Abstract
BACKGROUND
The roles of antagonistic activation of abdominal muscles and of intra-abdominal pressurization remain enigmatic, but are thought to be associated with both spinal unloading and spinal stabilization in activities such as lifting. Biomechanical analyses are needed to understand the function of intra-abdominal pressurization because of the anatomical and physiological complexity, but prior analyses have been over-simplified.
METHODS
To test whether increased intra-abdominal pressure was associated with reduced spinal compression forces for efforts that generated moments about each of the principal axis directions, a previously published biomechanical model of the spine and its musculature was modified by the addition of anatomically realistic three-layers of curved abdominal musculature connected by fascia to the spine. Published values of muscle cross-sectional areas and the active and passive stiffness properties were assigned. The muscle activations were calculated assuming minimized muscle stress and stretch for the model loaded with flexion, extension, lateral bending and axial rotation moments of up to 60 Nm, along with intra-abdominal pressurization of 5 or 10 kPa (37.5 or 75 mmHg) and partial bodyweight (340 N).
FINDINGS
The analysis predicted a reduction in spinal compressive force with increase in intra-abdominal pressurization from 5 to 10 kPa. This reduction at 60 Nm external effort was 21% for extension effort, 18% for flexion effort, 29% for lateral bending and 31% for axial rotation.
INTERPRETATION
This analysis predicts that intra-abdominal pressure produces spinal unloading, and shows likely muscle activation patterns that achieve this.
Keywords: Abdominal muscles, spinal loading, biomechanics
INTRODUCTION
The roles of abdominal muscles and of intra-abdominal pressure (IAP) remain enigmatic, especially the apparently antagonistic activation of abdominal muscles during extension efforts. This uncertainty has led to controversy about appropriate lifting techniques and rehabilitation exercises for people with back pain, and whether use of corsets has prophylactic value. Abdominal pressurization associated with abdominal muscle activation has been thought to be beneficial by producing spinal unloading during extension efforts [Morris et al. 1961, Arjmand and Shirazi-Adl 2006, Daggfeldt and Thorstensson 1997, Hemborg et al. 1985, Hodges et al. 2001]. Furthermore, it has been proposed that the added muscular stiffness associated with muscle co-activation provides increased stability of the trunk [Cholewicki et al. 1999a, Essendrop et al. 2002, Gardner-Morse and Stokes 1989, Hodges et al. 2003, Tesh et al. 1987]. Training of these muscles is included in exercise regimens for people with low back pain, based on these presumed beneficial effects. The supposed spinal unloading effect of IAP in lifting (extension) efforts results from the pressure acting on the diaphragm and pelvic floor (producing an extension moment) but must be offset against the flexion moment generated by the activation of abdominal musculature. However, it is thought that the resultant is a net extension moment [Morris et al. 1961], although the biomechanical basis for this has been questioned [McGill and Norman 1987]. The supposed stabilizing effect of activation of the abdominal wall muscles is a consequence of the stiffness of activated muscle [Bergmark 1989]. Experimental studies have supported this idea [Cresswell and Thorstensson 1994, Cholewicki et al. 1999b, Stokes et al. 2000]. Simplified biomechanical analyses of spinal buckling have also quantified the added stability [Cholewicki et al. 1999a, Gardner-Morse and Stokes 1989].
Experimentally, little or no decrease in dorsal muscle activation (where reduced muscle activation implies spinal unloading) has been reported in studies of live humans with voluntary augmentation or inhibition of abdominal muscle activation [Krag et al. 1986, Nachemson et al. 1986]. However, these contrived experiments are not necessarily a realistic representation of normal physiological recruitment of abdominal muscles. Increased spinal extension moment (implying spinal unloading) was recorded when intra-abdominal pressure was increased in experimental subjects by stimulating the phrenic nerve to induce diaphragm contraction [Hodges et al. 2001]. Therapeutically, one supposed effect of wearing a lumbar corset or belt is to facilitate abdominal pressurization, and hence produce spinal unloading and also increased stability [Ivancic et al. 2002, McGorry and Hsiang 1999, Miyamoto et al. 1999, Woodhouse et al. 1995, Cholewicki et al. 1999b].
Because live human subjects find it difficult to control abdominal pressurization in contrived experimental conditions, biomechanical analyses provide a way to explore the function of IAP. However, most prior analyses have represented the abdominal wall as an elastic membranous pressure vessel [Daggfeldt and Thorstensson 1997] or by straight line muscle paths that do not contain intra-abdominal pressure and therefore biomechanically over-simplify the anatomical and physiological complexity of the abdominal wall [Arjmand and Shirazi-Adl 2006, Stokes and Gardner-Morse 1999, Grenier and McGill 2007].
This paper reports on use of a new analysis of trunk biomechanics that includes an abdominal wall with all three muscle layers having realistic curved muscle paths added to a previously developed model [Stokes and Gardner-Morse 2001]. Relative to prior analyses of the biomechanics of intra-abdominal pressurization, this model includes a substantially more detailed representation of the lumbar spine and the dorsal musculature. In the present analyses, the abdominal muscles are curved (hence there is a relationship between their tension and the intra-abdominal pressure determined by force equilibrium) and have transverse stiffness properties and longitudinal stiffness that is dependent on the degree of activation. The model analyses were used to estimate the effects of raised intra-abdominal pressure on the compression loading of the spine in response to varying external loads applied in the cardinal planes, and to predict the associated trunk muscular activity needed to maintain spinal equilibrium. These analyses were used to test whether increased intra-abdominal pressure was associated with reduced spinal compression forces for efforts that generated moments about each of the principal axis directions.
METHODS
A biomechanical model of the spine and its musculature [Stokes and Gardner-Morse 2001] was modified by the addition of anatomically realistic curved abdominal wall musculature connected by fascia to the spine, and with transverse elastic (spring) elements connecting the contractile elements in a three-layer lattice. Curved abdominal muscles are required to contain intra-abdominal pressure. Important characteristics of the analysis included 111 symmetrical pairs of muscle `slips' (77 pairs of dorsal muscle slips including psoas, 11 pairs each of internal oblique, external oblique and transversus abdominis, and one pair representing rectus abdominis), and 5 lumbar vertebra (between the fixed pelvis, and rigid thorax) linked by flexible intervertebral joints.
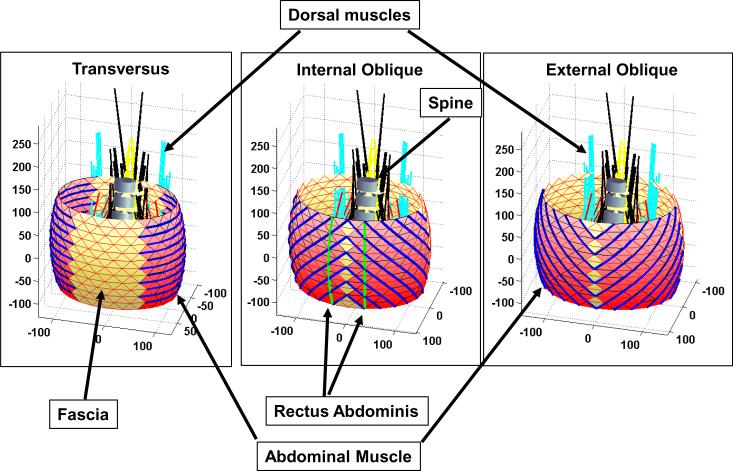
The geometry of the abdominal wall was simplified as three concentric elliptical barrel-shaped layers, with 10 mm spacing between them, representing the three muscle layers of the external obliques, internal obliques and transversus abdominis (Figure 1). The 10 mm spacing between muscle layers represented the estimated thickness of the muscle layers, as identified in Visible Human (http://www.nlm.nih.gov/research/visible/visible_human.html, accessed June 2010) cross sectional anatomy. Rectus abdominis was represented as a symmetrical pair of slips, each consisting of 12 elements to provide its curvature, and it was set into the middle layer of abdominal muscles (Figure 1). The concentric ellipses had major axes of 230, 250, and 270 mm and minor axes of 160, 180, and 200 mm, and a bulge of 10 mm, similar to dimensions given by Gatton [Gatton et al. 2001]. The height of the abdominal wall was equal to the height of the spine from T12 to S1, which was 196 mm.
Figure 1.
Three layers of abdominal musculature, dorsal muscles and lumbar spine as represented in the analytical model. Rectus abdominis is considered to be embedded within the middle layer (internal oblique). Axis dimensions are in mm.
Each concentric elliptical `barrel' section (layer) was divided into 13 elliptical strata of nodes separated vertically, and each stratum was specified by 24 'nodes' around the circumference. Interconnections (elements) within strata and between nodes in each stratum formed a triangular mesh. Although each element was straight, the nodes described a curved path for each muscle slip (Figure 1). The muscle layers were represented by 11 slips, and each slip consisted of between 2 and 12 straight-line elements between adjacent nodes. The numbers slips and elements were chosen to represent adequately the complex volume and curved geometry of these muscles. The contractile elements were either circumferential (to represent transversus abdominis muscle), or longitudinal (to represent rectus abdominis muscle), or helical (to represent the internal and external oblique muscles). Non-contractile elements were considered to be passive elastic elements representing connective tissue (fascia, etc.). Additional radial elements having high stiffness joined the concentric barrel sections. The radial elements were needed to maintain the 10 mm separation between the midlines of the three muscle layers, while transmitting the contact forces between them. For each abdominal wall muscle, each of the eleven slips was assigned one eleventh of the total physiological cross sectional area (PCSA) consistent with muscle volumes given by Stokes and Gardner-Morse [1999]. PCSA is the muscle volume divided by its length, and provides a measure of the average cross-sectional area and hence the force generating potential of a non-pennated muscle. The active muscle force in each muscle was constrained to have a value between zero and its PCSA multiplied by maximum stress equal to 0.46 MPa [Stokes and Gardner-Morse 2001].
Each muscle element was represented as a force generator, in parallel with a spring. The spring had an activation-dependent and constant component. The activation-dependent stiffness of each muscle was equal to a modulus multiplied by the degree of activation (between zero and one) and the muscle's cross-sectional area, and divided by its length [Bergmark 1989]. The modulus was equal to the maximum muscle stress (0.48 MPa), as derived from the form of the Hill's model length-tension relationship [Winters 1990]. The constant (passive) modulus was set to one tenth of the maximum muscle stress (hence passive stiffness was one tenth of the active stiffness at maximum activation, as an approximation of the length tension relationship of muscle, partitioned into active and passive components). The transverse connections between muscle slips, and the passive elastic elements representing fascia were assigned the same modulus as the passive muscle stiffness when in compression, and 100 times this value when in tension. The cross-sectional areas of these transverse muscle elements and the fascia corresponded to that of the muscle slips of internal oblique (the intermediate muscle layer). The sensitivity of the spinal compression forces to different values of muscular stiffness was evaluated empirically.
In the analyses, a value of intra-abdominal pressure was pre-specified as either 5 kPA or 10 kPa. The forces generated by the IAP acted on each node of the innermost abdominal layer. First, the forces were calculated for each triangular section of the abdominal wall, (pressure multiplied by triangle area) and then distributed between the three nodes forming that triangle. In addition to acting radially on the nodes of the inner-most muscle layer, the intra-abdominal pressure also produced upwards force on the diaphragm, and a downwards force on the pelvis. This force was calculated as the pressure multiplied by the area of the polygon formed by the nodes on the upper and lower elliptical surfaces (27600 mm2). The diaphragm was considered to be rigid (isometric) and attached to a rigid thorax, hence details of its structure and deformations were not included in the analyses.
The analysis was run with geometrical and other variables set to the presumed correct values (the `Baseline' model), and then sensitivity analyses were made to evaluate the effects of changing key parameter values. These analyses included variations in the angles of the oblique muscles (three different values of the helix angle relative to the horizontal), the amount of abdominal wall bulge (10 (baseline), 0 or 15 mm), and area of the diaphragm and pelvic floor. The helix angle, expressed as the angle of the muscle relative to the horizontal as seen at the front of the abdomen, averaged 45.5° in the `baseline' model, and this angle was changed to 37.5° and then 53° to assess the sensitivity to this factor.
Muscle forces were calculated, consistent with a 'cost function' that minimized the squares of both muscle stress and muscle length changes, while respecting static equilibrium consistent with forces applied to the trunk model [Stokes and Gardner-Morse 2001]. All calculations were performed by using the computing package Matlab (c) and its Optimization Toolbox routines quadprog.m and fmincon.m (Natick, MA, USA). This was achieved by minimizing a dimensionless cost function that included the weighted sum of squared muscle strains and the weighted sum of each muscle stress squared and normalized by its maximum muscle stress:
Where:
δlm = muscle length increase; lm = initial muscle length
σm = muscle stress (force per unit area); σmax = maximum muscle stress (=0.46 MPa)
w1, w2 = weighting factors
Constraints were imposed on the muscle forces (0 < force < 0.46*PCSA), and on the maximum permitted intervertebral motions (two degrees angular rotation, two mm of shear displacement) [Stokes and Gardner-Morse 2001]. Although muscle stretch was `penalized' by the cost function, the two-degrees and two mm constraints on intervertebral motion were imposed to prevent the model predicting probably spurious solutions having large relative movement of vertebrae and hence large intervertebral and passive elastic muscle forces. The optimization problem was solved iteratively since muscle stiffness depended on muscle activation. The ratio of w1 to w2 was initially set to 10 to `penalize' muscle proportional length changes by ten times more than the muscle stresses. This value was based on the value found by Stokes and Gardner-Morse [2001] that gave good agreement with measured EMG activity of muscles. The sensitivity of the calculated forces to this weighting factor was also determined empirically by setting the ratio to different values.
The muscle activations were determined for simulated abdominal pressurization (`Valsalva') with IAP of 5 and 10 kPa (37.5 and 75 mmHg), with a vertical force representing partial bodyweight above T12 (340 N) imposed on T12 at a distance 67 mm anterior to T12. Then, external moment producing efforts were simulated by sequentially adding moments in increments of 20 Nm up to 60 Nm about each principal axis direction (flexion, extension, lateral bending and axial torque). The intra-abdominal pressure was therefore 0.6 kPa per Nm when the pressure was 10 kPa and the generated moment was 60 Nm, consistent with observed in human subjects in standing posture by Grew [1980] and by Mairiaux [1988] who reported between 0.4 and 0.7 kPa per Nm for different effort directions. The maximum moment of 60 Nm was selected because it is the mean value for women making maximum voluntary efforts in axial rotation (other effort directions can generate higher moments [Stokes and Gardner-Morse 1995]). At each loading step, the muscular forces required to achieve static equilibrium at each node of the abdominal wall and at each vertebra were then calculated. This was done by solving for initially unknown displacements of each node and vertebra, from which the elastic forces and muscle activation forces required to satisfy static equilibrium were calculated.
RESULTS
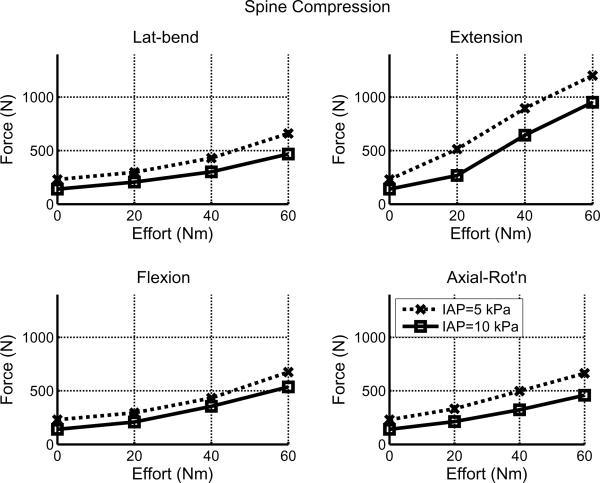
Greater intra-abdominal pressure was associated with lesser spinal compression force for all directions and magnitudes of efforts in the `Baseline' model. (Figure 2 and Table 1). In all four effort directions the spinal compressive force was less with the greater pressure (full line with squares in Figure 2). The reduction in intervertebral compression force with 60 Nm external moment was in the range 119 N (18%) for flexion to 250 N (21%) for extension, averaged over the six intervertebral levels from T12 to S1.
Figure 2.
Spinal loading averaged over the six intervertebral levels from T12 to S1 with 5 kPa and 10 kPa abdominal pressure in efforts in four principal moment directions (`Baseline' model).
Table 1.
Spinal compression forces (N) averaged over the six intervertebral levels from T12 to S1 and percentage differences from the `Baseline' values for efforts of 60 Nm in each of the four principal moment directions, with either 5 kPa or 10 kPa intra-abdominal pressure. The columns give values for the baseline model and 6 variations of the model.
| IAP | Baseline | 80% of `baseline' diaphragm area | Bulge 0 mm | Bulge 15 mm | Helix angle 53° | Helix angle 37.5° | |
|---|---|---|---|---|---|---|---|
| Lateral bend | 5 kPa | 660 | 676 (2 %) | 593 (−10 %) | 694 (−2 %) | 741 (12 %) | 575 (−13 %) |
| 10 kPa | 469 | 518 (10 %) | 473 (1 %) | 472 (1 %) | 601 (28 %) | 423 (−10 %) | |
| Extension | 5 kPa | 1202 | 1093 (−9 %) | 1209 (1 %) | 1141 (−5 %) | 984 (−18 %) | 1209 (1 %) |
| 10 kPa | 952 | 1004 (6 %) | 1010 (6 %) | 978 (3 %) | 967 (2 %) | 947 (−1 %) | |
| Flexion | 5 kPa | 656 | 715 (6 %) | 640 (−5 %) | 650 (−4 %) | 633 (−6 %) | 581 (−14 %) |
| 10 kPa | 537 | 581 (8 %) | 523 (−3 %) | 548 (2 %) | 592 (10 %) | 502 (−7 %) | |
| Axial Rotation | 5 kPa | 665 | 696 (5 %) | 701 (6 %) | 638 (−4 %) | 639 (−4 %) | 696 (5 %) |
| 10 kPa | 459 | 525 (14 %) | 524 (14 %) | 429 (−6 %) | 426 (−7 %) | 492 (8 %) |
The zero external moment conditions (zero effort, but partial bodyweight present) correspond to a Valsalva manoeuvre (Figure 2). These conditions produced average spinal compressive loading (averaged over the six intervertebral joints and the four moment directions) of 250 N with 5 kPa IAP, and 182 N with 10 kPa IAP. The calculated spinal compression force when the model was run without superimposed bodyweight or external moments was −25 N with 5 kPa IAP, and −61 N with 10 kPa IAP, thus indicating a small spinal distraction effect when the only forces were due to IAP together with the muscle forces required to produce IAP and to maintain spinal equilibrium.
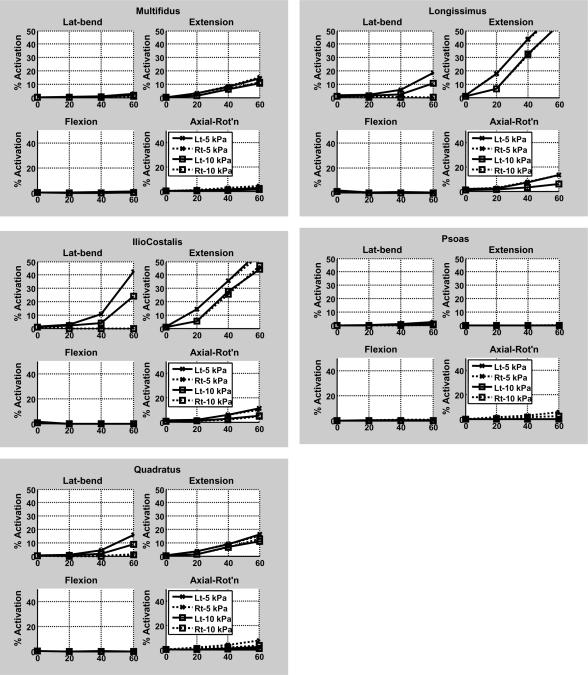
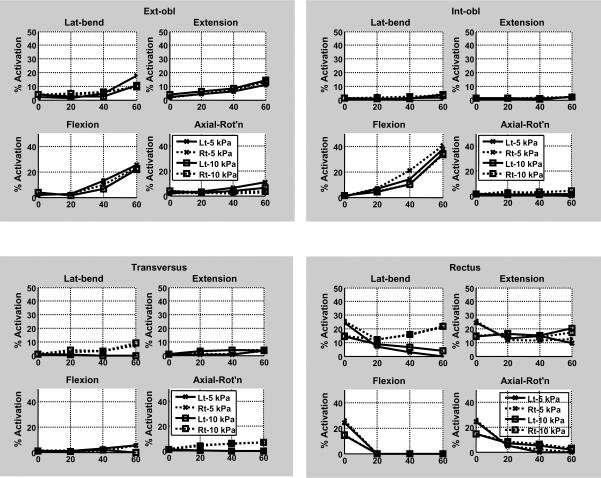
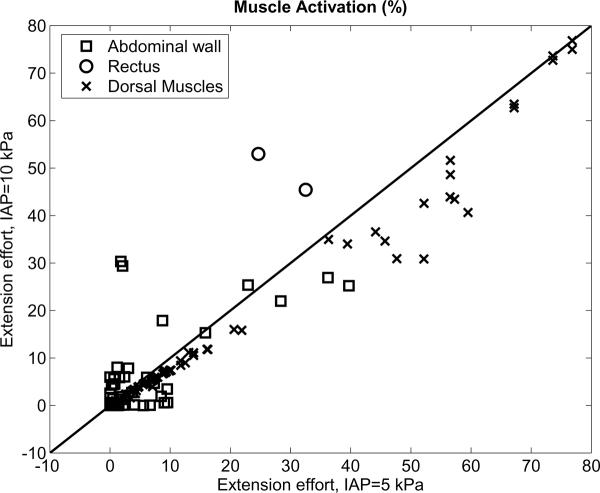
The lesser spinal loading with greater IAP was associated with less predicted dorsal muscle activation (Figures 3a and 4). Overall, for 60 Nm extension effort the extensor muscles were generally less activated at the higher IAP (10 kPa) than when IAP was 5 kPa IAP, and the abdominal muscles were mostly more activated to contain the higher IAP (Figures 3b and 4).
Figure 3.
Activation of trunk muscles in response to four different levels of external loads for efforts in four principal moment directions, as predicted by the analytical model. (a) Dorsal muscles (b) Abdominal muscles. For each panel the horizontal axis gives the magnitude of the effort (moment) in Nm. In the legend, `Lt' = left side muscles; `Rt' = right side muscles.
The finding of spinal `unloading' with increased IAP was relatively insensitive to variables in the model. Changing the abdominal wall geometry (helix angle and bulge) had a small effect on the calculated spinal compressive force (Table 1), with no consistent effects of increasing or decreasing the abdominal wall helix angle or bulge. When the area of diaphragm was reduced to 80% of the baseline value, thus lessening the area on which the intra-abdominal pressure acted (this has been cited as a possible source of variability in spinal `unloading' estimates [McGill and Norman 1987]), there was a small increase in spinal compression (between 2 and 14%), except for the case of 60 Nm extension effort with 5 kPa IAP, when a 9% decrease was calculated. Similarly, when the relative weighting of muscle stress and muscle strain was changed by a factor of 2 (from a ratio of 10 to a ratio of 5), there was a small change in the calculated pattern of muscle activation, associated with up to 15% increase in calculated spinal compressive force (this for the case of 60 Nm extension effort with 10 kPa IAP). When the longitudinal and transverse passive muscle stiffnesses were halved in the model, there was up to 7% increase in calculated spinal compression.
DISCUSSION
This model provides analytical estimates of the loading of the spine and trunk that indicate a lesser magnitude of spinal compression with higher intra-abdominal pressure (IAP). IAP acts on the diaphragm, pelvic floor and the contracted abdominal wall to `unload' the spine, while muscle and fascia tensions add to the spinal compression, but these analyses indicate that extension moment associated with the pressure acting on the diaphragm exceeds the flexion moment due to abdominal wall muscle forces. This `spinal unloading' effect was found consistently when using different assumptions about the angulation of the different abdominal muscle layers, the amount of abdominal wall bulging, and with different assumptions about the muscle activation strategy and the relative stiffness of abdominal wall components.
The present analysis is novel because it includes a detailed representation of the spinal articulations and musculature, the curved abdominal muscle paths, and the stiffness properties of fascia and of muscles both longitudinal and transverse to the contractile direction and all three muscular layers of the abdominal wall. This `pressure vessel' analysis provides more anatomically and physiologically accurate estimates of the spinal and trunk biomechanics than analyses with straight line muscles since in the absence of curvature the muscles do not contain the intra-abdominal pressure. The diaphragm must also be activated to support any pressure differential between abdomen and thorax [Hemborg et al. 1985]. In these analyses the diaphragm was considered to be rigid, so the stress in it and the relative roles of its activation and possible elastic strains associated with stretching of its tissue were not considered.
The representation of the abdominal wall in these analyses is considered to be substantially more realistic than previous analyses because it included the curved paths of abdominal muscles, which are required to contain intra-abdominal pressure (IAP). The muscle forces were calculated within the model (rather than relying on estimates from EMG, or from simplifying assumptions), thereby indicating how muscle activation strategies interact with the generated pressures and with spinal loading. Most prior analyses of trunk biomechanics have included estimates of muscular activation, based on electromyographic observations of a subset of the muscles, whereas in this analysis the muscle forces were calculated consistent with their roles to contain IAP, to generate external moments (efforts), and to maintain the spine in static equilibrium. As in all biomechanical models, assumptions were made about the strategy used to activate the redundant number of trunk muscles. Here, it was assumed that the muscles are activated to minimize the squared muscle stress and strain, as this strategy was reported as giving the best agreement with muscle activity measured by EMG [Stokes and Gardner-Morse 2001]. This assumption of optimized muscle activity may lead to a high estimate of the spinal load relieving effect, since suboptimal activation (greater muscle stresses, especially those associated with larger amounts of antagonistic activation) may act to increase spinal compression forces relative to the estimates from our analyses. The relative weights given in this cost function to muscle forces (stress) and stretch (strain) altered the muscle activation pattern, since minimizing stretch (and consequently the relative movements between vertebrae) required generally greater muscle forces.
This analysis indicated that performing a Valsalva manoeuvre would be associated with a small amount of spinal loading, but that greater abdominal pressure would produce lesser compressive force on the spine. This is contrary to experiments with live human subjects performing the Valsalva with pressures between 4 and 8 kPa IAP, where there was an increase in the spinal compression recorded by intradiscal pressure transducers, except that there was a reduction when they performed a strenuous extension effort [Nachemson et al. 1986]. It therefore appears that the experimental subjects performing a Valsalva manoeuvre generated more muscular activity, including antagonistic activity, in this less demanding tasks than the optimized values predicted by these analyses. This may have occurred also in other `contrived' experiments with human subjects that indicate a lesser or negligible effect of IAP on spinal loading.
The calculated spinal compression forces were in the range 250 N (with 5 kPa IAP and zero effort) to 1202 N (60 Nm extension effort). Based on intra-discal pressure measurements [Nachemson 1981], spinal compression forces range from 500 N (passive standing) to 2000 N (lifting activity). The analyses were done for two different IAP pressure magnitudes (5 and 10 kPa) that were in the physiological range. These intra-abdominal pressures were similar to those observed in human subjects in standing posture by Grew [1980] and by Mairiaux [1988] who reported between 0.4 and 0.7 kPa per Nm for different effort directions, and the pressure had an approximately linear increase with effort. Here, the pressure/moment ratio was 0.6 kPa per Nm when the pressure was 10 kPa and the generated moment was 60 Nm.
The main limitations in the analyses are considered to be the remaining simplifications of the abdominal wall, diaphragm and pelvic floor, and assumptions about transverse stiffness of the muscular layers of the abdomen. The abdominal wall was simplified as having elliptical shape. Considerations of the effects of individual variations and postural effects on abdominal wall shape and of the exact size and shape of respectively the diaphragm and the pelvic floor would be interesting topic for future work, but beyond the scope of the present study. However, the sensitivity studies (Table 1) suggested that the basic finding of spinal unloading was `robust'. Variations in the abdominal wall geometry, and of the area of the diaphragm (and hence the `extension' moment due to pressurization) did not have a major effect on the estimates of spinal loading. Also, by assuming an `optimal' pattern of muscular activation, the degree of activation relative to real in vivo levels, and hence the calculated spinal loading, may have been underestimated.
Muscle activation of the abdominal wall muscles was predicted to be in the range 0–40% of maximum voluntrary activation (Figure 3b), similar to published EMG data [Arjmand and Shirazi-Adl 2006, Cresswell et al. 1992, de Looze et al. 1999, McCook, et al. 2009, Thelen et al. 1995 ]. Differences include unexpectedly low activation of transversus, oblique muscles having low activation in axial rotation efforts, and rectus abdominis having low activation in flexion efforts. Apparently the obliques are activated in the analyses in order to contain the IAP, and these muscles also provided the flexion moment. The analyses predict greater activation of obliques, and lesser activation of rectus abdominis relative to EMG data reported by McCook et al. [2009]. The calculated activations are based on an `optimal' cost function that minimized muscular stress and strain, and the true physiological pattern of activation may not be optimal (hence higher activation). Also, the level of activation of these muscles is difficult to record via EMG, and it is difficult to elicit the value corresponding to maximum effort that is required to estimate the percentage of activation, complicating quantitative comparisons with individual muscles in the model.
Although the spinal unloading role of intra-abdominal pressurization is unproven, these analyses indicated that IAP has a spinal unloading effect over a range of magnitudes and directions of external moment generating efforts of the trunk. This is consistent with the extension moment generated by the IAP exceeding the flexion moment generated by tension in the abdominal wall. The reduction in spinal compression force was substantial - between 18% and 31% for different effort directions when pressure was increased from 5 to 10 kPa (37.5 to 70 mm Hg). The implications for trunk stiffening due to co-activation of trunk muscles and to considerations of spinal stability are yet to be quantified in this model. The `optimum' strategies that are widely assumed in biomechanics may be physiologically over-simplified, and in real life may be modified e.g. in the interests of joint stability. These analyses do provide information that is supportive of abdominal muscle training regimens and probably to the use of corsets prophylactically to reduce spinal loading during strenuous tasks.
CONCLUSIONS
A biomechanical analysis was made with a novel model that includes the curved paths of abdominal muscles, which are required to contain intra-abdominal pressure (IAP). The analyses indicated that IAP has a substantial spinal unloading effect for all directions of generated external moments. The unloading results from an extension moment generated by the IAP that exceeds the flexion moment generated by the abdominal wall muscle activation forces. These findings support the idea that intra-abdominal pressurization is beneficial because it unloads the spine.
Figure 4.
Comparison of muscle percent activation with 10 kPa and 5 kPa IAP, for 60 Nm extension effort. Each point on the graph represents values for one of the symmetrical muscle pairs used in the analysis. 'Abdominal wall' = obliques and transversus; 'Rectus' = rectus adominis; 'Dorsal Muscles' = 90 pairs of extensor muscles, including psoas.
Acknowledgements
This work was supported by the NIH grant R01 AR 40909. This sponsor had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arjmand N, Shirazi-Adl A. Role of intra-abdominal pressure in the unloading and stabilization of the human spine during static lifting tasks. Eur. Spine J. 2006;15(8):1265–75. doi: 10.1007/s00586-005-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmark A. Stability of the lumbar spine. A study in mechanical engineering. Acta Orthop. Scand. Suppl. 1989;230:1–54. doi: 10.3109/17453678909154177. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Juluru K, McGill SM. Intra-abdominal pressure mechanism for stabilizing the lumbar spine. J. Biomech. 1999a;32(1):13–7. doi: 10.1016/s0021-9290(98)00129-8. [DOI] [PubMed] [Google Scholar]
- Cholewicki J, Juluru K, Radebold A, Panjabi MM, McGill SM. Lumbar spine stability can be augmented with an abdominal belt and/or increased intra-abdominal pressure. Eur. Spine J. 1999b;8(5):388–95. doi: 10.1007/s005860050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell A, Thorstensson A. Changes in intra-abdominal pressure, trunk muscle activation and force during isokinetic lifting and lowering. Eur. J. Appl. Physiol. 1994;68:315–21. doi: 10.1007/BF00571450. [DOI] [PubMed] [Google Scholar]
- Cresswell AG, Grundstrom H, Thorstensson A. Observations on intra-abdominal pressure and patterns of abdominal intra-muscular activity in man. Acta. Physiol. Scand. 1992;144(4):409–18. doi: 10.1111/j.1748-1716.1992.tb09314.x. [DOI] [PubMed] [Google Scholar]
- Daggfeldt K, Thorstensson A. The role of intra-abdominal pressure in spinal unloading. J. Biomech. 1997;30(11–12):1149–55. doi: 10.1016/s0021-9290(97)00096-1. [DOI] [PubMed] [Google Scholar]
- de Looze MP, Groen H, Horemans H, Kingma I, van Dieën JH. Abdominal muscles contribute in a minor way to peak spinal compression in lifting. J. Biomech. 1999;32(7):655–62. doi: 10.1016/s0021-9290(99)00061-5. [DOI] [PubMed] [Google Scholar]
- Essendrop M, Andersen TB, Schibye B. Increase in spinal stability obtained at levels of intra-abdominal pressure and back muscle activity realistic to work situations. Appl. Ergon. 2002;33(5):471–6. doi: 10.1016/s0003-6870(02)00028-5. [DOI] [PubMed] [Google Scholar]
- Gardner-Morse MG, Stokes IAF. The effects of abdominal muscle co-activation on lumbar spine stability. Spine. 1989;23(1):86–92. doi: 10.1097/00007632-199801010-00019. [DOI] [PubMed] [Google Scholar]
- Gatton M, Pearcy M, Pettet G. Modelling the line of action for the oblique abdominal muscles using an elliptical torso model. J. Biomech. 2001;34(9):1203–7. doi: 10.1016/s0021-9290(01)00079-3. [DOI] [PubMed] [Google Scholar]
- Grenier SG, McGill SM. Quantification of lumbar stability by using 2 different abdominal activation strategies. Arch. Phys. Med. Rehabil. 2007;88(1):54–62. doi: 10.1016/j.apmr.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Grew ND. Intraabdominal pressure response to loads applied to the torso in normal subjects. Spine. 1980;5(2):149–54. doi: 10.1097/00007632-198003000-00009. [DOI] [PubMed] [Google Scholar]
- Hemborg B, Moritz U, Lowing H. Intra-abdominal pressure and trunk muscle activity during lifting. IV. The causal factors of the intra-abdominal pressure rise. Scand. J. Rehabil. Med. 1985;17(1):25–38. [PubMed] [Google Scholar]
- Hodges P, Kaigle Holm A, Holm S, Ekström L, Cresswell A, Hansson T, Thorstensson A. Intervertebral stiffness of the spine is increased by evoked contraction of transversus abdominis and the diaphragm: in vivo porcine studies. Spine. 2003;28(23):2594–601. doi: 10.1097/01.BRS.0000096676.14323.25. [DOI] [PubMed] [Google Scholar]
- Hodges PW, Cresswell AG, Daggfeldt K, Thorstensson A. In vivo measurement of the effect of intra-abdominal pressure on the human spine. J. Biomech. 2001;34(3):347–53. doi: 10.1016/s0021-9290(00)00206-2. [DOI] [PubMed] [Google Scholar]
- Ivancic PC, Cholewicki J, Radebold A. Effects of the abdominal belt on muscle-generated spinal stability and L4/L5 joint compression force. Ergonomics. 2002;45(7):501–13. doi: 10.1080/00140130210136035. [DOI] [PubMed] [Google Scholar]
- Krag MH, Byrne KB, Gilbertson LG, Haugh LD. Failure of intra-abdominal pressurization to reduce erector spinae loads during lifting tasks. North American Congress on Biomechanics; Montreal, Canada. 25–27 August 1986.1986. pp. 87–88. [Google Scholar]
- Mairiaux P, Malchaire J. Relation between intra-abdominal pressure and lumbar stress: effect of trunk posture. Ergonomics. 1988;31(9):1331–42. doi: 10.1080/00140138808966772. [DOI] [PubMed] [Google Scholar]
- McCook DT, Vicenzino B, Hodges PW. Activity of deep abdominal muscles increases during submaximal flexion and extension efforts but antagonist co-contraction remains unchanged. J. Electromyogr. Kinesiol. 2009;19(5):754–62. doi: 10.1016/j.jelekin.2007.11.002. [DOI] [PubMed] [Google Scholar]
- McGill SM, Norman RW. Reassessment of the role of intra-abdominal pressure in spinal compression. Ergonomics. 1987;30(11):1565–88. doi: 10.1080/00140138708966048. [DOI] [PubMed] [Google Scholar]
- McGorry RW, Hsiang SM. The effect of industrial back belts and breathing technique on trunk and pelvic coordination during a lifting task. Spine. 1999;24(11):1124–30. doi: 10.1097/00007632-199906010-00012. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Iinuma N, Maeda M, Wada E, Shimizu K. Effects of abdominal belts on intra-abdominal pressure, intra-muscular pressure in the erector spinae muscles and myoelectrical activities of trunk muscles. Clin. Biomech. 1999;14(2):79–87. doi: 10.1016/s0268-0033(98)00070-9. [DOI] [PubMed] [Google Scholar]
- Morris JM, Lucas DB, Bresler MS. The role of the trunk in stability of the spine. J. Bone Joint Surg. (Am) 1961;43(3):327–351. [Google Scholar]
- Nachemson AL. Disc pressure measurements. Spine. 1981;6(1):93–7. doi: 10.1097/00007632-198101000-00020. [DOI] [PubMed] [Google Scholar]
- Nachemson AL, Andersson BJ, Schultz AB. Valsalva maneuver biomechanics. Effects on lumbar trunk loads of elevated intraabdominal pressures. Spine. 1986;11(5):476–9. [PubMed] [Google Scholar]
- Stokes IAF, Gardner-Morse M. Quantitative anatomy of the lumbar musculature. J. Biomech. 1999;32:311–316. doi: 10.1016/s0021-9290(98)00164-x. [DOI] [PubMed] [Google Scholar]
- Stokes IA, Gardner-Morse M. Lumbar spinal muscle activation synergies predicted by multi-criteria cost function. J. Biomech. 2001;34(6):733–40. doi: 10.1016/s0021-9290(01)00034-3. [DOI] [PubMed] [Google Scholar]
- Stokes IA, Gardner-Morse M, Henry SM, Badger GJ. Decrease in trunk muscular response to perturbation with preactivation of lumbar spinal musculature. Spine. 2000:1957–64. doi: 10.1097/00007632-200008010-00015. [DOI] [PubMed] [Google Scholar]
- Stokes IAF, Gardner-Morse M. Lumbar spine maximum efforts and muscle recruitment patterns predicted by a model with multijoint muscles and joints with stiffness. J. Biomech. 1995;28(2):173–186. doi: 10.1016/0021-9290(94)e0040-a. [DOI] [PubMed] [Google Scholar]
- Tesh KM, Dunn JS, Evans JH. The abdominal muscles and vertebral stability. Spine. 1987;12(5):501–8. doi: 10.1097/00007632-198706000-00014. [DOI] [PubMed] [Google Scholar]
- Thelen DG, Schultz AB, Ashton-Miller JA. Co-contraction of lumbar muscles during the development of time-varying triaxial moments. J. Orthop. Res. 1995;13(3):390–8. doi: 10.1002/jor.1100130313. [DOI] [PubMed] [Google Scholar]
- Winters JM. Ch 5 in: Multiple muscle systems: Biomechanics and movement organization. Springer Verlag; New York: 1990. Hill-based muscle models: A systems engineering approach; pp. 69–93. [Google Scholar]
- Woodhouse ML, McCoy RW, Redondo DR, Shall LM. Effects of back support on intra-abdominal pressure and lumbar kinetics during heavy lifting. Hum. Factors. 1995;37(3):582–90. doi: 10.1518/001872095779049336. [DOI] [PubMed] [Google Scholar]