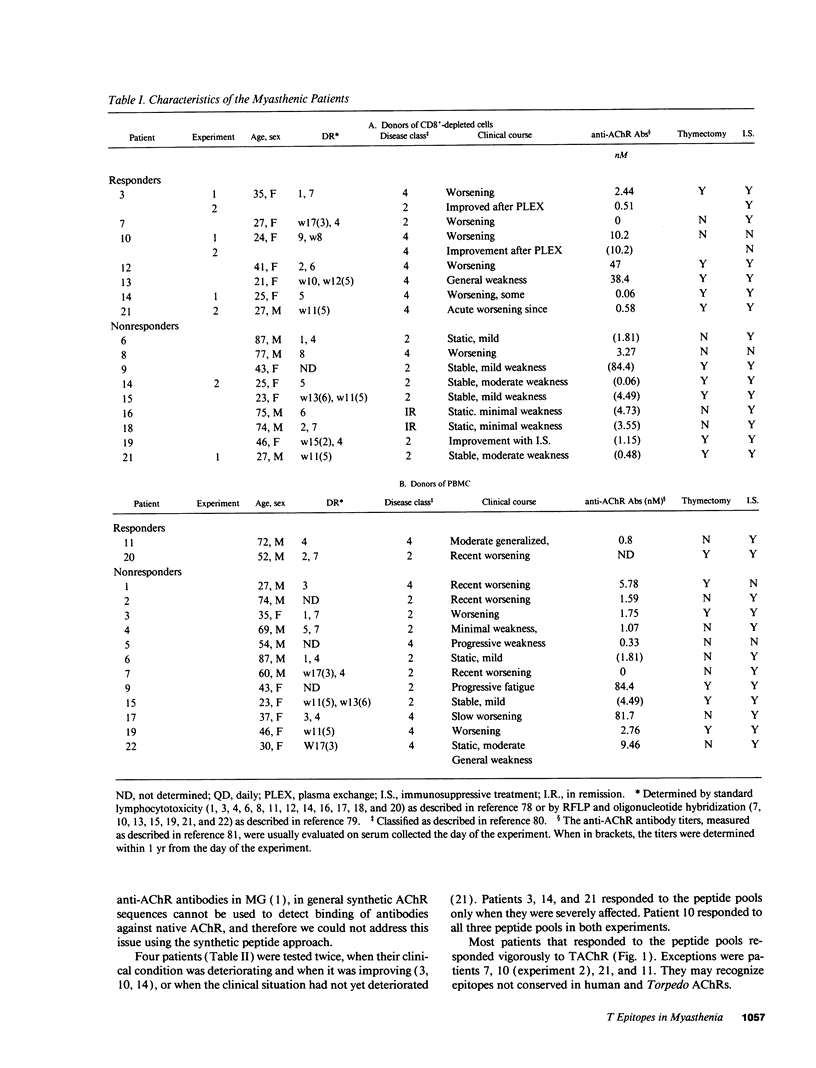
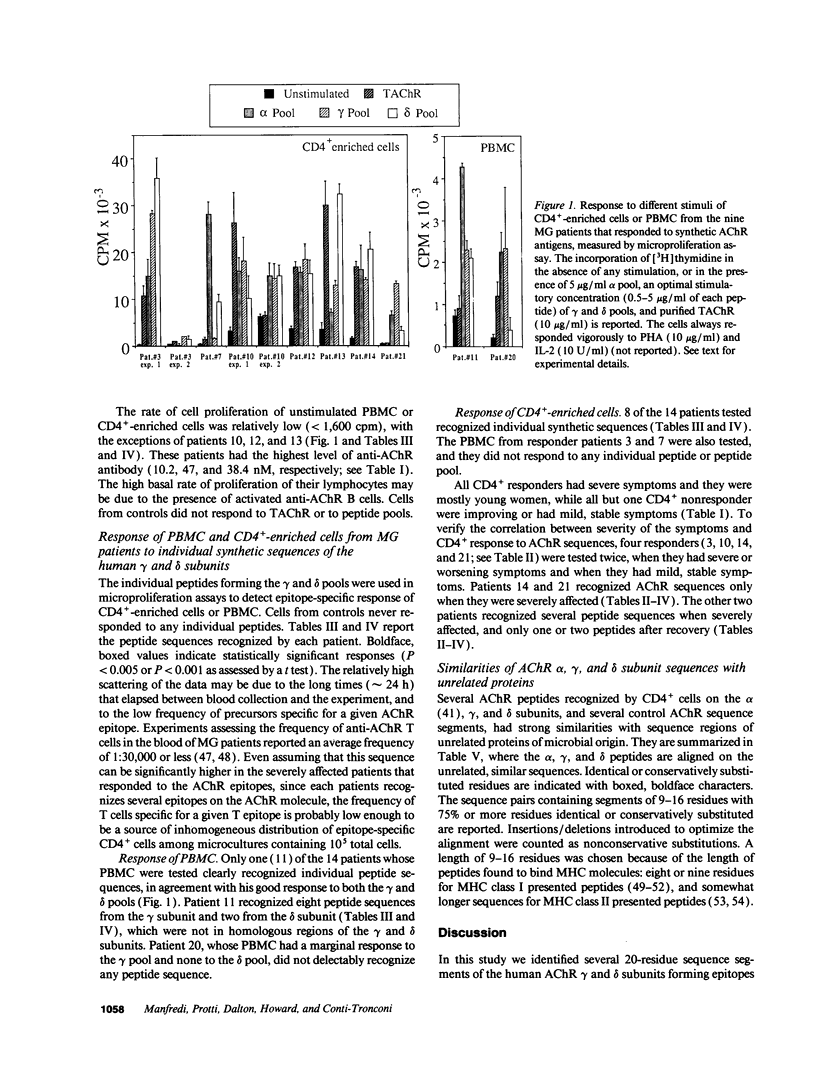
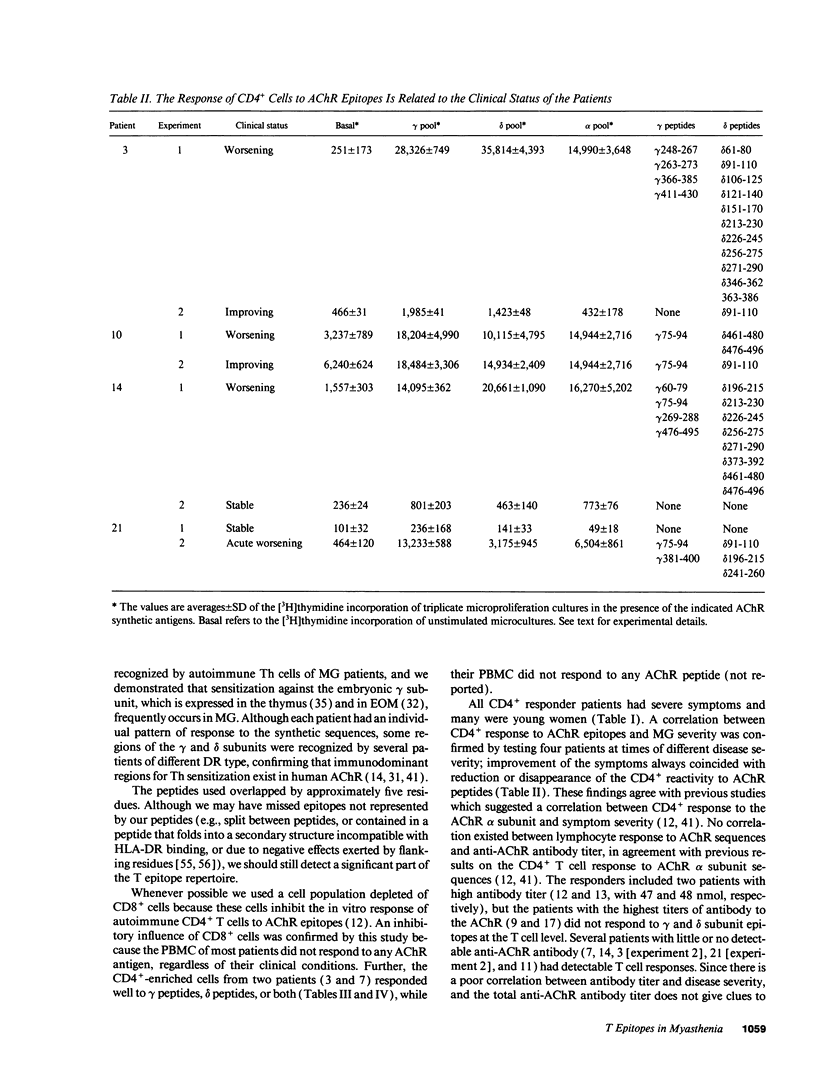
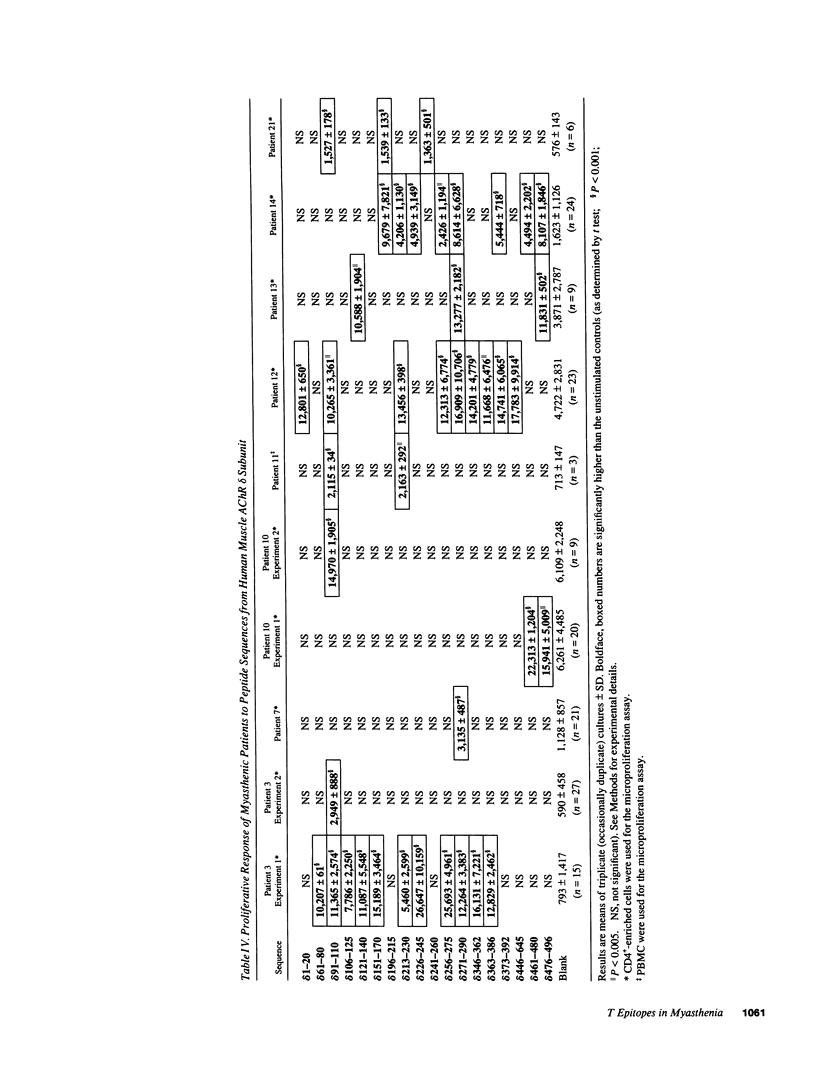
We tested the response of CD4+ cells and/or total lymphocytes from the blood of 22 myasthenic patients and 10 healthy controls to overlapping synthetic peptides, 20 residues long, to screen the sequence of the gamma and delta subunits of human muscle acetylcholine receptor (AChR). The gamma subunit is part of the AChR expressed in embryonic muscle and is substituted in the AChRs of most adult muscles by an epsilon subunit. The delta subunit is present in both embryonic and adult AChRs. Adult extrinsic ocular muscles, which are preferentially and sometimes uniquely affected by myasthenic symptoms, and thymus, which has a still obscure but important role in the pathogenesis of myasthenia gravis, express the embryonic gamma subunit. Anti-AChR CD4+ responses were more easily detected after CD8+ depletion. All responders recognized epitopes on both the gamma and delta subunits and had severe symptoms. In four patients the CD4+ cell response was tested twice, when the symptoms were severe and during a period of remission. Consistently, the response was only detectable, or larger, when the patients were severely affected.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert L. I., Papatestas A., Kark A., Osserman R. S., Osserman K. A histologic reappraisal of the thymus in myasthenia gravis. A correlative study of thymic pathology and response to thymectomy. Arch Pathol. 1971 Jan;91(1):55–61. [PubMed] [Google Scholar]
- Bellone M., Tang F., Milius R., Conti-Tronconi B. M. The main immunogenic region of the nicotinic acetylcholine receptor. Identification of amino acid residues interacting with different antibodies. J Immunol. 1989 Dec 1;143(11):3568–3579. [PubMed] [Google Scholar]
- Berzofsky J. A., Brett S. J., Streicher H. Z., Takahashi H. Antigen processing for presentation to T lymphocytes: function, mechanisms, and implications for the T-cell repertoire. Immunol Rev. 1988 Dec;106:5–31. doi: 10.1111/j.1600-065x.1988.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Besinger U. A., Toyka K. V., Hömberg M., Heininger K., Hohlfeld R., Fateh-Moghadam A. Myasthenia gravis: long-term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology. 1983 Oct;33(10):1316–1321. doi: 10.1212/wnl.33.10.1316. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Cease K. B., Ouyang C. S., Berzofsky J. A. Fine specificity of T cell recognition of the same peptide in association with different I-A molecules. J Immunol. 1989 Aug 1;143(3):771–779. [PubMed] [Google Scholar]
- Brutlag D. L., Dautricourt J. P., Maulik S., Relph J. Improved sensitivity of biological sequence database searches. Comput Appl Biosci. 1990 Jul;6(3):237–245. doi: 10.1093/bioinformatics/6.3.237. [DOI] [PubMed] [Google Scholar]
- Chavez R. A., Hall Z. W. Expression of fusion proteins of the nicotinic acetylcholine receptor from mammalian muscle identifies the membrane-spanning regions in the alpha and delta subunits. J Cell Biol. 1992 Jan;116(2):385–393. doi: 10.1083/jcb.116.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Morgutti M., Sghirlanzoni A., Clementi F. Cellular immune response against acetylcholine receptor in myasthenia gravis: I. Relevance to clinical course and pathogenesis. Neurology. 1979 Apr;29(4):496–501. doi: 10.1212/wnl.29.4.496. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B., Tzartos S., Lindstrom J. Monoclonal antibodies as probes of acetylcholine receptor structure. 2. Binding to native receptor. Biochemistry. 1981 Apr 14;20(8):2181–2191. doi: 10.1021/bi00511a017. [DOI] [PubMed] [Google Scholar]
- Demotz S., Grey H. M., Appella E., Sette A. Characterization of a naturally processed MHC class II-restricted T-cell determinant of hen egg lysozyme. Nature. 1989 Dec 7;342(6250):682–684. doi: 10.1038/342682a0. [DOI] [PubMed] [Google Scholar]
- Engel A. G. Myasthenia gravis and myasthenic syndromes. Ann Neurol. 1984 Nov;16(5):519–534. doi: 10.1002/ana.410160502. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Deres K., Metzger J., Jung G., Rammensee H. G. Identification of naturally processed viral nonapeptides allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J Exp Med. 1991 Aug 1;174(2):425–434. doi: 10.1084/jem.174.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Monden Y., Nakahara K., Hashimoto J., Kawashima Y. Antibody to acetylcholine receptor in myasthenia gravis: production by lymphocytes from thymus or thymoma. Neurology. 1984 Sep;34(9):1182–1186. doi: 10.1212/wnl.34.9.1182. [DOI] [PubMed] [Google Scholar]
- Gammon G., Sercarz E. How some T cells escape tolerance induction. Nature. 1989 Nov 9;342(6246):183–185. doi: 10.1038/342183a0. [DOI] [PubMed] [Google Scholar]
- Gorin M. B., Yancey S. B., Cline J., Revel J. P., Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984 Nov;39(1):49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Healy F., Drouet C., Romero P., Jaulin C., Maryanski J. L. Conversion of a self peptide sequence into a Kd-restricted neo-antigen by a Tyr substitution. J Exp Med. 1991 Dec 1;174(6):1657–1660. doi: 10.1084/jem.174.6.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlfeld R., Conti-Tronconi B., Kalies I., Bertrams J., Toyka K. V. Genetic restriction of autoreactive acetylcholine receptor-specific T lymphocytes in myasthenia gravis. J Immunol. 1985 Oct;135(4):2393–2399. [PubMed] [Google Scholar]
- Hohlfeld R., Kalies I., Kohleisen B., Heininger K., Conti-Tronconi B., Toyka K. V. Myasthenia gravis: stimulation of antireceptor autoantibodies by autoreactive T cell lines. Neurology. 1986 May;36(5):618–621. doi: 10.1212/wnl.36.5.618. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Manfredi A. A., Conti-Tronconi B. M. The 'embryonic' gamma subunit of the nicotinic acetylcholine receptor is expressed in adult extraocular muscle. Neurology. 1993 May;43(5):983–986. doi: 10.1212/wnl.43.5.983. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Jung S., Schluesener H. J. Human T lymphocytes recognize a peptide of single point-mutated, oncogenic ras proteins. J Exp Med. 1991 Jan 1;173(1):273–276. doi: 10.1084/jem.173.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski H. J., Maas E., Spiegel P., Ruff R. L. Why are eye muscles frequently involved in myasthenia gravis? Neurology. 1990 Nov;40(11):1663–1669. doi: 10.1212/wnl.40.11.1663. [DOI] [PubMed] [Google Scholar]
- Killen J. A., Hochschwender S. M., Lindstrom J. M. The main immunogenic region of acetylcholine receptors does not provoke the formation of antibodies of a predominant idiotype. J Neuroimmunol. 1985 Aug;9(3-4):229–241. doi: 10.1016/s0165-5728(85)80021-7. [DOI] [PubMed] [Google Scholar]
- Levinson A. I., Zweiman B., Lisak R. P. Immunopathogenesis and treatment of myasthenia gravis. J Clin Immunol. 1987 May;7(3):187–197. doi: 10.1007/BF00915723. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Shelton D., Fujii Y. Myasthenia gravis. Adv Immunol. 1988;42:233–284. doi: 10.1016/s0065-2776(08)60847-0. [DOI] [PubMed] [Google Scholar]
- Link H., Olsson O., Sun J., Wang W. Z., Andersson G., Ekre H. P., Brenner T., Abramsky O., Olsson T. Acetylcholine receptor-reactive T and B cells in myasthenia gravis and controls. J Clin Invest. 1991 Jun;87(6):2191–2196. doi: 10.1172/JCI115253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisak R. P., Levinson A. I., Zweiman B., Kornstein M. J. Antibodies to acetylcholine receptor and tetanus toxoid: in vitro synthesis by thymic lymphocytes. J Immunol. 1986 Aug 15;137(4):1221–1225. [PubMed] [Google Scholar]
- Luther M. A., Schoepfer R., Whiting P., Casey B., Blatt Y., Montal M. S., Montal M., Linstrom J. A muscle acetylcholine receptor is expressed in the human cerebellar medulloblastoma cell line TE671. J Neurosci. 1989 Mar;9(3):1082–1096. doi: 10.1523/JNEUROSCI.09-03-01082.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Manca F., Fenoglio D., Kunkl A., Cambiaggi C., Li Pira G., Celada F. B cells on the podium: regulatory roles of surface and secreted immunoglobulins. Immunol Today. 1988 Oct;9(10):300–303. doi: 10.1016/0167-5699(88)91321-7. [DOI] [PubMed] [Google Scholar]
- Martin R., Howell M. D., Jaraquemada D., Flerlage M., Richert J., Brostoff S., Long E. O., McFarlin D. E., McFarland H. F. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J Exp Med. 1991 Jan 1;173(1):19–24. doi: 10.1084/jem.173.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M. SCID mice as immune system models. Curr Opin Immunol. 1991 Apr;3(2):224–228. doi: 10.1016/0952-7915(91)90055-6. [DOI] [PubMed] [Google Scholar]
- McLane K. E., Wu X. D., Conti-Tronconi B. M. Structural determinants within residues 180-199 of the rodent alpha 5 nicotinic acetylcholine receptor subunit involved in alpha-bungarotoxin binding. Biochemistry. 1991 Nov 5;30(44):10730–10738. doi: 10.1021/bi00108a018. [DOI] [PubMed] [Google Scholar]
- Melms A., Schalke B. C., Kirchner T., Müller-Hermelink H. K., Albert E., Wekerle H. Thymus in myasthenia gravis. Isolation of T-lymphocyte lines specific for the nicotinic acetylcholine receptor from thymuses of myasthenic patients. J Clin Invest. 1988 Mar;81(3):902–908. doi: 10.1172/JCI113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina M., Takai T., Imoto K., Noda M., Takahashi T., Numa S., Methfessel C., Sakmann B. Molecular distinction between fetal and adult forms of muscle acetylcholine receptor. Nature. 1986 May 22;321(6068):406–411. doi: 10.1038/321406a0. [DOI] [PubMed] [Google Scholar]
- Nelson S., Conti-Tronconi B. M. Adult thymus expresses an embryonic nicotinic acetylcholine receptor-like protein. J Neuroimmunol. 1990 Sep-Oct;29(1-3):81–92. doi: 10.1016/0165-5728(90)90150-l. [DOI] [PubMed] [Google Scholar]
- Nicholas J. A., Levely M. E., Mitchell M. A., Smith C. W. A 16-amino acid peptide of respiratory syncytial virus 1A protein contains two overlapping T cell-stimulating sites distinguishable by class II MHC restriction elements. J Immunol. 1989 Nov 1;143(9):2790–2796. [PubMed] [Google Scholar]
- Noreen H. J., Davidson M. L., McCullough J., Bach F. H., Segall M. HLA class II typing by restriction fragment length polymorphism (RFLP) in unrelated bone marrow transplant patients. Transplant Proc. 1989 Feb;21(1 Pt 3):2968–2970. [PubMed] [Google Scholar]
- O'Sullivan D., Sidney J., Appella E., Walker L., Phillips L., Colón S. M., Miles C., Chesnut R. W., Sette A. Characterization of the specificity of peptide binding to four DR haplotypes. J Immunol. 1990 Sep 15;145(6):1799–1808. [PubMed] [Google Scholar]
- Ohno S. Many peptide fragments of alien antigens are homologous with host proteins, thus canalizing T-cell responses. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3065–3068. doi: 10.1073/pnas.88.8.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S. To be or not to be a responder in T-cell responses: ubiquitous oligopeptides in all proteins. Immunogenetics. 1991;34(4):215–221. doi: 10.1007/BF00215255. [DOI] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Howard J. F., Jr, Conti-Tronconi B. M. T cells in myasthenia gravis specific for embryonic acetylcholine receptor. Neurology. 1991 Nov;41(11):1809–1814. doi: 10.1212/wnl.41.11.1809. [DOI] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Howard J. F., Jr, Conti-Tronconi B. M. T cells in myasthenia gravis specific for embryonic acetylcholine receptor. Neurology. 1991 Nov;41(11):1809–1814. doi: 10.1212/wnl.41.11.1809. [DOI] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Howard J. F., Jr, Conti-Tronconi B. M. CD4+ T cell response to the human acetylcholine receptor alpha subunit in myasthenia gravis. A study with synthetic peptides. J Immunol. 1990 Feb 15;144(4):1276–1281. [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Howard J. F., Jr, Conti-Tronconi B. M. Immunodominant regions for T helper-cell sensitization on the human nicotinic receptor alpha subunit in myasthenia gravis. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7792–7796. doi: 10.1073/pnas.87.19.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Straub C., Wu X. D., Howard J. F., Jr, Conti-Tronconi B. M. Use of synthetic peptides to establish anti-human acetylcholine receptor CD4+ cell lines from myasthenia gravis patients. J Immunol. 1990 Mar 1;144(5):1711–1720. [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Wu X. D., Moiola L., Dalton M. W., Howard J. F., Jr, Conti-Tronconi B. M. Myasthenia gravis. CD4+ T epitopes on the embryonic gamma subunit of human muscle acetylcholine receptor. J Clin Invest. 1992 Oct;90(4):1558–1567. doi: 10.1172/JCI116024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protti M. P., Manfredi A. A., Wu X. D., Moiola L., Howard J. F., Jr, Conti-Tronconi B. M. Myasthenia gravis. T epitopes on the delta subunit of human muscle acetylcholine receptor. J Immunol. 1991 Apr 1;146(7):2253–2261. [PubMed] [Google Scholar]
- Reddehase M. J., Rothbard J. B., Koszinowski U. H. A pentapeptide as minimal antigenic determinant for MHC class I-restricted T lymphocytes. Nature. 1989 Feb 16;337(6208):651–653. doi: 10.1038/337651a0. [DOI] [PubMed] [Google Scholar]
- Reimann J., Rudolphi A., Claesson M. H. Selective reconstitution of T lymphocyte subsets in scid mice. Immunol Rev. 1991 Dec;124:75–95. doi: 10.1111/j.1600-065x.1991.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Richman D. P., Patrick J., Arnason B. G. Cellular immunity in myasthenia gravis. Response to purified acetylcholine receptor and autologous thymocytes. N Engl J Med. 1976 Mar 25;294(13):694–698. doi: 10.1056/NEJM197603252941304. [DOI] [PubMed] [Google Scholar]
- Roses A. D., Olanow C. W., McAdams M. W., Lane R. J. No direct correlation between serum antiacetylcholine receptor antibody levels and clinical state of individual patients with myasthenia gravis. Neurology. 1981 Feb;31(2):220–224. doi: 10.1212/wnl.31.2.220. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Sakai K., Sinha A. A., Mitchell D. J., Zamvil S. S., Rothbard J. B., McDevitt H. O., Steinman L. Involvement of distinct murine T-cell receptors in the autoimmune encephalitogenic response to nested epitopes of myelin basic protein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8608–8612. doi: 10.1073/pnas.85.22.8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadding G. K., Vincent A., Newsom-Davis J., Henry K. Acetylcholine receptor antibody synthesis by thymic lymphocytes: correlation with thymic histology. Neurology. 1981 Aug;31(8):935–943. doi: 10.1212/wnl.31.8.935. [DOI] [PubMed] [Google Scholar]
- Schoepfer R., Luther M., Lindstrom J. The human medulloblastoma cell line TE671 expresses a muscle-like acetylcholine receptor. Cloning of the alpha-subunit cDNA. FEBS Lett. 1988 Jan 4;226(2):235–240. doi: 10.1016/0014-5793(88)81430-3. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M., Vicini S., Hall Z. W. Myasthenic serum selectively blocks acetylcholine receptors with long channel open times at developing rat endplates. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2533–2537. doi: 10.1073/pnas.82.8.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri N., Gammon G., Horvath S., Miller A., Sercarz E. E. The choice between two distinct T cell determinants within a 23-amino acid region of lysozyme depends on their structural context. J Immunol. 1986 Aug 1;137(3):911–915. [PubMed] [Google Scholar]
- Shibahara S., Kubo T., Perski H. J., Takahashi H., Noda M., Numa S. Cloning and sequence analysis of human genomic DNA encoding gamma subunit precursor of muscle acetylcholine receptor. Eur J Biochem. 1985 Jan 2;146(1):15–22. doi: 10.1111/j.1432-1033.1985.tb08614.x. [DOI] [PubMed] [Google Scholar]
- Stroud R. M., McCarthy M. P., Shuster M. Nicotinic acetylcholine receptor superfamily of ligand-gated ion channels. Biochemistry. 1990 Dec 18;29(50):11009–11023. doi: 10.1021/bi00502a001. [DOI] [PubMed] [Google Scholar]
- Sun J. B., Harcourt G., Wang Z. Y., Hawke S., Olsson T., Fredrikson S., Link H. T cell responses to human recombinant acetylcholine receptor-alpha subunit in myasthenia gravis and controls. Eur J Immunol. 1992 Jun;22(6):1553–1559. doi: 10.1002/eji.1830220631. [DOI] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Sophianos D., Efthimiadis A. Role of the main immunogenic region of acetylcholine receptor in myasthenia gravis. An Fab monoclonal antibody protects against antigenic modulation by human sera. J Immunol. 1985 Apr;134(4):2343–2349. [PubMed] [Google Scholar]
- Tzartos S., Hochschwender S., Vasquez P., Lindstrom J. Passive transfer of experimental autoimmune myasthenia gravis by monoclonal antibodies to the main immunogenic region of the acetylcholine receptor. J Neuroimmunol. 1987 Jun;15(2):185–194. doi: 10.1016/0165-5728(87)90092-0. [DOI] [PubMed] [Google Scholar]
- Vacchio M. S., Berzofsky J. A., Krzych U., Smith J. A., Hodes R. J., Finnegan A. Sequences outside a minimal immunodominant site exert negative effects on recognition by staphylococcal nuclease-specific T cell clones. J Immunol. 1989 Nov 1;143(9):2814–2819. [PubMed] [Google Scholar]
- Van Bleek G. M., Nathenson S. G. Isolation of an endogenously processed immunodominant viral peptide from the class I H-2Kb molecule. Nature. 1990 Nov 15;348(6298):213–216. doi: 10.1038/348213a0. [DOI] [PubMed] [Google Scholar]
- Vincent A., Whiting P. J., Schluep M., Heidenreich F., Lang B., Roberts A., Willcox N., Newsom-Davis J. Antibody heterogeneity and specificity in myasthenia gravis. Ann N Y Acad Sci. 1987;505:106–120. doi: 10.1111/j.1749-6632.1987.tb51286.x. [DOI] [PubMed] [Google Scholar]
- Weinberg C. B., Hall Z. W. Antibodies from patients with myasthenia gravis recognize determinants unique to extrajunctional acetylcholine receptors. Proc Natl Acad Sci U S A. 1979 Jan;76(1):504–508. doi: 10.1073/pnas.76.1.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H., Hohlfeld R., Ketelsen U. P., Kalden J. R., Kalies I. Thymic myogenesis, T-lymphocytes and the pathogenesis of myasthenia gravis. Ann N Y Acad Sci. 1981;377:455–476. doi: 10.1111/j.1749-6632.1981.tb33753.x. [DOI] [PubMed] [Google Scholar]




