Abstract
Acute nicotine enhances multiple types of learning including trace fear conditioning but the underlying neural substrates of these effects are not well understood. Trace fear conditioning critically involves the medial prefrontal cortex and hippocampus, which both express nicotinic acetylcholine receptors (nAChRs). Therefore, nicotine could act in either or both areas to enhance trace fear conditioning. To identify the underlying neural areas and nAChR subtypes, we examined the effects of infusion of nicotine, or nicotinic antagonists dihydro-beta-erythroidine (DHβE: high-affinity nAChRs) or methyllycaconitine (MLA: low-affinity nAChRs) into the dorsal hippocampus, ventral hippocampus, and medial prefrontal cortex (mPFC) on trace and contextual fear conditioning. We found that the effects of nicotine on trace and contextual fear conditioning vary by brain region and nAChR subtype. The dorsal hippocampus was involved in the effects of nicotine on both trace and contextual fear conditioning but each task was sensitive to different doses of nicotine. Additionally, dorsal hippocampal infusion of the antagonist DHβE produced deficits in trace but not contextual fear conditioning. Nicotine infusion into the ventral hippocampus produced deficits in both trace and contextual fear conditioning. In the mPFC, nicotine enhanced trace but not contextual fear conditioning. Interestingly, infusion of the antagonists MLA or DHβE in the mPFC also enhanced trace fear conditioning. These findings suggest that nicotine acts on different substrates to enhance trace versus contextual fear conditioning, and that nicotine-induced desensitization of nAChRs in the mPFC may contribute to the effects of nicotine on trace fear conditioning.
Keywords: Working Memory, Learning, Addiction, Nicotine, Acetylcholine, Hippocampus
Nicotine enhances cognitive processes (Heishman et al., 2010; Kenney and Gould, 2008a; Swan and Lessov-Schlaggar, 2007) and symptoms of diseases that are sensitive to changes in cognition, such as Alzheimer’s disease, schizophrenia, and addiction, can be altered by nicotine administration (Buckingham et al., 2009; Winterer, 2010; Woodruff-Pak and Gould, 2002). For instance, the effects of nicotine on cognitive processes are thought to play an important role in both the development and maintenance of addiction (Gutkin et al., 2006; Kenney and Gould, 2008a; Patterson et al., 2009; Watkins et al., 2000). Initially, nicotine may enhance the formation of maladaptive drug-context associations and changes in cognition associated with abstinence may contribute to relapse (Brega et al., 2008; Gutkin et al., 2006; Patterson et al., 2010; Raybuck and Gould, 2009; Razani et al., 2004). We have found that nicotine alters contextual learning through hippocampus-mediated processes (Davis and Gould, 2009; Davis et al., 2007); however, it is unclear if the effects of nicotine on other types of learning are dependent on the same neural substrates. Nicotine can have diverse effects on different types of learning (Kenney and Gould, 2008a); this variability may be due to the involvement of different brain regions. One way to understand how nicotine affects learning is to identify the underlying brain regions involved in the effects of nicotine on different types of learning.
Trace fear conditioning is a contextual/spatial-independent form of forebrain-dependent learning (McEchron et al., 1998) that is sensitive to the effects of nicotine (Davis and Gould, 2007; Gould et al., 2004; Raybuck and Gould, 2009). Specifically, trace fear conditioning involves the association of temporally discontiguous conditioned stimuli (CS) and unconditioned stimuli (US). Trace fear conditioning is enhanced by acute nicotine, unlike delay cued fear conditioning in which the CS and US co-terminate (Gould et al., 2004). This task depends upon multiple brain regions that are not critically involved in delay cued fear conditioning, such as the hippocampus and medial prefrontal cortex (Büchel et al., 1999; Knight et al., 2004; McLaughlin et al., 2002; Quinn et al., 2008; Yoon and Otto, 2007). It is unknown, however, whether nicotine acts in these areas to alter trace fear conditioning.
The present studies used direct infusion of nicotine or high- or low-affinity nicotinic acetylcholine receptor (nAChR) antagonists dihydro-β-erythriodine (DHβE; antagonist for α4β2 nAChRs and other high-affinity nAChRs) and methyllycaconitine (MLA; antagonist for α7 nAChRs), respectively, into the dorsal hippocampus, ventral hippocampus, or medial prefrontal cortex of male C57BL6/J mice to investigate the neural areas and the nAChRs involved in the effects of acute nicotine on trace fear conditioning. To examine the specificity of the effects of nicotine on learning, contextual and delay cued fear conditioning were also examined.
Methods
Subjects
Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, Maine), 8–12 weeks old, were singly housed, maintained on a 12h light/dark cycle with lights on at 7:00 am, and allowed ad libitum access to food and water. Housing, surgical and behavioral procedures were approved by the Temple University Institutional Animal Care and Use Committee.
Materials
Drugs and Infusion
Nicotine hydrogen tartrate salt (reported as freebase), DHβE, and MLA were obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in physiological saline, which was used for control infusions. Drugs were directly infused through 22-gauge (dorsal and ventral hippocampus) or 33-gauge (medial prefrontal cortex) cannulas; smaller cannulas were used in the medial prefrontal cortex due the close proximity of the bilateral placements. Drug was infused at a rate of 0.50 μl/min and at 0.50 μl per side. Infusion cannulas were attached with polyethylene tubing (PE50; Plastics One, Roanoke, VA) to a 10 μl Hamilton syringe (Reno, NV), which was controlled by a microinfusion pump (KDS 100; KD Scientific, New Hope, PA). Injection cannulas were left in place for 30 seconds after infusion to allow for diffusion of drug away from cannula tip. Nicotine (0.045, 0.09, 0.18, or 0.35 μg/side) was infused immediately before training and/or testing and DHβE (4.50, 9.00, or 18.00 μg/side) or MLA (6.75, 13.50, or 27.00 μg/side) was infused 25 min before training and/or testing; doses and infusion times were based on previous work from our laboratory on the effects of nicotine infusion on contextual fear conditioning (Davis and Gould, 2007; Davis et al., 2007) and the effects of systemic administration of nicotinic antagonists on trace fear conditioning (Raybuck and Gould, 2009). Spread of infusion using this procedure has been previously estimated to be ~ 1mm3 (Lewis and Gould, 2007).
Apparatus
Training was conducted in conditioning chambers (6 3/4″ (w) X 7 3/8″ (d) X 5″ (h), model 307AW, Med Associates, St. Albans, VT) housed in sound attenuating cubicles. An 85 dB white noise CS was administered through speakers attached to the right wall of each chamber. A 69 dB background noise was provided by 50 mm ventilation fans, mounted on the right wall of each sound-attenuating cubicle. A 2 second, 0.57 mA footshock US was administered with a shock generator and scrambler (Med-Associates) through the stainless steel bar chamber floors. Stimulus administration was controlled by an IBM-compatible PC running Med-PC software (Med-Associates).
Testing of trace fear conditioning was conducted in four conditioning chambers situated in sound attenuating cubicles located in a different room than that used for training. The testing chambers (9 1/4″ (w) X 8″ (d) X 7 1/2″ (h)) were equipped with solid white plastic flooring, as opposed to stainless steel bars, Plexiglas panels for the front, rear and lid, and the stainless steel side panels with wall mountings that differed in shape, size, and color from those in the training chambers. Additionally, a novel olfactory cue (artificial vanilla extract) was applied to paper towels placed below each of the chamber floors. Background noise was provided by ventilation fans mounted on the right wall of the sound attenuating cubicles. For testing, the CS was generated with a Grason-Stradler noise generator (model 901B, West Concord, MA) attached to 3-inch speakers mounted on the left side of each of the conditioning chambers.
Procedure
Surgical
Mice were anesthetized with isoflurane (5% induction, 2% maintenance), placed in a mouse stereotaxic apparatus (David Kopf Instruments, Tujunga, CA), and guide cannulas (Plastics One, Roanoke, VA) were implanted in the dorsal hippocampus (A/P – 1.7, M/L ±1.5, D/V −2.3 mm), the ventral hippocampus (A/P –2.8, M/L ±3.0, D/V −4.0 mm), or the medial prefrontal cortex (A/P 1.7, M/L ±0.5, D/V −2.5 mm; Paxinos and Franklin, 2003). As controls, guide cannulas were also implanted above the dorsal hippocampus (A/P – 1.7, M/L ±1.5, D/V −0.76 mm), below the dorsal hippocampus (A/P – 1.7, M/L ±1.5, D/V −3.3 mm), medial to the ventral hippocampus (A/P –2.8, M/L ±1.5, D/V −4.0 mm), above the medial prefrontal cortex (A/P 1.7, M/L ±0.5, D/V −1.0 mm), and below the medial prefrontal cortex (A/P 1.7, M/L ±0.5, D/V –3.5 mm). Stainless steel stylets were placed in the guide cannulas to maintain patency during the minimal 5-day recovery period. All animals received the analgesic ketoprofen (2 mg/kg, sc; Fort Dodge Animal Health, Fort Dodge, IA) post-operatively. Five animals showed complications following implantation of ventral hippocampal cannulas and were excluded from further analysis.
Behavioral
Trace Fear Conditioning
Behavioral procedures were based on previous studies (Davis and Gould, 2007; Gould et al., 2004; Raybuck and Gould, 2009). Training of trace fear conditioning was conducted during a single 16-minute training session wherein the mice were presented with 5 CS-US pairings separated by a variable inter-trial-interval (average 120 seconds). CS-US pairings began with a 30 second, 85 dB, white noise CS presentation, followed by a 30 second trace interval, and terminated with the presentation of a 2 second, 0.57 mA, footshock US; mice remained in the chambers for 30 seconds following the last US presentation. During training, freezing before the first CS presentation was assessed as a measurement of baseline freezing, and freezing between the last and second to last CS-US parings was assessed as immediate freezing. Freezing was assessed at 10-second intervals (Gould and Wehner, 1999) and defined as the absence of all movement except for respiration (Blanchard and Blanchard, 1969). Twenty-four hours following training, mice were tested for both contextual and trace fear conditioning. To test for contextual associations formed during the training session, mice were placed in the training context and observed for freezing for five minutes. One to two hours later, mice were placed in the altered context testing chambers for six minutes; for the first three minutes no CS was presented and mice were scored for freezing to the altered context, a measure of generalized fear, then the CS was presented for three minutes and mice were scored for freezing. In addition to 5-pairing trace fear conditioning a subset of studies used a 2-pairing trace fear conditioning protocol, wherein mice received two CS-US pairings with a 30 second trace interval and a 120 second inter-trial interval. These training sessions lasted 6 minutes and 34 seconds.
Delay cued Fear Conditioning
Delay cued fear conditioning procedures were based on previous studies (Davis & Gould, 2007; Gould et al., 2004). Training of delay cued fear conditioning was conducted during a single 5 minute and 30 second training session wherein mice were presented with 2 CS-US pairings separated by a 120 second inter-trial interval. CS-US pairings consisted of a 30 second 85 dB white noise CS presentation that co-terminated with a 2 second 0.57 mA footshock US; mice remained in the chambers for 30 seconds following the last US presentation. Testing of both contextual and delay cued fear conditioning was performed as described for trace fear conditioning. In addition to the 2-pairing delay cued fear conditioning protocol, a subset of experiments used a 1 CS-US protocol with a 15 second CS to rule out a ceiling effect obscuring nicotine effects on freezing to the CS. In controls, the 1 CS-US pairing reduced freezing to the CS to 55.1% compared to 80.1% in mice that received two trials with a 30 second CS. This difference in freezing was significant (t(47) = 25.2, p < 0.0005); yet as discussed in the results nicotine did not have an effect on delay cued fear conditioning regardless of the number of trials.
Histology
All brains were post-fixed in formalin for at least 24 hours, sliced on a cryostat and stained with cresyl violet. Infusion sites were confirmed with either dye infusion, or by observing gliosis along the infusion cannula tracts. Placements outside of the target area were excluded from analysis. Placements are displayed alongside the behavioral data. Histological analysis of all dorsal hippocampal infusions showed only 3 were outside of the target area. For ventral hippocampal and medial prefrontal cortical infusions, no implantations were outside of the target area.
Analysis
Data were analyzed with one-way ANOVAs followed with Tukey’s HSD post-hoc tests, or with an independent-samples t-test (SPSS 17, Chicago, IL). Data sets not meeting homogeneity of variance assumption of the ANOVA, as tested with Levene’s Statistic, were followed up with a Games-Howell post-hoc test. Significance for post-hoc tests was p < 0.05.
Results
Baseline Freezing, Immediate Freezing, and Altered Context Freezing Data
For all experiments, baseline freezing measured prior to presentation of training stimuli, immediate freezing recorded during training, and altered context freezing, a measure of generalized freezing recorded prior to presenting the CS at testing were examined. For all experiments, no group differences were seen for baseline freezing or immediate freezing on training day. For all experiments except one, levels of altered context freezing were low (0–9% freezing) and no difference in altered context freezing was seen between drug groups. The one exception was that MLA infusion into the medial prefrontal cortex affected altered context freezing [F (3,35) = 5.407, p < 0.005]. Post-hoc analysis showed that the lower dose (6.75 μg/side) enhanced altered context freezing (vehicle mean = 0, SEM = 0.0; MLA 6.75 μg/side mean = 6.1, SEM = 1.84); this dose did not enhance trace fear conditioning. The overall lack of effect of drug on altered context freezing across experiments suggest that drug effects on trace fear conditioning were not due to changes in generalized contextual freezing.
Dorsal Hippocampus
Effects of dorsal hippocampal nicotine infusion on trace fear conditioning
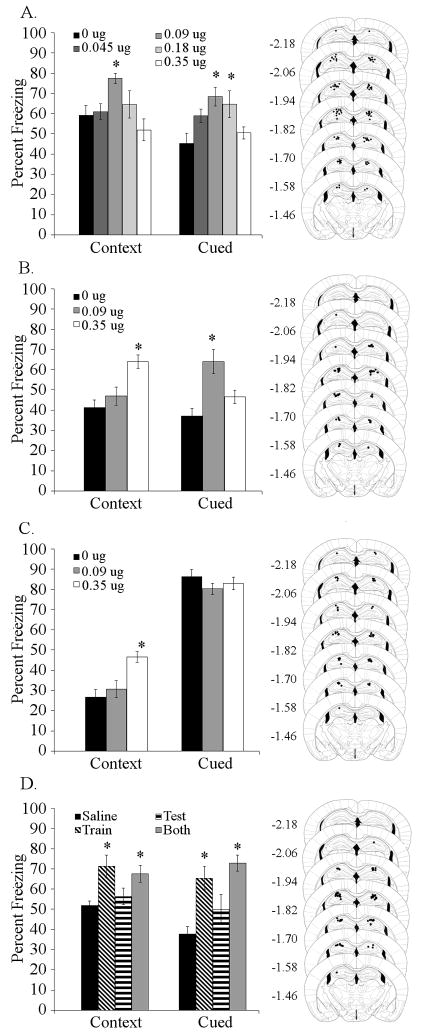
To determine if the dorsal hippocampus is involved in the effects of nicotine on trace fear conditioning, a range of doses of nicotine (0, 0.045, 0.09, 0.18, or 0.35 μg/side) were infused prior to training and testing of trace and contextual fear conditioning. Infusion of nicotine into the dorsal hippocampus affected both contextual [F (4,61) = 3.809, p < 0.01] and trace fear conditioning [F (4,61) = 4.683, p < 0.005]. Post-hoc analysis indicated that mice receiving 0.09 μg/side of nicotine froze more to the context than saline-infused control mice and that mice receiving 0.09 or 0.18 μg/side froze more to the CS than saline-infused controls (Figure 1A). Additionally, to determine if the effects of dorsal hippocampal nicotine infusion were particular to a 5-pairing trace fear conditioning protocol, nicotine (0, 0.09, or 0.35 μg/side) was infused prior to both training and testing of 2-pairing trace fear conditioning. Infusion of nicotine into the dorsal hippocampus affected contextual [F (2,21) = 10.467, p < 0.001] and trace [F (2,21) = 10.642, p < 0.001] fear conditioning. Post-hoc analysis revealed that the higher dose of nicotine (0.35 μg/side) enhanced contextual fear conditioning, and that the lower dose of nicotine (0.09 μg/side) enhanced trace fear conditioning (Figure 1B). Thus, different doses of nicotine in the dorsal hippocampus affect contextual and trace fear conditioning.
Figure 1. Effects of nicotine infusion into the dorsal hippocampus on fear conditioning.
A. Dorsal hippocampal nicotine infusion (0.09 μg/side contextual, 0.09 and 0.18 μg/side cued) at training and testing dose-dependently enhanced trace and contextual fear conditioning (n = 11–13). B. Infusion of 0.09 μg/side nicotine into the dorsal hippocampus enhanced 2-pairing trace fear conditioning, but not contextual conditioning, while 0.35 μg/side nicotine enhanced contextual but not trace conditioning (n = 8). C. Infusion of 0.35 μg/side nicotine into the dorsal hippocampus enhanced contextual but not 2-pairing delay cued fear conditioning (n= 8). D. Infusion of 0.09 μg/side nicotine into the dorsal hippocampus at training or at both training and testing enhanced both contextual and trace fear conditioning; however, infusion at testing had no effect (n = 8). Significant difference (p < 0.05) from the saline-infused control group denoted with (*); data are reported as mean ± standard error of the mean. Representations of cannula placements alongside data demonstrate that all infusions were directed into the dorsal hippocampus.
Effects of dorsal hippocampal nicotine infusion on delay cued fear conditioning
Infusion of nicotine into the dorsal hippocampus enhanced trace fear conditioning, however it is possible that this was driven by an effect of nicotine on stimulus processing that is not limited to trace fear conditioning. To determine if the effects of dorsal hippocampal nicotine infusion on trace fear conditioning were non-specific, nicotine was infused (0, 0.09, or 0.35 μg/side) into the dorsal hippocampus prior to training and testing of 2-pairing delay cued fear conditioning (i.e., no trace interval). Because delay cued fear conditioning involves different brain regions than trace fear conditioning (Büchel et al., 1999; Knight et al., 2004; McLaughlin et al., 2002; Misane et al., 2005; Quinn et al., 2008) and is not altered by systemic nicotine (Gould and Wehner, 1999; Gould et al., 2004), we expected no effect of nicotine infusion on delayed cued fear conditioning. Infusion of nicotine into the dorsal hippocampus affected contextual fear conditioning [F (2,21) = 9.596, p < 0.001], but not delay cued freezing. Post-hoc analysis demonstrated that mice treated with 0.35 μg/side nicotine froze more to the context than mice treated with saline or with 0.09 μg/side nicotine (Figure 1C). Additionally, to rule out the possibility that the lack of effect on delay cued fear conditioning was due to a ceiling effect, we used a less intensive conditioning protocol with 1 CS-US pairing and a 15 second CS duration to evaluate the effects of 0.09 μg/side nicotine infusion into the dorsal hippocampus prior to training and testing. Infusion of 0.09 μg/side nicotine prior to 1-pairing delay cued fear conditioning into the dorsal hippocampus had no effect (data not shown).
Effects of dorsal hippocampal nicotine infusion at training or testing of trace fear conditioning
To determine whether nicotine infusion into the dorsal hippocampus alters acquisition and/or retrieval of trace fear conditioning, an effective dose of nicotine (0 or 0.09 u/side) was infused at either training or testing, or on both days of trace fear conditioning (5 CS-US pairings). Infusion of nicotine affected contextual [F (2,21) = 10.642, p < 0.001] and trace [F (3,28) = 9.579, p < 0.001] fear conditioning. Post-hoc analysis revealed that nicotine infusion at training or at both training and testing enhanced contextual and trace fear conditioning but infusion of nicotine at testing had no effect (Figure 1D).
Effects of nicotine infusion above or below the dorsal hippocampus on trace fear conditioning
To determine if the effects of dorsal hippocampal nicotine infusion on trace fear conditioning were due to nicotine diffusion into areas above or below the dorsal hippocampus, nicotine (0 or 0.09 μg/side) was infused above the hippocampus into primary sensory cortex or below the dorsal hippocampus into the thalamus prior to training and testing of trace fear conditioning. Infusion of nicotine (0.09 μg/side) above or below the dorsal hippocampus had no effect (data not shown).
Effects of antagonism of nAChRs in the dorsal hippocampus on trace fear conditioning
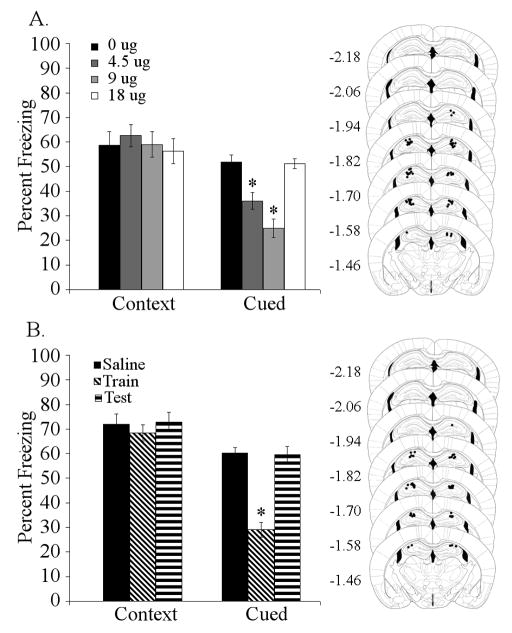
To determine if high-affinity nAChRs in the dorsal hippocampus are involved in trace and contextual fear conditioning, DHβE (0, 4.50, 9.00, or 18.00 μg/side) was infused into the dorsal hippocampus prior to both training and testing of trace fear conditioning (5-pairing). Infusion of DHβE into the dorsal hippocampus significantly affected trace [F (3,27) = 30.927, p < 0.001] fear conditioning, but had no effect on contextual fear conditioning. Post-hoc analysis revealed that mice treated with 4.50 or 9.00 μg/side DHβE showed deficits in trace fear conditioning compared to saline-infused controls, but mice treated with 18.00 μg/side DHβE were no different than controls (Figure 2A). Furthermore, to determine if deficits in trace fear conditioning produced by DHβE infusion into the dorsal hippocampus were due to effects on acquisition/consolidation or retrieval, DHβE (0 or 9 μg/side) was infused prior to either training or testing of trace fear conditioning (5-pairing). Infusion of DHβE into the dorsal hippocampus had a significant effect on trace [F (2,23) = 42.171, p < 0.001] but not contextual fear conditioning. Post-hoc analysis demonstrated that administration of DHβE into the dorsal hippocampus prior to training produced significant deficits in trace fear conditioning, but administration prior to testing had no effect (Figure 2B).
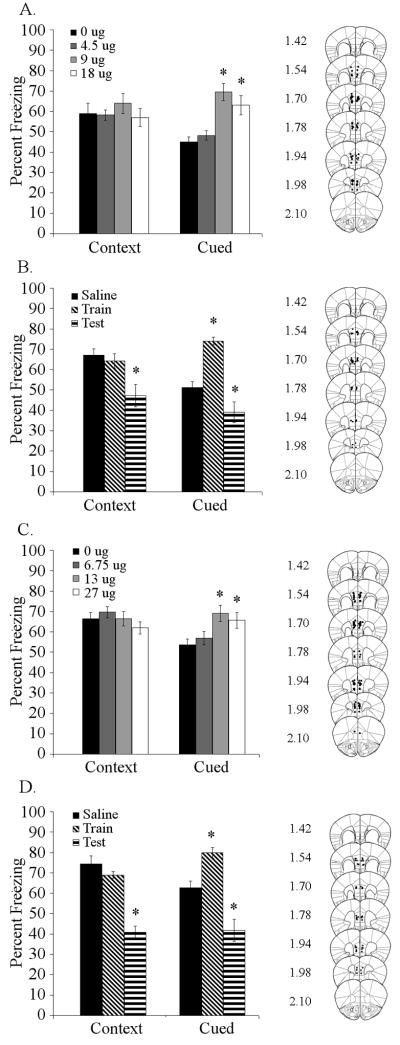
Figure 2. Effects of nicotinic antagonist infusion into the dorsal hippocampus on trace fear conditioning.
A. Antagonism of high-affinity nAChRs (DHβE: 4.50 and 9.00 μg/side) in the dorsal hippocampus at training and testing produced dose-dependent deficits in trace fear conditioning (n = 7 – 8). B. Infusion of 9.00 μg/side DHβE into the dorsal hippocampus at training produced deficits in trace conditioning, while infusion prior to testing had no effect (n = 8). Significant difference (p < 0.05) from control group denoted with (*); data are reported as mean ± standard error of the mean. Representations of cannula placements to right of data demonstrate that all infusions were directed into the dorsal hippocampus.
Acquisition of trace fear conditioning involves high-affinity nAChR signaling in the dorsal hippocampus. However, it is possible that low-affinity nAChRs in this brain area are also involved. To examine this, the low-affinity nAChR antagonist MLA (0, 6.75, 13.50, or 27.00 μg/side) was infused prior to training and testing of trace fear conditioning. Infusion of MLA had no effect (data not shown).
Ventral hippocampus
Effects of ventral hippocampal nicotine infusion on trace fear conditioning
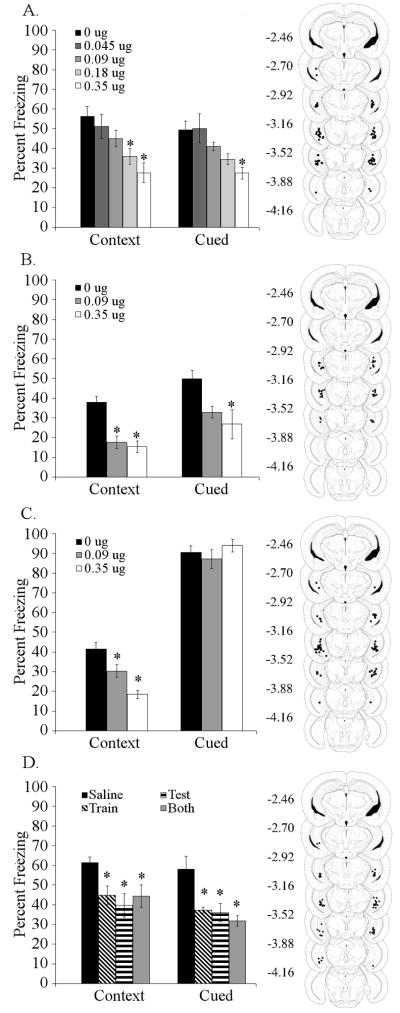
To determine if the ventral hippocampus is a site for the effects of nicotine on trace fear conditioning, nicotine was infused (0, 0.045, 0.09, 0.18, or 0.35 μg/side) prior to both training and testing of trace fear conditioning (5-pairing). Infusion of nicotine into the ventral hippocampus affected both contextual [F (4,32) = 5.758, p < 0.001] and trace [F (4,32) = 5.239, p < 0.005] fear conditioning. Post-hoc analysis revealed that ventral hippocampal nicotine infusion at doses of 0.18 and 0.35 μg/side produced deficits in contextual fear conditioning and that nicotine at 0.35 μg/side produced deficits in trace fear conditioning (Figure 3A). Additionally, to determine if the effects of ventral hippocampal nicotine infusion were particular to a 5-pairing trace fear conditioning protocol, nicotine (0, 0.09, 0.35 μg/side) was infused prior to both training and testing of 2-pairing trace fear conditioning. Infusion of nicotine into the ventral hippocampus affected contextual [F (2,22) = 18.950, p < 0.001] and trace [F (2,22) = 5.371, p < 0.05] fear conditioning. Post-hoc analysis revealed that infusion of 0.09 or 0.35 μg/side produced deficits in contextual fear conditioning, but only 0.35 μg/side of nicotine produced deficits in trace fear conditioning, although 0.09 μg/side approached significance (p = 0.0811, Figure 3B).
Figure 3. Effects of nicotine infusion into the ventral hippocampus on fear conditioning.
A. Infusion of nicotine into the ventral hippocampus at training and testing produced dose-dependent deficits in contextual (0.18 and 0.35 μg/side) and trace (0.35 μg/side) conditioning (n = 6 – 8). B. Infusion of nicotine into the ventral hippocampus produced deficits in contextual (0.09 and 0.35 μg/side) and trace (0.35 μg/side) conditioning in a 2-pairing trace fear conditioning paradigm (n = 8 – 9). C. Infusion of nicotine (0.09 or 0.35 μg/side) into the ventral hippocampus produced deficits in contextual fear conditioning, but had no effect on delay cued conditioning, using a 2-pairing 30 second CS training protocol (n = 8). D. Infusion of 0.35 μg/side nicotine into the ventral hippocampus at training or testing, or at both training and testing produced deficits in both trace and contextual fear conditioning (n = 8). Significant difference (p < 0.05) from the saline-infused control group denoted with (*); data are reported as mean ± standard error of the mean. Representations of cannula placements to right of data demonstrate that all infusions were directed into the ventral hippocampus.
Effects of ventral hippocampal nicotine infusion on delay cued fear conditioning
To determine if deficits in trace and contextual fear conditioning produced by nicotine infusion into the ventral hippocampus extend to delay cued fear conditioning (i.e., no trace interval), nicotine (0, 0.09, or 0.35 μg/side) was infused into the ventral hippocampus prior to training and testing of delay cued fear conditioning. Infusion of nicotine into the ventral hippocampus only affected contextual fear conditioning [F (3,27) = 10.768, p < 0.001]. Post-hoc analysis revealed that nicotine infusion at 0.09 or 0.35 μg/side produced deficits in contextual fear conditioning (Figure 3C). Furthermore, to address the possibility that deficits produced by infusion of 0.35 μg/side of nicotine into the ventral hippocampus could be masked by overtraining, a less intensive conditioning protocol with 1 CS-US pairing and a 15 second CS duration was used. Infusion of 0.35 μg/side of nicotine into the ventral hippocampus prior to training and testing produced selective deficits in contextual fear conditioning [t (14) = 7.341, p < 0.001] (data not shown).
Effects of nicotine in the ventral hippocampus at training or testing of trace fear conditioning
To determine if ventral hippocampal nicotine infusion affects processes supporting acquisition/consolidation or retrieval of trace and contextual fear conditioning, nicotine (0 or 0.35 μg/side) was infused into the ventral hippocampus prior to training and/or testing of trace fear conditioning (5-pairings). Infusion of 0.35 μg/side nicotine into the ventral hippocampus at training and/or testing affected contextual [F (2,19) = 16.019, p < 0.001] and trace [F (2,19) = 6.281, p < 0.01] fear conditioning. Post-hoc analysis revealed that nicotine infusion at training, testing, or on both days produced deficits in both contextual and trace fear conditioning (Figure 3D).
Effects of nicotine infusion medial to the ventral hippocampus on trace fear conditioning
To determine if nicotine produced deficits in trace fear conditioning by acting in regions beyond the ventral hippocampus, nicotine (0 or 0.35 μg/side) was infused 1.5 mm medial to the ventral hippocampus into the thalamus prior to both training and testing of trace fear conditioning. Infusion of nicotine (0.35 μg/side) medial to the ventral hippocampus had no effect (data not shown).
Effects of antagonism of nAChRs in the ventral hippocampus on trace fear conditioning
To determine if trace and contextual fear conditioning depend upon high-affinity nAChR signaling in the ventral hippocampus, DHβE (0, 4.50, 9.00 or 18.00 μg/side) was infused into the ventral hippocampus prior to training and testing. Infusion of DHβE into the ventral hippocampus had no effect (data not shown).
To determine if trace and contextual fear conditioning depend upon low-affinity nAChR signaling in the ventral hippocampus, MLA (0, 6.75, 13.5, or 27 μg/side) was infused into the ventral hippocampus prior to training and testing. Infusion of MLA into the ventral hippocampus had no effect (data not shown).
Medial Prefrontal Cortex
Effects of nicotine infusion into the medial prefrontal cortex on trace fear conditioning
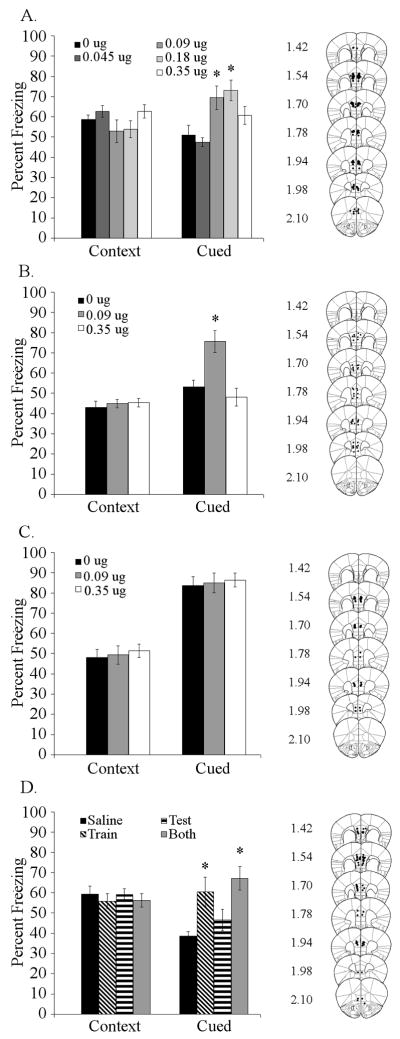
To determine if nicotine acts in the medial prefrontal cortex to alter trace or contextual fear conditioning, nicotine (0, 0.045, 0.09, 0.18, or 0.35 μg/side) was infused prior to training (5-pairing) and testing. Infusion of nicotine into the medial prefrontal cortex significantly affected trace fear conditioning [F (4,61) = 6.218, p < 0.001], but did not affect contextual fear conditioning. Post-hoc analysis revealed that mice treated with 0.09 μg/side or 0.18 μg/side showed enhanced trace fear conditioning compared to controls (Figure 4A). To determine if the effects of medial prefrontal cortical nicotine infusion were particular to a 5-pairing trace fear conditioning protocol, nicotine (0, 0.09, or 0.35 μg/side) was infused prior to both training and testing of 2-pairing trace fear conditioning. Infusion of nicotine affected trace fear conditioning [F (2,20) = 12.119, p < 0.001] in this task without affecting contextual fear conditioning. Post-hoc analysis revealed that 0.09 μg/side of nicotine enhanced 2-pairing trace fear conditioning, but 0.35 μg/side had no effect (Figure 4B).
Figure 4. Effects of nicotine infusion into the medial prefrontal cortex on fear conditioning.
A. Infusion of nicotine (0.09 and 0.18 μg/side) into the medial prefrontal cortex at training and testing enhanced trace fear conditioning, but not contextual fear conditioning ( n = 13 – 14). B. Infusion of 0.09 μg/side nicotine into the medial prefrontal cortex at training and testing enhanced trace fear conditioning, but not contextual fear conditioning in 2-pairing trace fear conditioning (n = 7 – 8). C. Infusion of nicotine (0.09 or 0.35 μg/side) had no effect on contextual or delay cued fear conditioning (2 CS-US pairings, CS 30 second; n = 8). D. Infusion of 0.09 μg/side nicotine into the medial prefrontal cortex at training or at training and testing enhanced trace conditioning, but infusion at testing had no effect (n = 8). Significant difference (p < 0.05) from the saline-infused control group denoted with (*); data are reported as mean ± standard error of the mean. Representations of cannula placements to right of data demonstrate that all infusions were directed into the medial prefrontal cortex.
Effects of medial prefrontal cortical nicotine infusion on delay cued fear conditioning
To determine if medial prefrontal cortical nicotine infusion affects delay cued fear conditioning, nicotine (0, 0.09, or 0.35 μg/side) was infused into the medial prefrontal cortex prior to training and testing of delay cued fear conditioning (i.e., no trace interval). There was no effect of nicotine infusion into the medial prefrontal cortex on contextual or delay cued fear conditioning (Figure 4C). To account for the possibility that effects of nicotine on delay cued fear conditioning might be obscured by high freezing levels, we also infused nicotine (0 or 0.09 μg/side) prior to training and testing of a 1 CS-US pairing, 15 second CS delay cued fear conditioning protocol. Infusion of nicotine into medial prefrontal cortex had no effect (data not shown).
Effects of medial prefrontal cortical nicotine infusion at training or testing of trace fear conditioning
To determine if medial prefrontal cortical nicotine infusion enhances acquisition/consolidation or retrieval of trace fear conditioning (5-pairing), nicotine (0 or 0.09 μg/side) was infused into the medial prefrontal cortex prior to training, testing, or both training and testing of trace fear conditioning. Infusion of nicotine affected trace fear conditioning [F (3,28) = 6.530, p < 0.005], but had no effect on contextual fear conditioning. Post-hoc analysis revealed that administration of nicotine at 0.09 μg/side enhanced trace fear conditioning if administered prior to training or both training and testing, but not prior to testing alone (Figure 4D).
Effects of nicotine infusion above or below the medial prefrontal cortical on trace fear conditioning
To determine if the effects of nicotine on trace fear conditioning were due to drug diffusion above or below the medial prefrontal cortex, nicotine (0 or 0.09 μg/side) was infused above the medial prefrontal cortex into primary motor cortex and cingulate cortex or below the medial prefrontal cortex into dorsal peduncular cortex and dorsal tenia tecta prior to training and testing of trace fear conditioning. Infusion of 0.09 μg/side nicotine above or below the medial prefrontal cortex had no effect (data not shown).
Effects of nAChR antagonism in the medial prefrontal cortex on trace fear conditioning
To determine if high-affinity nAChRs in the medial prefrontal cortex are involved in trace fear conditioning, DHβE (0, 4.50, 9.00, or 18.00 μg/side) was infused into the medial prefrontal cortex prior to training and testing of trace fear conditioning (5-pairing). DHβE affected trace fear conditioning [F (3,30) = 11.149, p < 0.001], but had no effect on contextual fear conditioning. Post-hoc analysis revealed that DHβE (9.00 and 18.00 μg/side) enhanced trace fear conditioning (Figure 5A). To determine if DHβE was affecting acquisition/consolidation or retrieval of trace fear conditioning, we administered DHβE (0 or 9.00 μg/side) at either training or testing of trace fear conditioning (5-pairing). Infusion of DHβE into the medial prefrontal cortex affected both contextual [F (2,23) = 7.548, p < 0.005] and trace fear conditioning [F (2,23) = 29.056, p < 0.001]. Post-hoc analysis revealed that DHβE infusion at training produced significant enhancement of trace fear conditioning without affecting contextual fear conditioning (Figure 5B). Alternately, administration of DHβE into the medial prefrontal cortex prior to testing produced deficits in both contextual and trace fear conditioning.
Figure 5. Effects of nicotinic antagonist infusion into the medial prefrontal cortex on trace fear conditioning.
A. Infusion of DHβE into the medial prefrontal cortex at training and testing dose-dependently (9.00 and 18.00 μg/side) enhanced trace fear conditioning but not contextual conditioning (n = 8 – 9). B. Infusion of 9.00 μg/side DHβE into the medial prefrontal cortex at training enhanced trace conditioning, but infusion at testing produced deficits in both trace and contextual conditioning (n = 8). C. Infusion of MLA (13.50 and 27.00 μg/side) into the medial prefrontal cortex at training and testing enhanced trace fear conditioning but did not affect contextual conditioning (n = 9 – 10). D. Infusion of 13.50 μg/side MLA into the medial prefrontal cortex at training enhanced trace conditioning, but infusion at testing produced deficits in both trace and contextual conditioning (n = 8). Significant difference (p < 0.05) from the saline-infused control group denoted with (*); data are reported as mean ± standard error of the mean. Representations of cannula placements alongside data demonstrate that all infusions were directed into the medial prefrontal cortex.
To determine if low-affinity nAChRs in the medial prefrontal cortex are also involved in trace fear conditioning, the low-affinity nAChR antagonist MLA (0, 6.75, 13.50, or 27.00 μg/side) was infused into the medial prefrontal cortex prior to training and testing of trace fear conditioning. Infusion of MLA affected trace [F (3,35) = 4.993, p < 0.005] but not contextual fear conditioning. Post-hoc analysis showed that the two higher doses of MLA (13.50 and 27.00 μg/side) enhanced trace fear conditioning (Figure 5C). To determine if MLA was affecting acquisition/consolidation or retrieval of trace fear conditioning, we administered MLA (0 or 13.50 μg/side) at either training or testing of trace fear conditioning. MLA infusion had a significant effect on both contextual [F (2,21) = 38.425, p < 0.001] and trace fear conditioning [F (2,21) = 26.808, p < 0.001]. Post-hoc analysis revealed that MLA infusion at training significantly enhanced trace fear conditioning without affecting contextual fear conditioning, but infusion of MLA into the medial prefrontal cortex at testing produced significant deficits in both contextual and trace fear conditioning (Figure 5D).
Discussion
Whereas the dorsal hippocampus, ventral hippocampus, and medial prefrontal cortex are all involved in trace fear conditioning, these areas may mediate different processes associated with trace fear conditioning. The present findings demonstrate that altering nAChR function in the dorsal hippocampus, ventral hippocampus, and medial prefrontal cortex produces different effects on trace fear conditioning (see Table 1 for summary). The dorsal hippocampus may be involved in the acquisition of trace fear conditioning (Burman et al., 2006; Esclassan et al., 2009; Gilmartin and McEchron, 2005a; McEchron et al., 1998; Quinn et al., 2005; Quinn et al., 2008) with disrupted dorsal hippocampal function more detrimental to trace fear conditioning than complete removal of the area (Czerniawski et al., 2009). The ventral hippocampus may be critically involved in the acquisition, maintenance, and/or expression of trace fear conditioning while also potentially involved in emotional and anxiolytic influences on learning (Esclassan et al., 2009; Yoon and Otto, 2007). Finally, the medial prefrontal cortex may be involved in acquisition and storage of both recent and remote trace fear conditioning memories (Blum et al., 2006; Gilmartin and McEchron, 2005b; Quinn et al., 2008; Runyan et al., 2004). Our findings that changes in nAChR function in these areas differentially altered trace fear conditioning further suggest that these areas mediate different processes.
Table 1. Summary of effects of nicotine and nicotinic antagonist infusion on trace, contextual, and delay fear conditioning.
Table represents effects of drug infusion into the dorsal hippocampus, ventral hippocampus, or medial prefrontal cortex on contextual and cued components of trace (5 pairing or 2 pairing) and delay (2 pairing or 1 pairing) fear conditioning. Enhancement denoted by (▲), deficits denoted by (▼). Effects occurring specifically at training or testing denoted by (tr) for training and (t) for testing, (-) denotes no effect.
| Trace (5-pr) | Trace (2-pr) | Delay (2-pr) | Delay (1-pr) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Drug | Context | Cued | Context | Cued | Context | Cued | Context | Cued |
| Dorsal Hippocampus | Nicotine | tr 0.09 ▲ | tr 0.09, 0.18 ▲ | 0.35 ▲ | 0.09 ▲ | 0.35 ▲ | - | - | - |
| DHβ E | - | tr 4.5, 9 ▼ | |||||||
| MLA | - | - | |||||||
| Ventral Hippocampus | Nicotine | tr,t .18, 0.35 ▼ | tr,t 0.35 ▼ | 0.09, 0.35 ▼ | 0.35 ▼ | 0.09, 0.35 ▼ | - | 0.35 ▼ | - |
| DHβ E | - | - | |||||||
| MLA | - | - | |||||||
| Medial Prefrontal Cortex | Nicotine | - | tr 0.09, 0.18 ▲ | - | 0.09 ▲ | - | - | - | - |
| DHβ E | t 9 ▼ | tr 9,18 ▲, t9 ▼ | |||||||
| MLA | t 13.5 ▼ | tr 13.5, 27 ▲, t 13.5 ▼ | |||||||
Dorsal Hippocampus
Infusion of nicotine into the dorsal hippocampus prior to training enhanced trace fear conditioning but infusion before testing had no effect. Additionally, infusion of the high-affinity nAChR antagonist DHβE before training, but not the low-affinity nAChR antagonist MLA, into the dorsal hippocampus disrupted trace fear conditioning. These results suggest that high-affinity nAChRs in the dorsal hippocampus may be involved in the acquisition of trace fear conditioning but not recall. Infusion of nicotine into the dorsal hippocampus also enhanced contextual fear conditioning, as we have previously reported (Davis and Gould, 2009; Davis et al., 2007). No effect was seen on delay cued fear conditioning demonstrating that these effects do not generalize to all types of learning. The similar effects of nicotine on both trace and contextual fear conditioning could suggest that nAChRs in the dorsal hippocampus are mediating similar processes for both types of learning, perhaps processing of contextual information that could support both tasks (Marchand et al., 2004). Current results, however, suggest that this is not the case. In support, the effective doses of nicotine infused into the dorsal hippocampus differed between the two types of conditioning. This difference could be related to nicotine acting on different dorsal hippocampal processes involved in each task, which could include different nAChR subtypes. Furthermore, infusion of DHβE in the dorsal hippocampus disrupted trace but not contextual fear conditioning. This further suggests that different dorsal hippocampal processes underlie trace and contextual fear conditioning One possibility is that during trace fear conditioning, the dorsal hippocampus maintains a representation of the CS across the trace interval (Burman and Gewirtz, 2007; Rodriguez and Levy, 2001), which can be enhanced by nicotine and depends on high-affinity nAChR signaling; while during contextual fear conditioning, the dorsal hippocampus maintains a representation of the context (Rudy et al., 2002), which can be enhanced by nicotine (Kenney and Gould, 2008b), but does not critically depend on nAChR signaling.
The finding that antagonism of dorsal hippocampal high-affinity nAChRs disrupts trace fear conditioning is surprising because we have previously shown that systemic administration of DHβE does not affect trace fear conditioning (Raybuck and Gould, 2009) and that β2 knockout (KO) mice show normal trace fear conditioning (Davis and Gould, 2007; Raybuck and Gould, 2009). This discrepancy could be due to multiple factors. Deficits induced by dorsal hippocampal DHβE infusion were dose-dependent, but our previous study only investigated the effects of a single systemically administered dose of DHβE (3 mg/kg) on trace fear conditioning (Raybuck and Gould, 2009); which while sufficient to precipitate withdrawal deficits in mice treated with chronic nicotine, may not have been sufficient to produce deficits in trace fear conditioning in nicotine-naïve mice. The lack of trace fear conditioning deficits in β2 KO mice (Davis and Gould, 2007; Raybuck and Gould, 2009) compared to the deficits observed with direct infusion of DHβE into the dorsal hippocampus suggests that there may be fundamental differences between transiently disrupting function of a brain region as with an antagonist and permanently altering a brain region with genetic KO techniques. For instance, compensatory developmental changes could occur in the β2 KO mice that allow for trace fear conditioning in these mice even though they do not have functional β2-containing nAChRs. Another potential difference is that DHβE, while selective for high affinity nAChRs, antagonizes both β2 and non β2-containing nAChRs such as α4β4 and α2β4 nAChRs (Harvey and Luetje, 1996; Williams and Robinson, 1984); antagonism of non β2-containing nAChRs in the dorsal hippocampus could disrupt trace fear conditioning.
Ventral Hippocampus
In contrast to the dorsal hippocampus, the present study found that nicotine infusion into the ventral hippocampus produced deficits in acquisition and retrieval of both trace and contextual fear conditioning. Infusion of MLA or DHβE into the ventral hippocampus had no effect. The difference in the effects of nicotinic drugs infused into the dorsal versus ventral hippocampus on trace and contextual fear conditioning could relate to differences in the connectivity of these areas. The ventral, but not the dorsal hippocampus, is reciprocally connected with the amygdala (Hoover and Vertes, 2007; Ishikawa and Nakamura, 2006; Pitkänen et al., 2000; Vertes, 2004). In fact it has been proposed that the ventral hippocampus acts as a conduit for information flow between the dorsal hippocampus and amygdala (Hobin et al., 2006; Maren and Fanselow, 1995; Maren and Hobin, 2007). In addition, the ventral hippocampus may mediate emotional or anxiety-related responses (Bannerman et al., 2003; Degroot and Treit, 2004; Kjelstrup et al., 2002; Pentkowski et al., 2006) through connections with the amygdala. Therefore, infusion of nicotine into the ventral hippocampus may alter hippocampus-mediated emotional/anxiety states that could disrupt acquisition and expression of both trace and contextual fear conditioning, though this requires further examination.
Delay cued fear conditioning was not altered by infusion of nicotine into the ventral hippocampus. A number of studies have shown that manipulation of ventral hippocampal function can have different effects on contextual and delay cued fear conditioning with some procedures affecting both and others only effecting contextual fear conditioning (Bast et al., 2001; Esclassan et al., 2009; Zhang et al., 2001). Thus, contextual and trace fear conditioning may be more sensitive than delay cued fear conditioning to the effects of infusion of nicotine into the ventral hippocampus.
Medial Prefrontal Cortex
Infusion of nicotine into the medial prefrontal cortex produced results different than those from the other areas examined in that only trace fear conditioning was altered. The specificity of these effects for trace fear conditioning are similar to results from a study by Blum, Herbert, and Dash (2006) that showed that inactivation of the medial prefrontal cortex disrupted recall of trace but not contextual fear conditioning. This area may be a long-term site of memory storage for trace fear conditioning (Quinn et al., 2008; Runyan et al., 2004) and nicotine may enhance storage of trace fear conditioning memories in the medial prefrontal cortex. Interestingly, infusion of either DHβE or MLA into the medial prefrontal cortex at training also enhanced trace fear conditioning. This matches a prior study showing enhanced trace fear conditioning in α7 KO mice (Davis and Gould, 2007). Because nicotine both activates and desensitizes nAChRs (Picciotto et al., 2008) and the antagonists had effects similar to nicotine, nicotine may enhance trace fear conditioning in part by desensitizing nAChRs in the medial prefrontal cortex. This finding is similar to prior results suggesting that nicotine ameliorated ethanol-induced learning deficits by desensitizing nAChRs in the cingulate cortex (Gulick and Gould, 2009).
One unexpected result was that infusion of MLA or DHβE into the medial prefrontal cortex at testing disrupted trace and contextual fear conditioning. It may be that different medial prefrontal cortical processes are involved in acquisition and recall/expression of learning and that these processes are differentially sensitive to nAChR antagonists. The medial prefrontal cortex is interconnected with the ventral hippocampus and amygdala (Hoover and Vertes, 2007; Ishikawa and Nakamura, 2006; Pitkänen et al., 2000; Vertes, 2004). Therefore, nAChR antagonists could disrupt medial prefrontal cortical function at testing by interfering with activity in areas involved in the expression of contextual and trace fear conditioning.
General Conclusions
The effects of direct infusion of nicotine and nicotinic antagonists show that nAChRs in the dorsal hippocampus, ventral hippocampus, and medial prefrontal cortex mediate different processes that underlie trace fear conditioning. In the dorsal hippocampus, nAChRs appear to be involved in the acquisition of trace fear conditioning. These effects may be independent of contextual processes as the effects of nicotinic agonists and antagonists infused into the dorsal hippocampus were different for contextual and trace fear conditioning. Infusion of nicotine into the ventral hippocampus may modulate both acquisition and expression of contextual and trace fear conditioning, though it is possible that the effects of infusion of nicotine into the ventral hippocampus are related to changes in anxiety (Bannerman et al., 2003; Degroot and Treit, 2004; Kjelstrup et al., 2002; Pentkowski et al., 2006). It is important to note that nicotine infusion into the dorsal hippocampus enhanced trace and contextual fear conditioning but infusion into the ventral hippocampus disrupted learning. The dorsal hippocampal infusion results match what is seen with systemic administration (Gould et al., 2004); this suggests that with systemic administration the dorsal hippocampal response is dominant over the ventral hippocampal response. An interesting question is under what conditions the ventral hippocampus would be dominant over the dorsal hippocampus. Finally, infusion of nicotine or nAChR antagonists into the medial prefrontal cortex enhanced trace fear conditioning, suggesting that desensitization of nAChRs in the medial prefrontal cortex could be involved in the effects of nicotine on trace fear conditioning.
Regional differences in nAChR composition, function, and cellular location could contribute to variability in the behavioral effects of infusing these drugs into different brain regions. For instance, dopamine is involved in trace fear conditioning (Runyan and Dash, 2004) and high- and low-affinity nAChRs can have different effects on dopamine release (Mameli-Engvall et al., 2006; Picciotto et al., 2008; Zhang et al., 2009). Furthermore, hippocampus and prefrontal cortex show different levels of nicotine stimulated dopamine release (Shearman et al., 2005; Shearman et al., 2008). Therefore, regional differences in nicotine-stimulated neurotransmitter release could contribute to these behavioral differences. Another possibility that needs further investigation is that regional differences in proteins that regulate nAChR function contribute to the behavioral differences associated with nicotine infusion into these areas. For example, nAChR function and desensitization is regulated by lynx1 and this protein is differentially expressed across cortical layers and hippocampal subregions (Ibañez-Tallon et al., 2002; Miwa et al., 1999; Miwa et al., 2006). An important future direction will be elucidating the underlying mechanisms responsible for the regional variability in the effects of nicotinic drugs on learning as this may also aid in understanding and treating diseases that involve changes in cognition and nAChR function such as Alzheimer’s disease, schizophrenia, and addiction.
Acknowledgments
The authors would like to acknowledge grant support from National Institute of Health (DA017949 TG, DA024787 TG, P50 CA143187: Caryn Lerman Ph.D). Additionally, JDR was supported by a NIH/NIDA training grant (T32DA07237) and is currently supported by (T32DA007262). Additionally the authors thank Kristy Cordero for assistance with histological analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bannerman DM, Grubb M, Deacon RMJ, Yee BK, Feldon J, Rawlins JNP. Ventral hippocampal lesions affect anxiety but not spatial learning. Behavioural Brain Research. 2003;139:197–213. doi: 10.1016/s0166-4328(02)00268-1. [DOI] [PubMed] [Google Scholar]
- Bast T, Zhang WN, Feldon J. The ventral hippocampus and fear conditioning in rats. Different anterograde amnesias of fear after tetrodotoxin inactivation and infusion of the GABA(A) agonist muscimol. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale. 2001;139:39–52. doi: 10.1007/s002210100746. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–4. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Brega AG, Grigsby J, Kooken R, Hamman RF, Baxter J. The impact of executive cognitive functioning on rates of smoking cessation in the San Luis Valley Health and Aging Study. Age and Ageing. 2008;37:521–5. doi: 10.1093/ageing/afn121. [DOI] [PubMed] [Google Scholar]
- Buckingham SD, Jones AK, Brown LA, Sattelle DB. Nicotinic acetylcholine receptor signalling: roles in Alzheimer's disease and amyloid neuroprotection. Pharmacological Reviews. 2009;61:39–61. doi: 10.1124/pr.108.000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman MA, Gewirtz JC. Hippocampal activity, but not plasticity, is required for early consolidation of fear conditioning with a short trace interval. The European Journal of Neuroscience. 2007;25:2483–90. doi: 10.1111/j.1460-9568.2007.05493.x. [DOI] [PubMed] [Google Scholar]
- Burman MA, Starr MJ, Gewirtz JC. Dissociable effects of hippocampus lesions on expression of fear and trace fear conditioning memories in rats. Hippocampus. 2006;16:103–13. doi: 10.1002/hipo.20137. [DOI] [PubMed] [Google Scholar]
- Büchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. The Journal of Neuroscience. 1999;19:10869–76. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Yoon T, Otto T. Dissociating space and trace in dorsal and ventral hippocampus. Hippocampus. 2009;19:20–32. doi: 10.1002/hipo.20469. [DOI] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. beta2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology. 2007;190:343–52. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. European Journal of Neuropharmacology. 2009;19(8):551–61. doi: 10.1016/j.euroneuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Kenney JW, Gould TJ. Hippocampal alpha4beta2 nicotinic acetylcholine receptor involvement in the enhancing effect of acute nicotine on contextual fear conditioning. The Journal of Neuroscience. 2007;27:10870–7. doi: 10.1523/JNEUROSCI.3242-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot A, Treit D. Anxiety is functionally segregated within the septo-hippocampal system. Brain Research. 2004;1001:60–71. doi: 10.1016/j.brainres.2003.10.065. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus. 2009;19:33–44. doi: 10.1002/hipo.20473. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the dentate gyrus and CA1 of the hippocampus exhibit inverse patterns of encoding during trace fear conditioning. Behavioral Neuroscience. 2005a;119:164–79. doi: 10.1037/0735-7044.119.1.164. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behavioral Neuroscience. 2005b;119:1496–510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behavioural Brain Research. 1999;102:31–9. doi: 10.1016/s0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Feiro O, Moore D. Nicotine enhances trace cued fear conditioning but not delay cued fear conditioning in C57BL/6 mice. Behavioural Brain Research. 2004;155:167–73. doi: 10.1016/j.bbr.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Gulick D, Gould TJ. The Hippocampus and Cingulate Cortex Differentially Mediate the Effects of Nicotine on Learning Versus on Ethanol-Induced Learning Deficits Through Different Effects at Nicotinic Receptors. Neuropsychopharmacology. 2009;34:2167–79. doi: 10.1038/npp.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkin BS, Dehaene S, Changeux J. A neurocomputational hypothesis for nicotine addiction. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1106–11. doi: 10.1073/pnas.0510220103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey SC, Luetje CW. Determinants of competitive antagonist sensitivity on neuronal nicotinic receptor beta subunits. The Journal of Neuroscience. 1996;16:3798–806. doi: 10.1523/JNEUROSCI.16-12-03798.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010 doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–82. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–79. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Ibañez-Tallon I, Miwa JM, Wang HL, Adams NC, Crabtree GW, Sine SM, Heintz N. Novel modulation of neuronal nicotinic acetylcholine receptors by association with the endogenous prototoxin lynx1. Neuron. 2002;33:893–903. doi: 10.1016/s0896-6273(02)00632-3. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Ventral hippocampal neurons project axons simultaneously to the medial prefrontal cortex and amygdala in the rat. Journal of Neurophysiology. 2006;96:2134–8. doi: 10.1152/jn.00069.2006. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Molecular Neurobiology. 2008a;38:101–21. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Nicotine enhances context learning but not context-shock associative learning. Behavioral Neuroscience. 2008b;122:1158–65. doi: 10.1037/a0012807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KG, Tuvnes FA, Steffenach H, Murison R, Moser EI, Moser M. Reduced fear expression after lesions of the ventral hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10825–30. doi: 10.1073/pnas.152112399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Cheng DT, Smith CN, Stein EA, Helmstetter FJ. Neural substrates mediating human delay and trace fear conditioning. The Journal of Neuroscience. 2004;24:218–28. doi: 10.1523/JNEUROSCI.0433-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MC, Gould TJ. Reversible inactivation of the entorhinal cortex disrupts the establishment and expression of latent inhibition of cued fear conditioning in C57BL/6 mice. Hippocampus. 2007;17:462–70. doi: 10.1002/hipo.20284. [DOI] [PubMed] [Google Scholar]
- Mameli-Engvall M, Evrard A, Pons S, Maskos U, Svensson TH, Changeux J, Faure P. Hierarchical control of dopamine neuron-firing patterns by nicotinic receptors. Neuron. 2006;50:911–21. doi: 10.1016/j.neuron.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Marchand AR, Luck D, Di Scala G. Trace fear conditioning: a role for context? Archives Italiennes de Biologie. 2004;142:251–63. [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. The Journal of Neuroscience. 1995;15:7548–64. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Hobin JA. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learning & Memory. 2007;14:318–24. doi: 10.1101/lm.477007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–46. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behavioral Neuroscience. 2002;116:37–47. [PubMed] [Google Scholar]
- Misane I, Tovote P, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15:418–26. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Ibanez-Tallon I, Crabtree GW, Sánchez R, Sali A, Role LW, Heintz N. lynx1, an endogenous toxin-like modulator of nicotinic acetylcholine receptors in the mammalian CNS. Neuron. 1999;23:105–14. doi: 10.1016/s0896-6273(00)80757-6. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Stevens TR, King SL, Caldarone BJ, Ibanez-Tallon I, Xiao C, Fitzsimonds RM, Pavlides C, Lester HA, Picciotto MR, Heintz N. The prototoxin lynx1 acts on nicotinic acetylcholine receptors to balance neuronal activity and survival in vivo. Neuron. 2006;51:587–600. doi: 10.1016/j.neuron.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and Alcohol Dependence. 2010;106:61–4. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry. 2009;65:144–9. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The Mouse Brain in Stereotaxic Coordinates: Compact. 2. Academic Press; 2003. [Google Scholar]
- Pentkowski NS, Blanchard DC, Lever C, Litvin Y, Blanchard RJ. Effects of lesions to the dorsal and ventral hippocampus on defensive behaviors in rats. The European Journal of Neuroscience. 2006;23:2185–96. doi: 10.1111/j.1460-9568.2006.04754.x. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in Neurobiology. 2008;84:329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000;911:369–91. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15:665–74. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learning & Memory. 2008;15:368–72. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice--a role for high-affinity beta2 subunit-containing nicotinic acetylcholine receptors. The European Journal of Neuroscience. 2009;29:377–87. doi: 10.1111/j.1460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razani J, Boone K, Lesser I, Weiss D. Effects of cigarette smoking history on cognitive functioning in healthy older adults. The American Journal of Geriatric Psychiatry. 2004;12:404–11. doi: 10.1176/appi.ajgp.12.4.404. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Levy WB. A model of hippocampal activity in trace conditioning: where's the trace? Behavioral Neuroscience. 2001;115:1224–38. doi: 10.1037//0735-7044.115.6.1224. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behavioral Neuroscience. 2002;116:530–8. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiology of Learning and Memory. 2004;82:65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. The Journal of Neuroscience. 2004;24:1288–95. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman E, Fallon S, Sershen H, Lajtha A. Nicotine-induced monoamine neurotransmitter changes in the brain of young rats. Brain Research Bulletin. 2008;76:626–39. doi: 10.1016/j.brainresbull.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Shearman E, Rossi S, Sershen H, Hashim A, Lajtha A. Locally administered low nicotine-induced neurotransmitter changes in areas of cognitive function. Neurochemical Research. 2005;30:1055–66. doi: 10.1007/s11064-005-7132-9. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychology Review. 2007;17:259–73. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse (New York, NY) 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: acute positive reinforcement and withdrawal. Nicotine & Tobacco Research. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Williams M, Robinson JL. Binding of the nicotinic cholinergic antagonist, dihydro-beta-erythroidine, to rat brain tissue. The Journal of Neuroscience. 1984;4:2906–11. doi: 10.1523/JNEUROSCI.04-12-02906.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer G. Why do patients with schizophrenia smoke? Current Opinion in Psychiatry. 2010;23:112–9. doi: 10.1097/YCO.0b013e3283366643. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Gould TJ. Neuronal nicotinic acetylcholine receptors: involvement in Alzheimer's disease and schizophrenia. Behavioral and Cognitive Neuroscience Reviews. 2002;1:5–20. doi: 10.1177/1534582302001001002. [DOI] [PubMed] [Google Scholar]
- Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiology of Learning and Memory. 2007;87:464–75. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang L, Liang Y, Siapas AG, Zhou F, Dani JA. Dopamine signaling differences in the nucleus accumbens and dorsal striatum exploited by nicotine. The Journal of Neuroscience. 2009;29:4035–43. doi: 10.1523/JNEUROSCI.0261-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WN, Bast T, Feldon J. The ventral hippocampus and fear conditioning in rats: different anterograde amnesias of fear after infusion of N-methyl-D-aspartate or its noncompetitive antagonist MK-801 into the ventral hippocampus. Behavioural Brain Research. 2001;126:159–74. doi: 10.1016/s0166-4328(01)00256-x. [DOI] [PubMed] [Google Scholar]