Abstract
Clock gene expression has been observed in tissues of the hypothalamic-pituitary-gonadal (HPG) axis. While the contribution of hypothalamic oscillators to the timing of reproductive biology is well known, the role of peripheral oscillators like those in the ovary is less clear. Circadian clocks in the ovary may play a role in the timing of ovulation. Disruption of the clock in ovarian cells or desynchrony between ovarian clocks and circadian oscillators elsewhere in the body may contribute to the onset and progression of various reproductive pathologies. Here we review evidence for clock function in the ovary across multiple species and offer a novel perspective on the role of this clock in normal ovarian physiology and in diseases that negatively impact fertility.
Keywords: rat, mouse, ovulation, rhythm, clock gene, reproduction
The Molecular Clock and Reproductive Physiology
Over the past 20 years, the field of circadian biology has come of age, primarily as a consequence of two critical developments: unraveling much of the molecular machinery that generates the near 24h oscillations and the clear demonstration that clocks exist in cells throughout the body. By now we have a good idea of how central clocks in the brain are synchronized to the environment; however, we know almost nothing about the mechanisms through which signals from the brain synchronize the clocks that exist in most peripheral organs or how peripheral clocks contribute to the functions of the organs in which they reside. It is likely that such contributions will be highly organ-specific. In all probability, elucidating the relationships between circadian rhythmicity and organ function will lead to important new insights into the physiology and pathology of specific organ systems. While it has been known for some time that many aspects of reproductive function are strongly circadian, autonomous rhythmicity in reproductive structures has only recently been explored. Though the majority of evidence from mammals suggests that some male reproductive structures (e.g. testis) do not contain cell autonomous circadian clocks [1, 2], circadian clocks are present in accessory structures such as the extra-testicular ducts [3]. Circadian clocks are also present in the cells of the ovary [4–7], uterus [8–10] and oviduct [11].
The fundamental basis for circadian rhythms of physiology is a molecular clock consisting of interlocked transcriptional/translational feedback loops (for review see [12, 13]). The transcription factors CLOCK (or its paralog neuronal Per-Arnt-Sim (PAS) domain containing protein; NPAS2) and BMAL1 (brain and muscle arnt-like protein 1) heterodimerize and promote rhythmic transcription of the period (per1, per2) and cryptochrome (cry1, cry2) gene families. Once translated in the cytoplasm, PER and CRY proteins heterodimerize and are phosphorylated by the casein kinases (CK1ε,δ). The modified PER:CRY complexes translocate to the nucleus and repress the activity of the CLOCK:BMAL1 complex. Over several hours, PER:CRY complexes are degraded and eventually the CLOCK:BMAL1 complex is released from feedback inhibition. Though the precise kinetics of each reaction are poorly understood, it is clear that the entire process requires approximately 24h to complete, thus defining the period of the oscillator. While the period of the oscillator is relatively constant around a mean of 24h regardless of tissue, the phase of peak clock gene expression varies greatly in a cell- and tissue- dependent fashion. For instance, the rhythm of clock gene expression in the lung peaks during the early night, while that of the liver generally peaks around midnight [14]. The circadian clock gene products are basic helix-loop-helix transcription factors and as such can drive rhythmic expression of “clock-controlled genes” (CCGs) through binding at E-box promoter sequences. Recent evidence points to a multitude of genes involved in cell signaling pathways, cellular metabolism and cell cycle regulation as putative CCGs [15–17]. The cell-type specific nature of the oscillations and downstream CCG expression is likely to be critical for normal physiological function in the various components of the system.
A majority of central and peripheral tissues contain coupled cellular oscillators [18, 19], which influence both endocrine and neuroendocrine processes [20–23]. For example, the neural oscillator in the suprachiasmatic nucleus of the hypothalamus (SCN) plays a well established role in the timing of the preovulatory luteinizing hormone (LH) surge [22, 24]. However, normal function appears to rely on an integrated “circadian system” that synchronizes a multitude of autonomous central and peripheral clocks [19].
In the last decade, the development of rat and mouse transgenic reporter lines [14, 25–28] has enhanced our ability to study the role of cell autonomous circadian clocks in mammalian physiology. To maintain physiological “rheostasis” (a term Mrosovsky has introduced to describe regulation with rhythmic steady states as opposed to the constant steady states of homeostatically regulated systems [29]), the circadian system requires adaptive “phase-synchrony” between the numerous tissue- and cell- specific circadian oscillators.
To better understand how this concept applies to the hypothalamic-pituitary-gonadal (HPG) axis, experiments are needed to elucidate the clocks’ role in the physiology of each organ and cell type. Here we describe evidence for the existence of circadian clocks in the ovaries of invertebrates, non-mammalian vertebrates and mammals with an emphasis on birds and rodents. We describe the cellular distribution and functional characteristics of the ovarian clock and review the evidence for the role of this oscillator in the timing of ovarian physiology. Finally, we discuss the potential relevance of this work to the study of diseases that adversely affect female fertility. Although this review focuses on the function of the clock in the ovary, it should be clear that this tissue is but one major component of the HPG axis. For a broader discussion of the role of the circadian clock in mammalian reproductive biology as it relates to the HPG axis and general influences on fertility and fecundity, we refer the reader to several recent reviews on the topic [30–32]. In what follows we have used “clock genes” to refer to any of the genes that have been shown to participate in the feedback loop that generates basic circadian oscillations.
The Circadian Clock in the Ovary: Form and Function
Invertebrates
Clock gene expression has been observed in the ovaries of the silkworm (Bombyx mori; [33]), prawn (Macrobrachium rosenbergii; [34]) and the fruit fly (Drosophila melanogaster; [35–39]). Although circadian rhythms and/or functional consequences of clock gene expression have not been described in the silkworm or prawn, these parameters have been described in the fruit fly. In Drosophila ovaries, clock genes (period and timeless) are present but not rhythmically expressed [38, 39]. Mutant flies lacking functional period (per01) or timeless (tim01) gene expression produce fewer mature oocytes and progeny after mating with a wild-type male [38]. These effects may be due to the absence of clock genes or to inbreeding depression in per01 mutant flies [39]. More evidence is required before any conclusions can be made regarding the importance of the circadian clock in Drosophila ovaries, although it is worth noting that circadian rhythmicity is essential for proper testis function in some insects [40–42]. Given the robust nature of circadian rhythms of egg-laying in Drosophila, it is surprising that the ovary lacks a circadian clock [43].
Non-Mammalian Vertebrates
Examination of the oviposition-ovulation cycle in domestic hens (Gallus domesticus [44, 45] and Japanese quail (Coturnix Japonica; [46–48]) suggests a multi-oscillatory system that includes a central clock driving circadian rhythms of body temperature and an “ovulatory” clock responsible for the timing of oviposition [48]. In domestic hens and quail, the rhythm of ovulation-oviposition is entrained to the daily L:D cycle such that egg-laying occurs in the early morning (chicken) or middle of the afternoon (quail; [45–47]). In both species this rhythm persists in constant light with a period near 27h [46, 47]. The rhythm of ovulation-oviposition in birds depends in part on the timing of LH secretion [45–47] which in turn depends on rhythmic sensitivity of the hypothalamus to circulating progesterone (for review see [44]). However, the mechanism for temporal regulation of hypothalamic sensitivity is unknown, and evidence of hierarchical control of ovulation in birds by central and peripheral oscillators is limited [49–51].
Intriguing evidence for the contribution of a functional clock within the avian ovary comes from work by Underwood and colleagues [46]. These authors examined the rhythm of core body temperature (CBT) and oviposition in intact or ovariectomized (OVX) Japanese quail housed in either constant light (LL) or constant darkness (DD). In quail, as in many endotherms, there is a robust circadian rhythm of CBT, peaking around the middle of the subjective day [46]. The rhythm of CBT is entrained to the 12:12 L:D cycle and free-runs (persists with an endogenous period) in constant conditions. In LL at 10 and 100 lux, the circadian rhythm of CBT free-runs with a period >24h and is synchronized with the rhythm of oviposition. When placed in DD, animals cease to ovulate and the rhythm of CBT free-runs with a period closer to 22h. Surprisingly, ovariectomy abolished rhythms of CBT when birds were placed in LL. Although birds have photoreceptors in both their eyes and brains (for review see [52]), blocking visual cues with opaque cones affixed to the eyes resulted in two simultaneous rhythms of CBT, one with a period <24h and another >24h associated with the rhythm of oviposition. These data strongly suggest the influence of multiple dissociable circadian oscillators on the rhythm of CBT [46]. Perhaps one oscillator, (in the basal hypothalamus?) drives the short period rhythm and another oscillator (in the ovary?), produces the longer period rhythm associated with oviposition. While the source of the long period ovary-dependent oscillation remains unknown, one possibility is that the ovarian clock drives a long period (>24h) rhythm of steroid hormone secretion that in turn induces the observed rhythm in CBT.
Evidence for the existence of a clock in the ovary itself was recently reported in Japanese quail and domestic hens [47, 53]. Expression of clock genes including per2, per3, clock and bmal1 was detected in multiple quail tissues including the liver, lung, heart, kidney, spleen and ovary [53]. Furthermore, strong diurnal rhythms of quail per2 (qPer2) and qPer3 mRNA expression in the granulosa and thecal cells of fully mature F1 follicles were observed [47]. Rhythms of qPer2 and a tendency toward cycling in qPer3, clock and bmal1 were also detected in F1 follicles from quail housed in constant light. Diurnal rhythms of the cholesterol transporter steroidogenic acute regulatory protein (StAR) and the steroid biosynthetic enzyme 3β-hydroxysteroid dehydrogenase (3-βhsd) were observed in F1 follicles [47]. Analysis of the promoter region of StAR in transiently transfected primary cultures of chick granulose cells revealed transcriptional activation of StAR gene expression by the CLOCK:BMAL1 heterodimer [47]. It seems likely that the ovary in quail (and domestic hens) contains a circadian clock, specifically within granulosa and thecal cells, which plays a role in the timing of steroidogenesis. As Ball suggests [48], this supports the novel hypothesis that the ovarian clock in birds may contribute to the circadian rhythm of ovulation by driving rhythmic positive steroid hormone feedback on the hypothalamus. The gross similarities among rhythms of ovulation-oviposition in birds, ovulation during the estrous cycle in rodents and the menstrual cycle in humans (for review see [44, 45] and Box 1) suggest that the circadian clock in cells of the mammalian follicle could play a similar role in ovarian physiology.
Box 1. Comparative physiology of preovulatory LH secretion and the timing of ovulation.
Circadian rhythms of ovulation in birds and rodents are linked to the timing of LH secretion, itself driven by circadian pacemakers in the SCN (for review see [44, 45]). Although control of the LH surge by the circadian clock in the SCN has been demonstrated in rodents (e.g. rats, mice and hamsters), a similar role for this structure remains unclear in women. However, diurnal rhythms of LH secretion during the menstrual cycle, which correlate strongly with the circadian rhythm of serum cortisol and the light:dark cycle, have been repeatedly observed in female subjects [93–95]. Further, it has been reported that ovulation occurs within 5–15 hours of peak LH secretion, further supporting the notion that the timing of ovulation in women may be linked to the circadian system [94]. Future experiments may provide definitive evidence linking the activity of pacemaker neurons in the SCN with the timing of ovarian physiology in women. Unraveling these relationships may prove critical to understanding the role of circadian clock function in diseases that negatively impact fertility.
Mammals
Clock gene expression has been described in the ovaries of rats [4–7, 54], mice [31, 55] and ruminants (ovine, bovine; [56]). In situ methods were used to localize clock gene expression to the granulosa (GC), thecal (TC) and luteal (LC) cells of the rat ovary [4, 5]. Analyses with quantitative real-time (qPCR) indicated that per1 and per2 mRNA expression peaked in the early night, was rhythmic regardless of estrous cycle phase and persisted when rats were placed in constant darkness [5]. Expression of per1 and per2 mRNA was also observed in interstitial glandular tissue, corpora lutea, pre-antral, antral and pre-ovulatory follicles. Furthermore, high-power confocal microscopy measured time-dependent shuttling of PER1 and PER2 proteins between the cytoplasm and nucleus in luteinized granulosa and thecal cells [5]. Within the same year circadian rhythms of per2 and bmal1 mRNA were reported in rat GCs and TCs [4]. Further, real-time PCR revealed a significant increase in bmal1 expression at ZT18 (ZT12=lights off in a 12:12 L:D cycle) on proestrus, suggesting an effect of the preovulatory LH surge, which occurs between ZT12-18, on the ovarian clock [4]. To explore this, the authors treated hypophysectomized immature female rats with a priming dose of equine chorionic gonadotrophin (eCG) followed by treatment with human chorionic gonadotrophin (hCG). Treatment with eCG alone, which induces development of immature follicles, failed to produce a significant increase or robust diurnal rhythm of per2 or bmal1 expression. However, subsequent treatment with hCG, which acts at LH/CG receptors and is known to induce follicular rupture, produced a robust diurnal rhythm of clock gene expression in the rat ovary [4]. These data suggest that the rat ovary contains gonadotrophin-sensitive circadian oscillators.
In vitro evidence for a cell autonomous circadian clock in specific cell types within the ovary was first reported by He and colleagues [6, 54]. They monitored real-time bioluminescence in dexamethasone (DEX) synchronized primary cultures of GCs and LCs from per2-luciferase (per2-luc) transgenic rats. A circadian rhythm of per2-luc expression was observed in LCs from pregnant rats or immature rats primed with eCG and hCG, whereas naïve GCs failed to show persistent high amplitude rhythms [54]. In a subsequent study, these authors examined the response of primary GC cultures to gonadotrophin treatment [6], but did not detect rhythms of per1 mRNA expression in GCs from immature follicles. However, per1 mRNA was rhythmic in LCs from pubertal rats and could be stimulated by gonadotrophins through CRE-mediated transactivation [6]. These data indicate that follicular cells are able to express cell autonomous circadian rhythmicity, and that while these oscillators can be affected by gonadotrophins, the effect depends on the differentiated state of the cell (e.g. GC vs. LC).
In a recent study our laboratory examined the response of the ovarian circadian clock of rats to phase-shifts of the 12:12 L:D cycle and found that entrainment requires humoral, but not neural, inputs [7]. Further, circadian rhythms of per1-luciferase gene expression were measured in individual follicular cells taken from gonadotrophin-primed immature rats and maintained as mixed granulosa/thecal cell monolayer cultures (Figure 1a). In addition, phase-dependent responses to LH or FSH treatment were observed [7]. The stimulatory effects of gonadotrophins on the circadian clock in cultured granulosa cells agree with results reported by others [6]. Thus, follicular cells may normally be entrained to the environment by humoral cues, most likely pituitary gonadotrophins, acting in a phase-dependent manner. However, these data do not provide evidence for a functional link between the clocks in the ovary and ovarian physiology. To address this, it will be necessary to examine the effects on ovarian physiology of disrupting or eliminating the circadian clock. Though complicated by significant caveats, evidence suggests that global clock gene mutations (e.g. clockΔ19 mutant, bmal1−/− or per1/2 double KOs) fail to alter follicular growth and morphology (see [30, 57–59] and Box 2). The use of tissue and cell specific targeted genetic deletion of clock gene expression may substantially improve our understanding of how individual circadian oscillators within the HPG axis contribute to reproductive physiology.
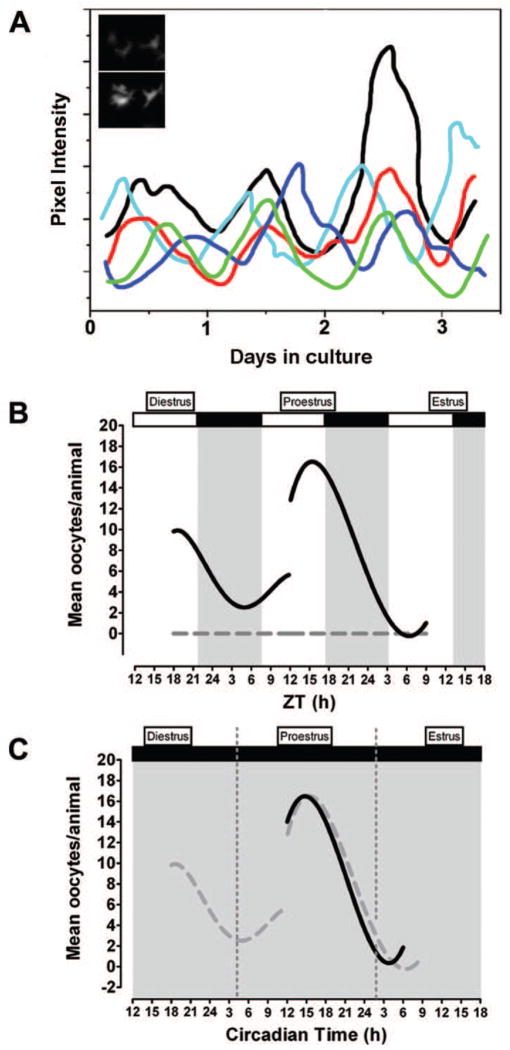
Figure 1.
A circadian clock in the rat ovary may contribute to the timing of ovulation. (a) Schematic representation showing circadian rhythms of period1-luciferase gene expression in individual granulosa/thecal cells. Inset graph: Images of trough (top panel) and peak (bottom panel) per1-luciferase gene expression in a representative granulosa/thecal cell recorded with an intensified CCD camera. Based on data from [7]. Schematic representation of (b) diurnal and (c) circadian rhythms of ovulation in response to exogenous LH in the absence of endogenous LH secretion. Animals housed under (b) a 12:12 L:D cycle or (c) constant dim light were injected with the GnRH receptor antagonist Cetrorelix on diestrus or proestrus to suppress endogenous LH secretion followed by timed injections of equine LH (solid black lines). LH-treatment during the subjective night on both diestrus and proestrus (L:D) or proestrus alone resulted in more frequent ovulation and significantly more oocytes/ovulation. Animals treated with sterile saline (gray dashed line in (b)) failed to ovulate regardless of injection time. The open and solid bars at the top of the figure indicate the light and dark portions of the L:D cycle. The solid gray background in (c) indicates that animals were maintained under constant dim light. Dashed gray lines in (f) are data from (b) re-plotted to emphasize the similarity of the results. Panels (b, c) modified from [60].
Box 2. The effects of clock gene mutations on reproductive physiology.
Several recent studies describe the effects of disrupting circadian clock function on mammalian reproductive physiology [10, 30, 57, 59, 96, 97]. Reduced fertility and fecundity have been reported in middle-aged, but not young, per1−/− and per2−/−mice [98]. Examination of dominant negative clockΔ19 mutant mice reveals prolonged estrous cycles with extended periods of estrus and a reduced amplitude or complete absence of a proestrous LH surge [10, 57]. These mice have morphologically normal ovaries, regularly mate and produce fertilizable ova, though a significant proportion of embryos and full-term fetuses are reabsorbed [57]. Evidence suggests that irregular estrous cycles in clock mutant mice are due to abnormal rhythms of vasopressin (AVP) expression in SCN neurons and reduced AVP1a receptor in SCN target regions in the hypothalamus [99].
Several investigators have observed significantly impaired fertility and fecundity in bmal1−/− mice [58, 59, 80]. Bmal1−/− mice display decreased steroidogenesis due to reduced expression of steroidogenic acute regulatory protein (a rate limiting enzyme in steroid hormone synthesis [80]). Although bmal1−/− mice exhibit abnormally long estrous cycles, they produce viable and fertilizable ova [58]. Compared with wild-type littermates, these animals display a higher incidence of implantation failure due to reduced progesterone secretion from the corpus luteum [58]. It is worth noting that StAR and bmal1 are apparently arrhythmic in corpora lutea, suggesting that regulation of steroidogenesis by bmal1 occurs through a non-clock associated pathway.
An important caveat to these results is worth noting. Most of the studies examining the effects of clock gene mutations on fertility have been conducted with mice housed in 12:12 L:D cycles. Thus, these experiments fail to account for the impact of photic masking, which may override the effects of genetic mutations. A recent report examining the effects of the clockΔ19 mutation in mice housed in constant dark revealed a significant increase in abnormal cycling and pregnancy losses [10]. Further, scrutiny reveals a trend towards a reduced number of oocytes and fertilized embryos in the oviducts from bmal1−/− mice on the third day after mating with a wild-type male [58]. Thus, while the preponderance of evidence suggests that follicular maturation and ovarian morphology remain normal in both clock and bmal1 mutant mice held in L:D cycles, it is possible that the sensitivity of the ovarian follicle to gonadotrophins is impaired by the circadian deficits.
To investigate a potential physiological role for the clock in the rat ovary, we suppressed endogenous LH secretion with a selective gonadotrophin releasing hormone (GnRH) receptor antagonist (Cetrorelix; CET) and examined the phasic response of the ovary (i.e. ovulation) to exogenous gonadotrophin treatment (Figure 1b, 1c [60]). Animals kept in a 12:12 L:D cycle that were treated with CET on the afternoon of diestrus or the early morning of proestrus displayed a robust diurnal rhythm of ovulation in response to exogenous LH such that animals ovulated more often when injected during the dark phase on both days (Figure 1b). A repeat of this experiment with rats kept in constant dim light revealed an equally robust circadian rhythm of ovulation with a peak during the subjective night (Figure 1c). These data imply that the circadian responsiveness of the ovary, independent of the timing of endogenous gonadotrophin secretion, contributes to the timing of ovulation by setting a window of sensitivity to LH. Additional experiments are needed to discriminate between the role of clocks in the ovary itself and/or the possible contribution of other endocrine or neural inputs to this rhythm of ovarian sensitivity.
A Paradigm Shift: How the Circadian Clock in the Ovary May Contribute to the Timing of Ovulation
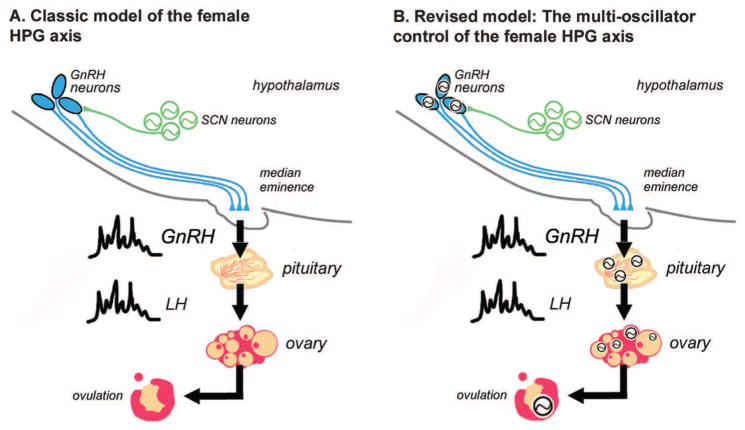
Since the elegant work of Everett and Sawyer, it has been clear that a neural timing system drives the rhythmic secretion of LH and subsequent ovulation [61, 62]; see Figure 2a). However, it was approximately 22 years before the central circadian clock was found to be localized in the SCN [63]. Since then, lesions of the SCN have been shown to disrupt the timing of LH secretion, ovulation and normal cycling in female rats [64, 65]. These and many other reports strongly support the classical paradigm for the timing of ovulation in rodents ([24]; Figure 2a). That is, the circadian oscillator in the SCN drives rhythmic patterns of GnRH release, which in turn stimulates LH secretion on the afternoon of proestrus. This preovulatory surge of LH initiates a cascade of events leading to follicular rupture and oocyte release [66, 67]. However, recent studies suggest that the GnRH neuron may also be an autonomous circadian oscillator [68–71]. Rhythms of clock gene expression have been observed in cultured immortalized GnRH neurons [68, 69] and in mouse GnRH neurons in vivo [70]. There is even limited evidence supporting circadian clock gene expression in pituitary cells [72–76]. These data, together with the evidence presented above for the participation of the ovary’s own clock in the control of ovulation, support a novel paradigm: rather than responding to a linear hierarchical system, events like ovulation which are controlled by the HPG axis are timed by a multi-oscillatory system that depends on synchronization among its coupled components (Figure 2b).
Figure 2.
A paradigm shift: How the multi-oscillator HPG axis controls the timing of reproductive physiology. (a) Classic model for hypothalamic-pituitary-gonadal (HPG) axis regulation of mammalian reproductive physiology. On the afternoon of proestrus, the circadian clock in the SCN drives rhythmic release of GnRH that stimulates rhythmic secretion of LH. Circulating LH then stimulates the ovarian follicle leading to rupture and oocyte release. According to the classic model, the timing of events in this system is dependent solely on timing cues from the pacemaker neurons in the SCN. (b) A revised model for the “multi-oscillator circadian system” in the HPG axis emphasizing the existence of circadian oscillators in each component of the axis. Synchronization between SCN pacemakers, GnRH neurons, pituitary cells and ovarian cells is necessary for proper timing of physiological events controlled by the HPG. Based on this model, we hypothesize that disrupting circadian phase relationships among these tissues will have negative effects on reproductive physiology. Green circles containing sine waves represent SCN pacemaker neurons. Black circles containing sine waves represent rhythmic circadian clock gene expression within the cells of each tissue or region. Potential feedback relationships among the oscillators have been omitted for clarity. Gonadotrophin releasing hormone (GnRH), luteinizing hormone (LH), Suprachiasmatic nucleus (SCN).
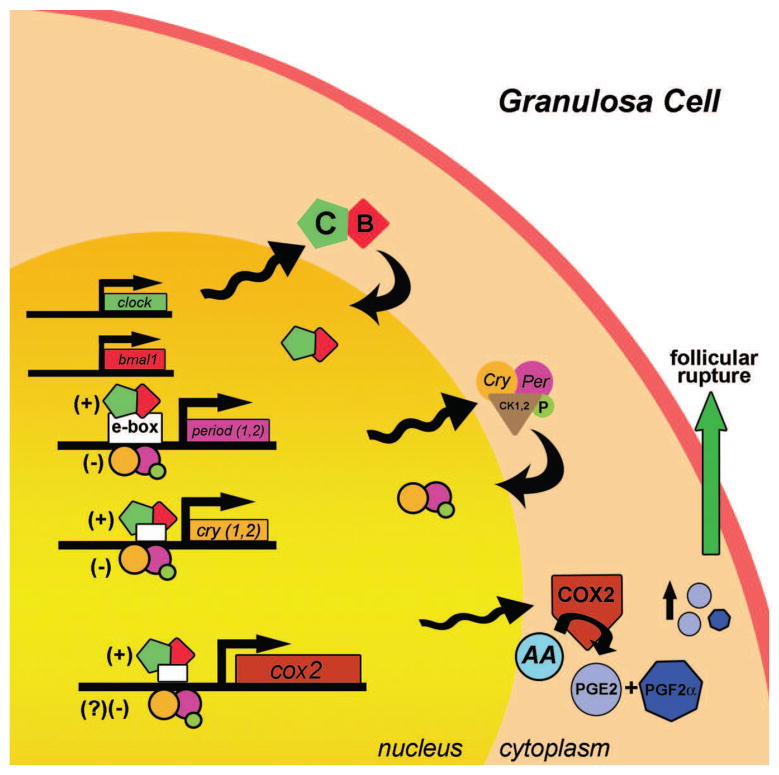
To understand the role of the ovarian clock in the timing of ovulation, it will be necessary to uncover the links between the clock, clock-controlled gene expression and the molecular processes associated with follicular rupture. The proestrous LH surge initiates a complex and generally well understood cascade of molecular events resulting in weakening and eventual rupture of the follicular wall [66, 67]. The response has been described as an “inflammatory” reaction involving the activation and secretion of enzymes such as cyclooxygenase-2, matrix metalloproteinases and prostaglandins from the cells lining the follicular wall [66, 67]. Thus, one possible link between the circadian clock in the ovary and the timing of ovulation could involve an increase in prostanoid signaling [66, 67]. Prostaglandin E2 and prostaglandin F2α are involved in the inflammatory response that precedes rupture of the follicular lumen [77]. The activity of the enzyme cyclooxygenase-2 (cox2) is the rate limiting step in prostaglandin (PG) synthesis. It was recently reported that cox2 transcription is partially regulated through E-box DNA binding sequences located in its promoter region that are known targets of the CLOCK:BMAL1 transactivator complex [78, 79]. The timing of cox2 expression relative to ovulation is highly conserved, such that cox2 mRNA begins to increase approximately 10h before ovulation in several species [77]. While the presence of an E-box consensus sequence in the cox2 promoter does not prove that cox2 transcription is regulated by the core clock, E-box mediated transcription is required for activation of cox2 expression by gonadotrophins [78]. In light of these data, we propose a working model (Figure 3) in which CLOCK:BMAL1 heterodimers bind to and activate clock controlled transcription of cox2 mRNA on the day of ovulation. An increase in COX2 and in turn PG synthesis, in anticipation of the LH surge, would provide a window for follicular rupture induced by the surge. In addition to the process of follicular rupture, recent evidence suggests circadian clock control of steroidogenesis, a facet of ovarian physiology critical for reproductive success [58, 80]. The timing of follicular rupture and steroidogenesis may both be regulated by the circadian clock in the ovary and act in concert to facilitate the timing of ovulation.
Figure 3.
The molecular clock in mammalian follicular cells may drive the expression of clock-controlled genes necessary for ovulation. Circadian clock genes, including activators [BMAL1 (B); CLOCK (C)] and repressors [period (per) and cryptochrome (cry)], are rhythmically expressed and phosphorylated by casein kinases in granulosa cells. Cyclooxygenase-2 (cox2), the rate limiting enzyme for prostanoid synthesis, has E-box sequences in its promoter region, and evidence suggests that CLOCK:BMAL1 heterodimers can bind to and activate cox2 transcription [78, 79]. Circadian rhythms of cox2 mRNA expression may result in rhythmic accumulation of COX2 enzyme. In turn, rhythms of COX2 enzyme expression may lead to rhythmic synthesis and accumulation of PGE2 and PGF2α. Increased levels of prostanoid synthesis, particularly in response to a surge in LH secretion, are associated with follicular rupture. Thus, circadian rhythms of cox2 mRNA synthesis might indirectly contribute to the timing of ovulation by establishing a ready pool of LH-inducible prostaglandins. Transactivation by BMAL1:CLOCK is indicated by (+); repression of BMAL1:CLOCK activity by PER:CRY is indicated by (−). Arrowheads attached to sine waves indicate rhythmic transcription/translation. Curved arrows indicate nuclear translocation. Abbreviations: arachidonic acid (AA); prostaglandin E2 (PGE2); prostaglandin F2α (PGF2α); phosphorylation (P); Casein kinase 1,2 (CK1,2).
Circadian Clocks in the HPG and Disorders of the Female Reproductive System: A Working Hypothesis
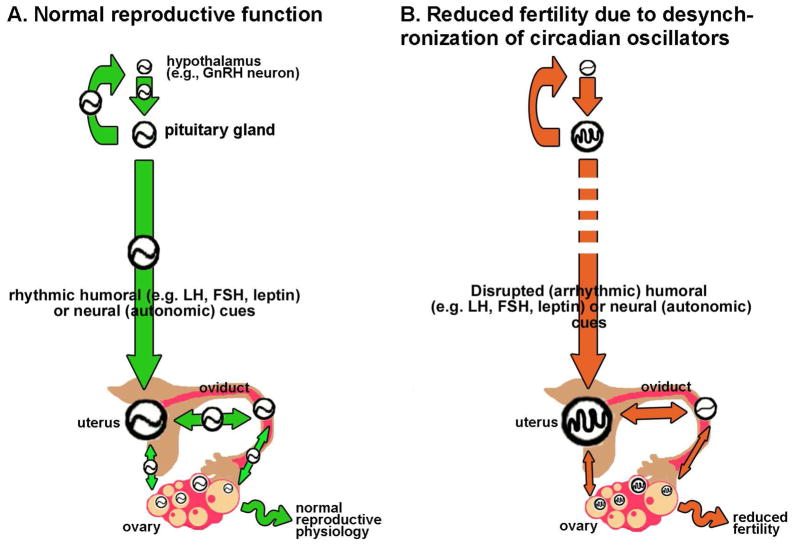
Disruption of the circadian system in the HPG may be a causative factor in diseases affecting fertility. We know that conditions resulting in disrupted coordination or synchronization within and between circadian clocks in the brain and periphery can have significant negative impacts on health. For example, transient phase shifts associated with transmeridian travel and rotating shift work activate the stress axis, and increase cancer risk, sleep/mood disorders and the incidence of digestive disorders [81–84]. It has been suggested that these effects are directly linked to desynchronization within and between circadian clocks in the brain and periphery [85]. These conditions are also known to affect reproductive physiology and fertility in humans, including but not limited to altered menstrual cycles, increased menstrual pain, altered follicular phase length, changes in the level of FSH secretion, low birth weights and greater incidence of spontaneous abortion [32, 86]. Further, a recent genetic screen in Finland revealed that a polymorphism of the clock gene arntl (bmal1; arntl TT) increased the percentage of miscarriages [87]. Normal reproductive physiology may well depend on coordination within and between circadian clocks in the HPG axis (Figure 4a). Diseases like polycystic ovarian syndrome (PCOS), endometriosis or various malignant endocrine tumors which are common and have adverse effects on fertility, could be caused or exacerbated by desynchronization between circadian clocks in the ovary, pituitary, uterus, oviduct and mediobasal hypothalamus ([32, 88, 89]; Figure 4b). As an example, the etiology of PCOS may be indirectly linked to the timing of the circadian clock. While considerable debate surrounds the exact etiology of the disease, it is generally believed that excessive androgen secretion during development is an underlying factor [90]. Recent evidence suggests an interaction between androgen receptor stimulation and the timing of circadian rhythms of behavior, itself dependent on rhythms of clock gene expression in SCN neurons [91, 92]. Persistent stimulation by circulating androgens during development may alter the phase or period of the clock in central and peripheral tissues, resulting in potentially harmful desynchrony among constituent oscillators of the HPG axis (Figure 4b). A shift away from the classic “top-down” view of the timing of reproductive physiology should inspire researchers to examine the effects of perturbing tissue specific clocks through global, targeted or conditional genetic ablation on the etiology of complex disorders of the reproductive system.
Figure 4.
A synchronized circadian system in the HPG may be necessary for rheostasis. (a) We suggest that rheostasis in the reproductive system depends on synchronization within and between circadian clocks in the HPG axis. For normal function circadian clocks in hypothalamic neuroendocrine cells (e.g. GnRH neurons), pituitary gland, uterus, oviduct and ovary (represented by circles containing a sine wave) must be appropriately synchronized. Regular temporal cues [indicated as green arrows in (a)] originating in the SCN and transduced by nervous and humoral outputs to the periphery to drive coordination of central and peripheral clocks. Temporal cues originating in peripheral oscillators (e.g. the uterus) may also alter timing in nearby peripheral oscillators. For clarity, several additional links and feedback circuits have been omitted from this schematic. (b) We hypothesize that disrupting normal synchronization, either by reducing the amplitude or robustness of circadian clocks in target tissues [indicated by circles containing abnormal waveforms] or by altering the rhythmicity or amplitude of temporal cues [indicated by red arrows] of central or peripheral origin may exacerbate (or even cause) diseases associated with reduced fertility.
Concluding remarks
We have highlighted current evidence for the existence of circadian clocks in the ovaries of several species. In addition, we have summarized what little is known regarding the physiological function of ovarian clocks. We propose a revised paradigm in which circadian clocks at each level of the HPG axis contribute to the timing of reproductive events, with specific emphasis on the possible role of the ovary’s circadian clock in the timing of ovulation. Finally, we hypothesize that synchronization within and between these clocks contributes to rheostasis in the reproductive system whereas disruption of this synchrony may exacerbate, or in some cases even cause, disease that adversely affects fertility.
Glossary
- Suprachiasmatic nucleus (SCN)
Central circadian clock located in the mediobasal hypothalamus just above the optic chiasm
- Luteinizing hormone (LH)
Gonadotrophin released from the anterior pituitary gland by stimulation from gonadotrophin releasing hormone (GnRH) from the hypothalamus. The surge of LH on proestrus stimulates follicular rupture
- Follicle stimulating hormone (FSH)
Gonadotrophin released from the anterior pituitary gland by stimulation from gonadotrophin releasing hormone (GnRH). FSH stimulates growth and maturation of the ovarian follicle
- Zeitgeber time (ZT)
Time scale relative to exogenous light:dark cycle: ZT0 = light on and ZT12 = lights off
- Circadian time (CT)
Subjective time scale dependent on the animal’s activity: CT0 = activity end and CT12 = activity onset
- Granulosa cell (GC)
Cells that surround the oocyte and follicular antrum in multiple layers. Granulosa cells are physically separated from thecal cells by the basal lamina. Granulosa cells synthesize and secrete estradiol and are luteinized following stimulation by the LH surge on proestrus
- Thecal cell (TC)
Cells located in the theca interna between the innermost granulosa cells and the non-hormone secreting epithelial cells located in the theca externa. They normally produce progesterone that is primarily converted to androgens, secrete estrogen and are luteinized following stimulation by the LH surge on proestrus
- Luteal cell (LC)
Progesterone-secreting cells of the corpus luteum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Morse D, Cermakian N, Brancorsini S, Parvinen M, Sassone-Corsi P. No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol Endocrinol. 2003;17:141. doi: 10.1210/me.2002-0184. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez JD, Chen D, Storer E, Sehgal A. Non-cyclic and developmental stage-specific expression of circadian clock proteins during murine spermatogenesis. Biol Reprod. 2003;69:81–91. doi: 10.1095/biolreprod.102.011833. [DOI] [PubMed] [Google Scholar]
- 3.Bebas P, Goodall CP, Majewska M, Neumann A, Giebultowicz JM, Chappell PE. Circadian clock and output genes are rhythmically expressed in extratesticular ducts and accessory organs of mice. Faseb J. 2009;23:523–533. doi: 10.1096/fj.08-113191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karman BN, Tischkau SA. Circadian clock gene expression in the ovary: Effects of luteinizing hormone. Biol Reprod. 2006;75:624–632. doi: 10.1095/biolreprod.106.050732. [DOI] [PubMed] [Google Scholar]
- 5.Fahrenkrug J, Georg B, Hannibal J, Hindersson P, Gras S. Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology. 2006;147:3769–3776. doi: 10.1210/en.2006-0305. [DOI] [PubMed] [Google Scholar]
- 6.He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. Gonadotropic regulation of circadian clockwork in rat granulosa cells. Mol Cell Biochem. 2007;302:111–118. doi: 10.1007/s11010-007-9432-7. [DOI] [PubMed] [Google Scholar]
- 7.Yoshikawa T, Sellix M, Pezuk P, Menaker M. Timing of the ovarian circadian clock is regulated by gonadotrophins. Endocrinology. 2009;9:4338–47. doi: 10.1210/en.2008-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura TJ, Moriya T, Inoue S, Shimazoe T, Watanabe S, Ebihara S, Shinohara K. Estrogen differentially regulates expression of Per1 and Per2 genes between central and peripheral clocks and between reproductive and nonreproductive tissues in female rats. J Neurosci Res. 2005;82:622–630. doi: 10.1002/jnr.20677. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura TJ, Sellix MT, Menaker M, Block GD. Estrogen directly modulates circadian rhythms of PER2 expression in the uterus. Am J Physiol Endocrinol Metab. 2008;295:E1025–1031. doi: 10.1152/ajpendo.90392.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dolatshad H, Campbell EA, O’Hara L, Maywood ES, Hastings MH, Johnson MH. Developmental and reproductive performance in circadian mutant mice. Hum Reprod. 2006;21:68–79. doi: 10.1093/humrep/dei313. [DOI] [PubMed] [Google Scholar]
- 11.Kennaway DJ, Varcoe TJ, Mau VJ. Rhythmic expression of clock and clock-controlled genes in the rat oviduct. Mol Hum Reprod. 2003;9:503–507. doi: 10.1093/molehr/gag067. [DOI] [PubMed] [Google Scholar]
- 12.Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 15.Ko GY, Shi L, Ko ML. Circadian regulation of ion channels and their functions. J Neurochem. 2009;110:1150–1169. doi: 10.1111/j.1471-4159.2009.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borgs L, Beukelaers P, Vandenbosch R, Belachew S, Nguyen L, Malgrange B. Cell “circadian” cycle: new role for mammalian core clock genes. Cell Cycle. 2009;8:832–837. doi: 10.4161/cc.8.6.7869. [DOI] [PubMed] [Google Scholar]
- 18.Herzog ED, Schwartz WJ. A neural clockwork for encoding circadian time. J Appl Physiol. 2002;92:401. doi: 10.1152/japplphysiol.00836.2001. [DOI] [PubMed] [Google Scholar]
- 19.Davidson AJ, Yamazaki S, Menaker M. SCN: ringmaster of the circadian circus or conductor of the circadian orchestra? Novartis Found Symp. 2003;253:110–121. discussion 121–115, 281–114. [PubMed] [Google Scholar]
- 20.Hastings M, O’Neill JS, Maywood ES. Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol. 2007;195:187–198. doi: 10.1677/JOE-07-0378. [DOI] [PubMed] [Google Scholar]
- 21.Guilding C, Piggins HD. Challenging the omnipotence of the suprachiasmatic timekeeper: are circadian oscillators present throughout the mammalian brain? Eur J Neurosci. 2007;25:3195–3216. doi: 10.1111/j.1460-9568.2007.05581.x. [DOI] [PubMed] [Google Scholar]
- 22.Kriegsfeld LJ, Silver R. The regulation of neuroendocrine function: Timing is everything. Horm Behav. 2006;49:557–574. doi: 10.1016/j.yhbeh.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnefont X. Circadian timekeeping and multiple timescale neuroendocrine rhythms. J Neuroendocrinol. 2010;3:209–16. doi: 10.1111/j.1365-2826.2010.01955.x. [DOI] [PubMed] [Google Scholar]
- 24.de la Iglesia HO, Schwartz WJ. Minireview: timely ovulation: circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology. 2006;147:1148–1153. doi: 10.1210/en.2005-1311. [DOI] [PubMed] [Google Scholar]
- 25.LeSauter J, Yan L, Vishnubhotla B, Quintero JE, Kuhlman SJ, McMahon DG, Silver R. A short half-life GFP mouse model for analysis of suprachiasmatic nucleus organization. Brain Res. 2003;964:279–287. doi: 10.1016/s0006-8993(02)04084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2:LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo SH, Ko CH, Lowrey PL, Buhr ED, Song EJ, Chang S, Yoo OJ, Yamazaki S, Lee C, Takahashi JS. A non-canonical E-box enhancer drives mouse Period2 circadian oscillations in vivo. Proc Natl Acad Sci U S A. 2005;102:2608–2613. doi: 10.1073/pnas.0409763102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilsbacher LD, Yamazaki S, Herzog ED, Song EJ, Radcliffe LA, Abe M, Block G, Spitznagel E, Menaker M, Takahashi JS. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice invivo. Proc Natl Acad Sci USA. 2002;99:489. doi: 10.1073/pnas.012248599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mrosovsky N. Rheostasis: the physiology of change. Oxford University Press; 1990. [Google Scholar]
- 30.Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392. doi: 10.1530/rep.1.00614. [DOI] [PubMed] [Google Scholar]
- 31.Dolatshad H, Davis FC, Johnson MH. Circadian clock genes in reproductive tissues and the developing conceptus. Reprod Fertil Dev. 2009;21:1–9. doi: 10.1071/rd08223. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney MM. Shift work, jet lag, and female reproduction. Int J Endocrinol. 2010;2010:813764. doi: 10.1155/2010/813764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwai S, Thi Dieu Trang L, Sehadova H, Takeda M. Expression analyses of casein kinase 2alpha and casein kinase 2beta in the silkmoth, Bombyx mori. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:38–46. doi: 10.1016/j.cbpb.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Yang JS, Dai ZM, Yang F, Yang WJ. Molecular cloning of Clock cDNA from the prawn, Macrobrachium rosenbergii. Brain Res. 2006;1067:13–24. doi: 10.1016/j.brainres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Lorenz L, Yu QN, Hall JC, Rosbash M. Spatial and temporal expression of the period gene in Drosophila melanogaster. Genes Dev. 1988;2:228–238. doi: 10.1101/gad.2.2.228. [DOI] [PubMed] [Google Scholar]
- 36.Saez L, Young MW. In situ localization of the per clock protein during development of Drosophila melanogaster. Mol Cell Biol. 1988;8:5378. doi: 10.1128/mcb.8.12.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardin PE. Analysis of period mRNA cycling in Drosophila head and body tissues indicates that body oscillators behave differently from head oscillators. Mol Cell Biol. 1994;14:7211. doi: 10.1128/mcb.14.11.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaver LM, Rush BL, Gvakharia BO, Giebultowicz JM. Noncircadian regulation and function of clock genes period and timeless in oogenesis of Drosophila melanogaster. J Biol Rhythms. 2003;18:463–472. doi: 10.1177/0748730403259108. [DOI] [PubMed] [Google Scholar]
- 39.Kotwica J, Larson MK, Bebas P, Giebultowicz JM. Developmental profiles of PERIOD and DOUBLETIME in Drosophila melanogaster ovary. J Insect Physiol. 2009;55:419–425. doi: 10.1016/j.jinsphys.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Gvakharia BO, Kilgore JA, Bebas P, Giebultowicz JM. Temporal and spatial expression of the period gene in the reproductive system of the codling moth. J Biol Rhythms. 2000;15:4–12. doi: 10.1177/074873040001500102. [DOI] [PubMed] [Google Scholar]
- 41.Beaver LM, Gvakharia BO, Vollintine TS, Hege DM, Stanewsky R, Giebultowicz JM. Loss of circadian clock function decreases reproductive fitness in males of Drosophila melanogaster. Proc Natl Acad Sci U S A. 2002;99:2134–2139. doi: 10.1073/pnas.032426699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kotwica J, Bebas P, Gvakharia BO, Giebultowicz JM. RNA interference of the period gene affects the rhythm of sperm release in moths. J Biol Rhythms. 2009;24:25–34. doi: 10.1177/0748730408329109. [DOI] [PubMed] [Google Scholar]
- 43.TM, Hari Dass S, Sharma VK. Egg-laying rhythm in Drosophila melanogaster. J Genet. 2008;87:495–504. doi: 10.1007/s12041-008-0072-9. [DOI] [PubMed] [Google Scholar]
- 44.Sharp PJ, MacNamee MC, Talbot RT, Sterling RJ, Hall TR. Aspects of the neuroendocrine control of ovulation and broodiness in the domestic hen. J Exp Zool. 1984;232:475–483. doi: 10.1002/jez.1402320314. [DOI] [PubMed] [Google Scholar]
- 45.Silver R. Circadian and interval timing mechanisms in the ovulatory cycle of the hen. Poultry Science. 1986;65:2355. doi: 10.3382/ps.0652355. [DOI] [PubMed] [Google Scholar]
- 46.Underwood H, Siopes T, Edmonds K. Eye and gonad: role in the dual-oscillator circadian system of female Japanese quail. Am J Physiol. 1997;272:R172–182. doi: 10.1152/ajpregu.1997.272.1.R172. [DOI] [PubMed] [Google Scholar]
- 47.Nakao N, Yasuo S, Nishimura A, Yamamura T, Watanabe T, Anraku T, Okano T, Fukada Y, Sharp PJ, Ebihara S, Yoshimura T. Circadian clock gene regulation of steroidogenic acute regulatory protein gene expression in preovulatory ovarian follicles. Endocrinology. 2007;148:3031–3038. doi: 10.1210/en.2007-0044. [DOI] [PubMed] [Google Scholar]
- 48.Ball GF. The ovary knows more than you think! New views on clock genes and the positive feedback control of luteinizing hormone. Endocrinology. 2007;148:3029–3030. doi: 10.1210/en.2007-0570. [DOI] [PubMed] [Google Scholar]
- 49.Ikegami K, Katou Y, Higashi K, Yoshimura T. Localization of circadian clock protein BMAL1 in the photoperiodic signal transduction machinery in Japanese quail. J Comp Neurol. 2009;517:397–404. doi: 10.1002/cne.22165. [DOI] [PubMed] [Google Scholar]
- 50.Yasuo S, Watanabe M, Okabayashi N, Ebihara S, Yoshimura T. Circadian clock genes and photoperiodism: Comprehensive analysis of clock gene expression in the mediobasal hypothalamus, the suprachiasmatic nucleus, and the pineal gland of Japanese Quail under various light schedules. Endocrinology. 2003;144:3742–3748. doi: 10.1210/en.2003-0435. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura T. Molecular mechanism of the photoperiodic response of gonads in birds and mammals. Comp Biochem Physiol A Mol Integr Physiol. 2006;144:345–350. doi: 10.1016/j.cbpa.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 52.Doyle S, Menaker M. Circadian photoreception in vertebrates. Cold Spring Harb Symp Quant Biol. 2007;72:499–508. doi: 10.1101/sqb.2007.72.003. [DOI] [PubMed] [Google Scholar]
- 53.Yoshimura T, Suzuki Y, Makino E, Suzuki T, Kuroiwa A, Matsuda Y, Namikawa T, Ebihara S. Molecular analysis of avian circadian clock genes. Molecular Brain Research. 2000;78:207. doi: 10.1016/s0169-328x(00)00091-7. [DOI] [PubMed] [Google Scholar]
- 54.He PJ, Hirata M, Yamauchi N, Hashimoto S, Hattori MA. The disruption of circadian clockwork in differentiating cells from rat reproductive tissues as identified by in vitro real-time monitoring system. J Endocrinol. 2007;193:413–420. doi: 10.1677/JOE-07-0044. [DOI] [PubMed] [Google Scholar]
- 55.Johnson MH, Lim A, Fernando D, Day ML. Circadian clockwork genes are expressed in the reproductive tract and conceptus of the early pregnant mouse. Reprod Biomed Online. 2002;4:140. doi: 10.1016/s1472-6483(10)61931-1. [DOI] [PubMed] [Google Scholar]
- 56.Cushman RA, Allan MF, Jones SA, Rupp GP, Echternkamp SE. Localization of Period 1 mRNA in the ruminant oocyte and investigations of its role in ovarian function. Anim Reprod Sci. 2007;99:93–105. doi: 10.1016/j.anireprosci.2006.04.057. [DOI] [PubMed] [Google Scholar]
- 57.Miller BH, Olson SL, Turek FW, Levine JE, Horton TH, Takahashi JS. Circadian clock mutation disrupts estrous cyclicity and maintenance of pregnancy. Curr Biol. 2004;14:1367–1373. doi: 10.1016/j.cub.2004.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ratajczak CK, Boehle KL, Muglia LJ. Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology. 2009;150:1879–1885. doi: 10.1210/en.2008-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boden M, Varcoe T, Voultsios A, Kennaway D. Reproductive biology of female Bmal1 null mice. Reproduction. 2010;39:1077–90. doi: 10.1530/REP-09-0523. [DOI] [PubMed] [Google Scholar]
- 60.Sellix MT, Yoshikawa T, Menaker M. A circadian egg timer gates ovulation. Curr Biol. 2010;20:R266. doi: 10.1016/j.cub.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Everett JW, Sawyer CH, Markee JE. A neurogenic timing factor in control of the ovulatory discharge of luteinizing hormone in the cyclic rat. Endocrinology. 1949;44:234–250. doi: 10.1210/endo-44-3-234. [DOI] [PubMed] [Google Scholar]
- 62.Everett JW, Sawyer CH. A 24-hour periodicity in the “LH-release apparatus” of female rats, disclosed by barbiturate sedation. Endocrinology. 1950;47:198. doi: 10.1210/endo-47-3-198. [DOI] [PubMed] [Google Scholar]
- 63.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wiegand SJ, Terasawa E, Bridson WE. Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology. 1978;102:1645. doi: 10.1210/endo-102-5-1645. [DOI] [PubMed] [Google Scholar]
- 65.Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 66.Espey LL, Richards JS. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod. 2002;67:1662–1670. doi: 10.1095/biolreprod.102.005173. [DOI] [PubMed] [Google Scholar]
- 67.Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. doi: 10.1146/annurev.physiol.64.081501.131029. [DOI] [PubMed] [Google Scholar]
- 68.Chappell PE, White RS, Mellon PL. Circadian gene expression regulates pulsatile gonadotropin-releasing hormone (GnRH) secretory patterns in the hypothalamic GnRH-secreting GT1-7 cell line. J Neurosci. 2003;23:11202–11213. doi: 10.1523/JNEUROSCI.23-35-11202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gillespie JM, Chan BP, Roy D, Cai F, Belsham DD. Expression of Circadian Rhythm Genes in GnRH-Secreting GT1-7 Neurons. Endocrinology. 2003;12:5285–92. doi: 10.1210/en.2003-0802. [DOI] [PubMed] [Google Scholar]
- 70.Hickok JR, Tischkau SA. In vivo circadian rhythms in gonadotropin-releasing hormone neurons. Neuroendocrinology. 2010;91:110–120. doi: 10.1159/000243163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao S, Kriegsfeld LJ. Daily changes in GT1-7 cell sensitivity to GnRH secretagogues that trigger ovulation. Neuroendocrinology. 2009;89:448–457. doi: 10.1159/000192370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Leclerc GM, Boockfor FR. Pulses of prolactin promoter activity depend on a noncanonical E-box that can bind the circadian proteins CLOCK and BMAL1. Endocrinology. 2005;146:2782–2790. doi: 10.1210/en.2005-0100. [DOI] [PubMed] [Google Scholar]
- 73.Bose S, Boockfor FR. Episodes of Prolactin Gene Expression in GH3 Cells Are Dependent on Selective Promoter Binding of Multiple Circadian Elements. Endocrinology. 2010;151:2287–96. doi: 10.1210/en.2009-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Olcese J, Sikes HE, Resuehr D. Induction of PER1 mRNA expression in immortalized gonadotropes by gonadotropin-releasing hormone (GnRH): involvement of protein kinase C and MAP kinase signaling. Chronobiol Int. 2006;23:143–150. doi: 10.1080/07420520500521996. [DOI] [PubMed] [Google Scholar]
- 75.Resuehr D, Wildemann U, Sikes H, Olcese J. E-box regulation of gonadotropin-releasing hormone (GnRH) receptor expression in immortalized gonadotrope cells. Mol Cell Endocrinol. 2007;278:36–43. doi: 10.1016/j.mce.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Resuehr HE, Resuehr D, Olcese J. Induction of mPer1 expression by GnRH in pituitary gonadotrope cells involves EGR-1. Mol Cell Endocrinol. 2009;311:120–125. doi: 10.1016/j.mce.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 77.Sirois J, Sayasith K, Brown KA, Stock AE, Bouchard N, Dore M. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum Reprod Update. 2004;10:373–385. doi: 10.1093/humupd/dmh032. [DOI] [PubMed] [Google Scholar]
- 78.Morris JK, Richards JS. An E-box region within the prostaglandin endoperoxide synthase-2 (PGS-2) promoter is required for transcription in rat ovarian granulosa cells. J Biol Chem. 1996;271:16633–16643. doi: 10.1074/jbc.271.28.16633. [DOI] [PubMed] [Google Scholar]
- 79.Liu J, Antaya M, Boerboom D, Lussier JG, Silversides DW, Sirois J. The delayed activation of the prostaglandin G/H synthase-2 promoter in bovine granulosa cells is associated with down-regulation of truncated upstream stimulatory factor-2. J Biol Chem. 1999;274:35037–35045. doi: 10.1074/jbc.274.49.35037. [DOI] [PubMed] [Google Scholar]
- 80.Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A. The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms. 2008;23:26–36. doi: 10.1177/0748730407311254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rafnsson V, Tulinius H, Jonasson JG, Hrafnkelsson J. Risk of breast cancer in female flight attendants: a population-based study (Iceland) Cancer Causes Control. 2001;12:95–101. doi: 10.1023/a:1008983416836. [DOI] [PubMed] [Google Scholar]
- 82.Costa G. Shift work and occupational medicine: an overview. Occup Med (Lond) 2003;53:83–88. doi: 10.1093/occmed/kqg045. [DOI] [PubMed] [Google Scholar]
- 83.Filipski E, King VM, Li X, Granda TG, Mormont MC, Liu X, Claustrat B, Hastings MH, Levi F. Host circadian clock as a control point in tumor progression. J Natl Cancer Inst. 2002;94:690. doi: 10.1093/jnci/94.9.690. [DOI] [PubMed] [Google Scholar]
- 84.Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004;64:7879–7885. doi: 10.1158/0008-5472.CAN-04-0674. [DOI] [PubMed] [Google Scholar]
- 85.Reddy AB, Field MD, Maywood ES, Hastings MH. Differential resynchronisation of circadian clock gene expression within the suprachiasmatic nuclei of mice subjected to experimental jet lag. Journal of Neuroscience. 2002;22:7326. doi: 10.1523/JNEUROSCI.22-17-07326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shechter A, Boivin DB. Sleep, Hormones, and Circadian Rhythms throughout the Menstrual Cycle in Healthy Women and Women with Premenstrual Dysphoric Disorder. Int J Endocrinol. 2010;2010:259345. doi: 10.1155/2010/259345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kovanen L, Saarikoski ST, Aromaa A, Lonnqvist J, Partonen T. ARNTL (BMAL1) and NPAS2 gene variants contribute to fertility and seasonality. PLoS One. 5:e10007. doi: 10.1371/journal.pone.0010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Legro RS, Spielman R, Urbanek M, Driscoll D, Strauss JF, 3rd, Dunaif A. Phenotype and genotype in polycystic ovary syndrome. Recent Prog Horm Res. 1998;53:217–256. [PubMed] [Google Scholar]
- 89.Franks S, Gilling-Smith C, Gharani N, McCarthy M. Pathogenesis of polycystic ovary syndrome: evidence for a genetically determined disorder of ovarian androgen production. Hum Fertil (Camb) 2000;3:77–79. doi: 10.1080/1464727002000198731. [DOI] [PubMed] [Google Scholar]
- 90.Nisenblat V, Norman RJ. Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2009;16:224–231. doi: 10.1097/MED.0b013e32832afd4d. [DOI] [PubMed] [Google Scholar]
- 91.Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148:5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53:422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kerdelhue B, Brown S, Lenoir V, Queenan JT, Jr, Jones GS, Scholler R, Jones HW., Jr Timing of initiation of the preovulatory luteinizing hormone surge and its relationship with the circadian cortisol rhythm in the human. Neuroendocrinology. 2002;75:158–163. doi: 10.1159/000048233. [DOI] [PubMed] [Google Scholar]
- 94.Garcia JE, Jones GS, Wright GL., Jr Prediction of the time of ovulation. Fertil Steril. 1981;36:308–315. [PubMed] [Google Scholar]
- 95.Seibel MM. Luteinizing hormone and ovulation timing. J Reprod Med. 1986;31:754–759. [PubMed] [Google Scholar]
- 96.Kennaway DJ. The role of circadian rhythmicity in reproduction. Hum Reprod Update. 2005;11:91. doi: 10.1093/humupd/dmh054. [DOI] [PubMed] [Google Scholar]
- 97.Kennaway DJ, Boden MJ, Voultsios A. Reproductive performance in female Clock(Delta19) mutant mice. Reprod Fertil Dev. 2005;16:801–810. doi: 10.1071/rd04023. [DOI] [PubMed] [Google Scholar]
- 98.Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction. 2008;135:559–568. doi: 10.1530/REP-07-0434. [DOI] [PubMed] [Google Scholar]
- 99.Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod. 2006;75:778–784. doi: 10.1095/biolreprod.106.052845. [DOI] [PubMed] [Google Scholar]