Abstract
Chronic psychosocial stress produces an array of adverse health consequences that are highly comorbid, including emotional eating, affective disorders, and metabolic syndrome. The consumption of high caloric diets (HCD) is thought to provide comfort in the face of unrelenting psychosocial stress. Using social subordination in female rhesus monkeys as a model of continual exposure to daily stressors in women, we tested the hypothesis that subordinate females would consume significantly more calories from a HCD compared to dominant females, and this pattern of food intake would be associated with reduced cortisol release and reduced frequency of anxiety- like behaviors. Food intake, parameters of cortisol secretion, and socio-emotional behavior were assessed for 3 weeks during a no choice phase when only a low caloric diet (LCD) was available and during a choice condition when both a LCD and HCD were available. While all animals preferred the HCD, subordinate females consumed significantly more of the HCD than did dominant females. A flattening of the diurnal cortisol rhythm and a greater increase in serum cortisol to an acute social separation occurred during the diet choice condition in all females. Furthermore, the rate of anxiety- like behavior progressively declined during the 3-week choice condition in subordinate but not dominant females. These data provide support for the hypothesis that daily exposure to psychosocial stress increases consumption of calorically dense foods. Furthermore, consumption of HCDs may be a metabolic stressor that synergizes with the psychosocial stress of subordination to further increase the consumption of these diets.
Keywords: social subordination, psychosocial stress, food intake, anxiety, cortisol
Introduction
Chronic exposure to stressful life events, resulting in a dysregulation of the limbic-hypothalamic – pituitary – adrenal (LHPA) axis, leads to a number of adverse health outcomes [1], including mood disorders [2–4]. The maladies induced from stressor exposure are often comorbid such that the incidence of depression and anxiety is typically associated with eating disorders [5, 6]. For example, animal studies show that chronic stressors [7, 8] or corticotropin releasing factor (CRF) administration [9–12] attenuates intake of standard laboratory low calorie diets (LCD). Indeed, food intake and body weight in rats are reduced by repeated restraint stress [13, 14], chronic variable stress [15], or exposure to a predator [16]. These effects are also observed in more naturalistic settings, as social subordination is associated with reduced intake of standard diets and lower body weights in rats [15, 17] and rhesus monkeys [18–20]. These data are consistent with observations from clinical studies showing anorexia in women is often associated with abnormal LHPA function [21].
However, these well-documented anorexic effects of stressor exposure are at odds with the data linking mood disorders to obesity [22–26]. From an evolutionary standpoint, consumption and storage of calories can enable an individual to more adequately deal with stress exposure. These behaviors can become deleterious, however, if the stress becomes chronic and consumption of high caloric diets (HCD) is excessive. Limited data in humans support the well-accepted folklore of a relation between stress and comfort food ingestion [27–31]. Data from a number of animal models show that stress leads to increased calorie consumption under some circumstances. Rats exposed to physical stressors preferentially consume a palatable, HCD versus a LCD, following a fast [32], gain weight, and show an increase in abdominal fat mass [33, 34]. The importance of chronic LHPA activation as a key determinant of changes in food choice and intake is supported by observations that corticosterone dose-dependently increases consumption of palatable diets, and increases abdominal adipose tissue [34, 35].
Studies using more naturalistic stressors support these observations. Intermittent social defeat consistently increases food intake and visceral fat in male hamsters [36, 37] while the stress associated with group housing produces obesity in female hamsters [38]. Food intake and metabolism are affected differently during and following the removal of the psychosocial stress of subordination in rats. When housed in the visible burrow system, subordination increases corticosterone, suppresses food intake, and decreases both fat and lean body mass [17, 39]. Once removed from social housing and transferred to single caging, previously subordinate rats become hyperphagic and accumulate more fat in visceral regions compared to dominant rats. Finally, socially housed subordinate female rhesus monkeys consume less of a typical low fat, high fiber monkey diet compared with dominant animals [40], consistent with their lower body weights [20]. However, when given access to a diet high in fat or high in fat or sugar, subordinate females eat significantly more calories from a high fat as well as a high sugar diet than do dominant females [41].
The fundamental question is why some foods are preferred during stressor exposure. One possibility is that consumption of calorically dense diets acts centrally on neuropeptide systems that influence the expression of anxiety and other adverse consequences of stress. Indeed, corticosterone-induced increases in sucrose ingestion significantly attenuate CRF expression in the PVN [33]. Furthermore, activation of the LHPA axis following restraint is diminished in rats given a choice between lard and/or sugar vs. chow [42–44]. The opportunity to choose between diets is a key feature of the effect, suggesting that choice or control is more important than just calories [6, 42]. It is unclear what signals mediate this effect, but there is evidence that calorically dense foods may alleviate the adverse behavioral effects of stressor exposure [45]. Importantly, withdrawal of a preferred HCD increases locomotor and anxiety-like behavior in rodents [46–48]. Thus, it is important to understand whether preferred consumption of a HCD reduces anxiety and if this is associated with a reduction in glucocorticoids.
The present study used socially housed female rhesus monkeys to determine whether food choice and the availability of a HCD affects diurnal and stress-induced LHPA activity and whether this is associated with a reduction in anxiety-like behavior. We hypothesized that subordinate monkeys would prefer and consume more of the HCD than dominant monkeys. Furthermore, we predicted the increased consumption of the HCD by subordinates would normalize parameters of cortisol secretion and reduce the frequency of anxiety-like behaviors.
Materials and Methods
The subjects were 10 adult female rhesus monkeys (Macaca mulatta) born and raised at the Yerkes National Primate Research Center Field Station at Emory University. The previously ovariectomized adult female monkeys (n = 10) were housed in an indoor-outdoor enclosures as described previously [20]. The study was conducted from Oct to Dec 2008 coincident with an approximate sunrise at 0700 and sunset at 1800 hr. Their indoor light cycle was fixed at lights on at 0700 hr and off at 1900 hr. The ten subjects were housed in one of two small groups of five females each and been previously been subjects in feeding studies [41], the latest of which ended 5 weeks prior to the initiation of the present study. Unless otherwise noted, animals were fed standard Purina monkey chow (Lab Diets, #5038, see below for description). The Emory University Animal Care and Use Committee in accordance with the Animal Welfare Act and the U.S. Department of Health and Human Services “Guide for Care and Use of Laboratory Animals” approved this protocol.
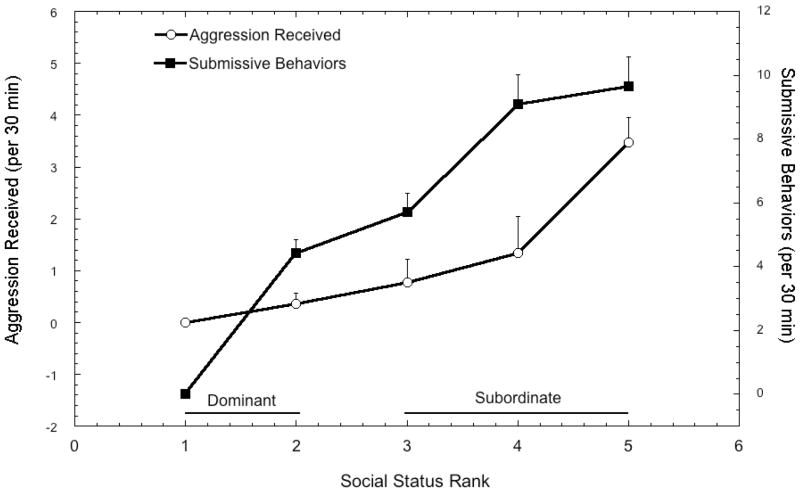
Rhesus monkeys provide an ethologically valid, translational model in which to study adverse health effects that are comorbid with psychosocial stress. Lower ranking animals receive proportionately more aggression from higher-ranking group mates and these subordinates terminate these interactions by emitting submissive behavior [49]. Given the recurrent exposure to harassment from more dominant females, subordinate females have larger adrenal glands [50] and show a greater cortisol response to social challenges [51]. In addition, pharmacological tests using a dexamethasone suppression [20, 41, 52–55] or ACTH challenge [55] show subordinate females are hypercortisolemic. The use of social subordination in macaques is a well established model to study the adverse effects of psychosocial stress on cardiovascular disease [56], addictive behavior [57], central monoamine changes [55, 58, 59], reproductive compromise [56, 60], immune compromise [53, 61], and an increase in anxiety-like or displacement behaviors [41, 62] known to be stress dependent [63]. Group dominance ranks were determined based on the outcome of dyadic interactions between females where a female clearly emitted a submissive response to another animal [49]. As detailed in Results in Figure 1, females ranked 1 and 2 were classified as dominant and females ranked 3–5 were considered subordinate [50] to increase power beyond that provided by two subjects per dominance rank position. Groups had been formed and dominance ranks stable for 96 months prior to the initiation of this study.
Figure 1.
Mean ± SEM rates of aggressive behavior received and submission behavior emitted in females at each social dominance rank throughout the course of the study. Rates of aggression received and submission emitted were significantly higher (p < 0.002) in animals categorized as subordinate females (ranks 3 – 5) compared with those categorized as dominant (ranks 1 and 2).
Food intake data for each subject was recorded 24 hours per day, 7 days each week by use of automated feeding devices that have been previously validated [41]. Briefly, the feeders were enclosed in a waterproof box and then attached to the front of the cage; one dispenser in the indoor side and another located outdoors to allow two sources of ad lib access to food. The opening of the dispenser, which the monkeys must reach through to obtain a food pellet, contains an AVID reader (American Veterinary Identification Devices, Norco CA) that identifies each monkey from the unique microchips implanted subcutaneously in both wrists. Detection by the reader activates the dispenser and one pellet is delivered per attempt. The dispensers are hardwired to a computer that captures the ID of the animal and the time when the pellet is delivered. Using an Ethernet Connection the data was retrieved remotely. The containers that hold the food pellets were refilled every 2 hours or as needed by research and animal care staff. The validation of the feeding system [41] showed that dominant females do not restrict access by subordinate animals to the feeders. Furthermore, the data showed that dominant animals rarely (~1% of the time) take a pellet of food that subordinate females obtained. Thus, this automated feeding system allows the continuous quantitation of calories consumed by individual monkeys housed in a social group setting.
Each group of animals had access to two different diets by means of the automated feeders. The study was divided into two 3-week periods, during which animals had either a choice between a high caloric (HCD) or low caloric diet (LCD) or no choice with only the LCD being available. The order of choice vs. no choice was counterbalanced between the two groups of monkeys. At the completion of the first 3-week treatment phase, the diets were immediately switched to the other condition, such that no time elapsed between the conditions. The LCD was the regular Purina monkey diet (diet #5038) that was re-pelleted by Research Diets (C40040; New Brunswick, NJ) so that it could be dispensed by the automated feeders. The LCD contains 3.61 kcal/gram and the calories were distributed as 16% from protein, 12% from fat, and 72% from carbohydrates. Of the total 2.59 kcal/gram of carbohydrates, 2.44 were derived from fiber and 0.15 from sugar. The HCD (D07091204, Research Diets) contained 5.42 kcal/gram and it was composed of 20% from protein, 40% from fat, and 40% from carbohydrates. Of the total 1.62 kcal/gram of carbohydrates, 0.6 were derived from fiber and 1.02 from sugars. Thus, the HCD contains significantly more fat (derived from lard and soybean oil) and more sugar than the LCD. Both diets had similar amounts of cholesterol and the appropriate minerals and vitamins required to maintain the health of the animals.
The effects of diet on plasma cortisol were assessed in two ways. Because the diurnal cortisol rhythm is flattened in humans exposed to chronic stressors [64–66], we assessed diurnal cortisol by collecting samples during week 2 of each phase. Samples were obtained at 0730, 1200, and 1800 hr. In addition, the change in cortisol to an acute stressor was examined on the second day during week 3 of each phase. Because the temporary removal of a female rhesus monkey from her social group for 30 minutes to an unfamiliar location is an acute stressor significantly increasing serum cortisol [67], each subject was removed from her social group and confined in a transfer box (18 × 15 × 26 inches) for a 40-minute period. These boxes are used to transfer animals between the home caging to a procedural cage. Because animals are typically in these cages for no more than 5 minutes, we reasoned that containment in the transfer box for 40 min would constitute a stressor. A blood sample was obtained at time 0 (0730 hr) and 40 min later, after which females were returned to their social groups. A third sample was obtained at 1200 hr to assess recovery from the stressor. We chose the 4-hour time point as it matched the noon time sample collected as a part of the diurnal cortisol assessment.
Behavioral data was obtained three times during each of the three weeks for the choice and no choice conditions. Using a standard ethogram [20], observation sessions of 30 min were done in the mornings between 0830 and 1030. Behaviors included affiliation (proximity, grooming), aggression (open mouth threat with, slap or bite, chase), and submission (grimace, withdraw). In addition, macaques exhibit a specific set of behaviors in stress-eliciting situations that are considered anxiety-like, as they are relieved by benzodiazepines [68–71]. Thus, these behaviors, including yawns, body shakes, self-scratches, self-explore, and pacing were also measured.
The females had been trained for conscious venipuncture using procedures previously described [72] to allow for the collection of blood without anesthesia. The samples were assayed for cortisol in the Biomarker Core Laboratory at Yerkes using a commercially prepared kit (Beckman Coulter, Webster, TX). The assay had an inter- and intra-coefficient of variation of 4.50% at 4.22 μg/dl and 8.74% at 19.68 μg/dl, respectively.
Repeated measures analysis of variance models evaluated the main and interaction effects of social status (dominant vs. subordinates), diet choice (LCD vs. Choice of LCD and HCD), weeks, days, and time of day. The behavioral data was transformed with a log10 to correct for lack of homogeneity of variance. Bivariate correlations were performed on some variables to evaluate their linear relationship. Statistical tests having a probability of p<0.05 were considered significant.
Results
Social Status Categorization
Figure 1 shows rates of aggression received and submissive behavior emitted for monkeys at each social dominance rank position. These data reflect agonistic behavior throughout the two 3-week study phases, independent of diet. Categorizing females ranked 1 and 2 as dominant and those ranked 3 through 5 as subordinate results in a significant main effect of status for aggression received (F 1, 8 = 19.97, p = 0.002) and submissive behaviors emitted (F 1, 8 = 39.92, p < 0.001). In addition, there was a significant correlation of rank (1 – 5) with the frequency of aggression received (r8 = 0.95, p < 0.001) and submissive behavior emitted (r8 = 0.93, p < 0.001), such that 90% of the variance in aggression received and 86% of the variance in submission emitted was accounted for by rank.
Food intake
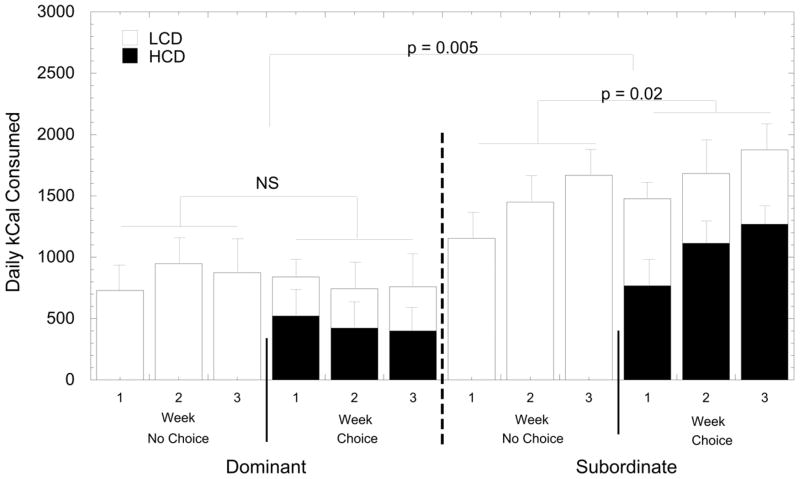
Subordinate females consumed significantly more calories than did dominant females during both the choice and no choice conditions (Figure 2; F1, 8= 14.32, p= 0.005). Furthermore, there was a significant interaction between social status and diet condition (F1, 8 = 25.82, p = 0.001), as subordinate females consumed significantly more calories during the choice compared to the no choice condition (p = 0.02), whereas total calories consumed by dominant females did not vary significantly between the two conditions (p = 0.18). The effect of status by diet interaction also varied significantly across the three weeks (status by week interaction: F2, 16 = 4.83, p = 0.023). Subordinate females gradually increased the total calories consumed each week regardless of the diet condition whereas dominant females consumed similar number of calories in each week of both conditions. Females consumed significantly more calories during the daytime (0600 – 1800 hr) compared with the nighttime during both conditions (Table 1; F1, 8 = 70.14, p < 0.001), and this too varied significantly by social status as subordinates consumed more calories at night than did dominant females (F1, 8 = 6.22, p = 0.037) in a similar pattern during the choice and no choice condition (F1, 8 = 0.34, p = 0.58).
Figure 2.
Mean daily kcal consumed (± SEM) by dominant and subordinate females during three weeks of the no choice condition when only the LCD was available (open bars) and the three-week choice condition when the LCD (open bars) and HCD (closed bars) were available. “NS” indicates the indicated comparison was not significant (p > 0.05).
Table 1.
Mean ±SEM kcal consumed for each three-week diet phase during the daytime (0600 – 1800 hr) and nighttime (1800 – 0600 hr) dominant (n = 4) and subordinate females (n = 6).
| Kcal Consumed in Each Diet Condition | |||||||
|---|---|---|---|---|---|---|---|
| No Choice (LCD only) | Choice | ||||||
| Social Status | Diet | Daytime* | Nighttime | Total | Daytime* | Nighttime | Total |
| Dominant | LCD | 703 ± 153a | 147 ± 46a | 850 ± 162 | 284 ± 1162 | 50 ± 51a | 334 ± 159 |
| HCD | - | - | - | 413 ± 209b | 35 ± 28a | 447 ± 221 | |
| Total | 703 ± 153 | 147 ± 4 | 850 ± 1621 | 696 ± 138 | 85 ± 56 | 781 ± 1421 | |
| Subordinate | LCD | 1248 ± 125b | 175 ± 37a | 1424 ± 132 | 450 ± 95b | 179 ± 42c | 628 ± 130 |
| HCD | - | - | - | 931 ± 170c | 119 ± 23b | 1050 ± 181 | |
| Total | 1248 ± 125 | 175 ± 37 | 1424 ± 1322 | 1380 ± 112 | 298 ± 47 | 1678 ± 1153 | |
The asterisk indicates that calorie consumption occurred more during the day than at night (p< 0.001). Different letters within each diet condition (e.g., choice – daytime) indicates calories consumed by dominant vs. subordinates were significantly different (p < 0.05). Also shown is the total kcal consumed during each phase for daytime and nighttime periods as well as a total daily average. Different numerical superscripts for total calories consumed throughout a 24-hour period indicate groups differed significantly.
During the choice condition (Figure 2, Table 1), the consumption of a specific diet varied significant between the daytime and nighttime. All females consumed more calories from the HCD compared with the LCD during the day (671 ± 134 vs. 366 ± 75) whereas no preference was shown for either diet at night (114 ± 33 vs. 77 ± 148; F1, 8= 9.81, p= 0.014). Furthermore, there was no status by diet interaction (F1, 8 = 0.45, p= 0.52) suggesting that both dominant and subordinate females preferred the HCD (Table 1). However, this preference for the HCD was largely due to subordinates, as the percentage of kcal derived from the HCD for dominant females across the three weeks was 53.1% (± 13.1), 50.2% (±14.54), and 50.0% (±12.2) whereas percentages of HCD intake for subordinates was 48.3% (±9.3), 63.5% (±11.6), and 70.0% (±10.7). Considering consumption of the LCD only during the choice versus the no choice condition (Figure 2, Table 1), subordinate females consumed significantly more calories than the dominant females (Table 1; F1, 8 = 9.04, p = 0.017) in both diet conditions, and this status difference was unaffected by the availability of the HCD during the choice phase (F1, 8 = 0.88, p = 0.37). Not surprisingly all females consumed more of the LCD during the no choice compared with the choice condition (F1, 8 = 19.50, p = 0.002).
Table 2 shows body weights at the start and end of each diet phase as well as the change in weight during these intervals. With respect to absolute body weight, there was no effect of diet (F1, 8 = 1.00, p = 0.35) or diet by status interaction (F1, 8 = 1.37, p = 0.28). However, an examination of the change in body weight from the start to the end of each phase indicated a diet effect (F1, 8 = 11.06, p = 0.01) that was significantly affected by status (F1, 8 = 8.46, p = 0.02). Subordinate females gained more weight than dominant females when on the choice phase (p < 0.05).
Table 2.
Mean ± SEM body weights (kg) at the start and end of each three-week diet phase for dominant and subordinate females. Also shown are changes in body weight.
| Status | Choice |
No Choice (LCD only) |
||||
|---|---|---|---|---|---|---|
| Start | End | Change | Start | End | Change | |
| Dominant | 8.74 ± 0.72 | 8.63 ± 0.70 | −0.11 ± 0.03 | 8.74 ± 0.72 | 8.65 ± 0.67 | −0.13 ± 0.08 |
| Subordinate | 8.89 ± 0.59 | 9.05 ± 0.57 | 0.16 ± 0.03* | 8.86 ± 0.58 | 8.72 ± 0.55 | −0.14 ± 0.07 |
The asterisk indicates the change in weight for the subordinates during the choice condition was significantly greater than other values (p < 0.05).
Diurnal cortisol and the cortisol response to an acute stressor
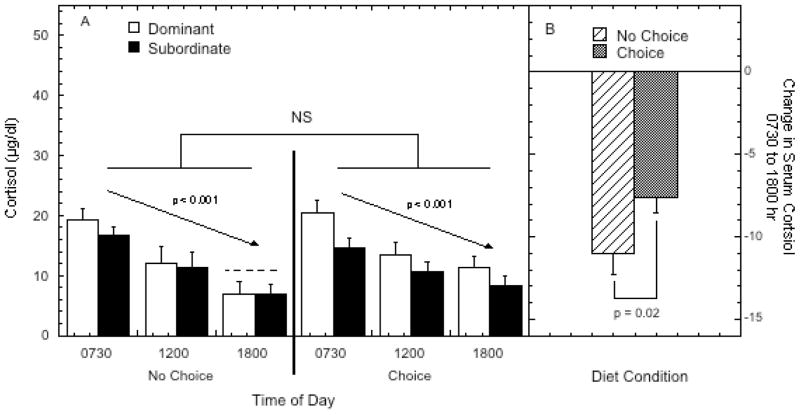
As illustrated in Figure 3, females have higher levels of cortisol in the morning (730 hour) that declined significantly through the midday (1200 hr) and early evening (1800 hr; F 2, 16 = 33.84, p < 0.001). This pattern was not significantly influenced by social status (F 1, 8 = 0.85, p = 0.45), diet condition (F 1, 8 = 0.66, p = 0.44) or a diet by status interaction (F 1, 8 = 1.39, p = 0.27). However, as illustrated in panel B of Figure 3, the change in serum cortisol from the morning to the evening was significantly blunted during the choice condition (F 1, 8 = 8.53, p = 0.02) and this effect was independent of social status (F 1, 8 = 0.14, p = 0.91).
Figure 3.
Panel A shows mean ± SEM serum diurnal cortisol (μg/dl) concentrations at 0730, 1200, 1800 hours during week two of each diet phase for dominant (open bars) and subordinate females (closed bars). The dashed line above the bars for the 1800 hr time point for the no choice condition illustrates the mean of serum cortisol for the 1800 hr time point for the choice condition. Panel B shows the change in serum cortisol from 0730 to 1800 hr for both diet conditions, collapsed across dominance status. “NS” indicates the indicated comparison was not significant (p > 0.05).
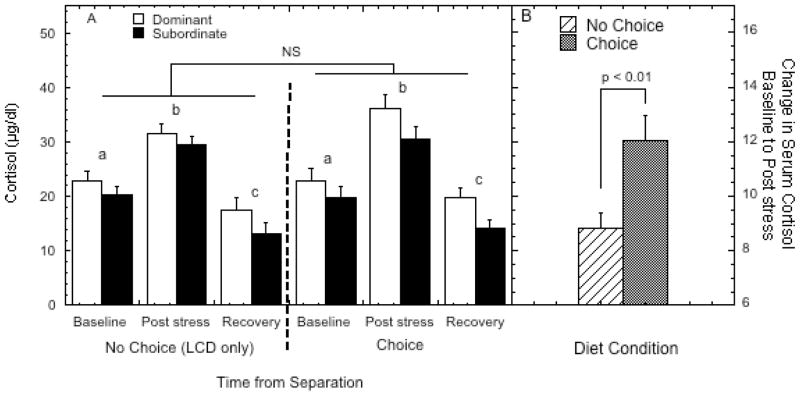
Serum cortisol was significantly elevated by the social separation test before returning to below baseline levels several hours after the test (Figure 4A; F 2, 16 = 142.37, p < 0.001) but this pattern was not significantly affected by diet condition (F 2, 16 = 2.73, p = 0.09), status (F 2, 16 = 0.62, p = 0.55), or diet by status interaction (F 2, 16 = 0.60, p = 0.56). However, as illustrated in Figure 4B, the increase in serum cortisol from baseline to the sample obtained immediately following the 40-minute separation was significantly greater during the choice compared to the no choice condition (F 1, 8 = 12.85, p = 0.007). This diet-induced difference was not significantly affected by status (F 1, 8 = 2.75, p = 0.136). The recovery in serum cortisol, reflecting the difference between the post stress value and the sample obtained at noon, some 2.5 hrs following the test was not affected by diet availability (F 1, 8 = 3.92, p = 0.06) or status (F 1, 8 = 0.36, p = 0.56). However, serum cortisol at this noon sample (16.17 ± 1.12 μg/dl) was nonetheless significantly higher than the noon value obtained during the diurnal sampling (11.85 ± 1.28 μg/dl; F 1, 8 = 35.80, p < 0.001).
Figure 4.
Panel A shows the mean ± SEM serum cortisol levels (μg/dl) prior to the separation (“Baseline”), immediately following a 40 minutes social separation (“Post stress”), and 2.5 hours after being returned to the group (“Recovery”) for the dominant (open bars) and subordinate monkeys (closed bars) during both the no choice and choice phases. Different letters indicate time points are significantly different (post-hoc test, p < 0.001) within each diet condition. “NS” indicates the interaction of status, time, and diet condition in serum cortisol during the social separation test was not significant (p > 0.05). Panel B shows the change in serum cortisol from baseline to post stress samples for both diet conditions, collapsed across dominance status.
Effects of diet availability on behavior
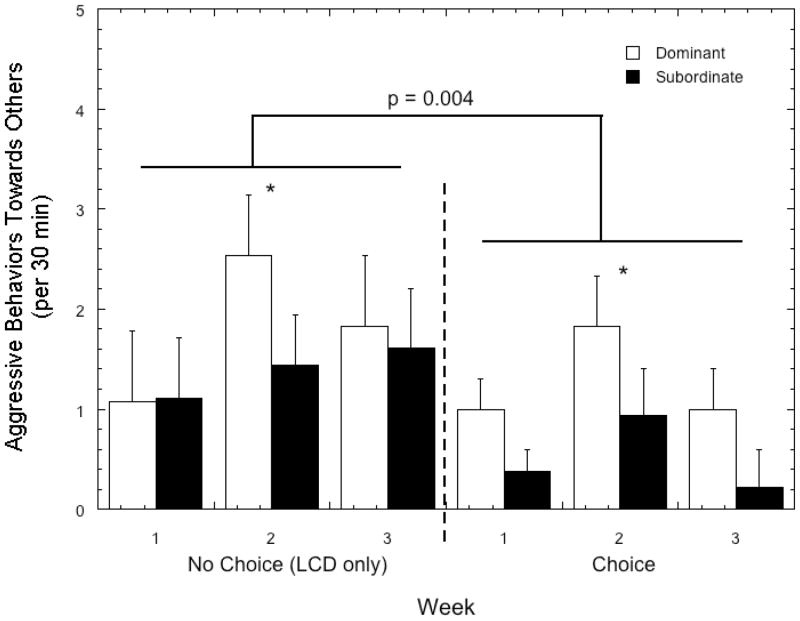
As illustrated in Figure 5, rates of aggressive behavior directed at other females were significantly higher during the no choice condition when only LCD was available (F 1, 8 = 16.16, p = 0.004) and this diet-dependent pattern was not influenced by status (F 1, 8 = 0.27, p = 0.62). Furthermore, although dominant animals initiated more aggressive interactions towards group mates (1.56 ± 0.39) when compared with subordinate females (0.95 ± 0.32), the difference was not significant (F 1, 8 = 3.69, p = 0.09). Inexplicably, rates of aggression directed towards others were significantly higher during week 2 compared with weeks 1 and 3 (Figure 5; F 1, 8 = 3.97, p = 0.04), a pattern that was attenuated by the availability of the choice diet (F 1, 8 = 3.46, p = 0.56). This increase in aggression directed at group mates during week 2 was most prominent in dominant females during both diet conditions (Figure 5; F 1, 8 = 16.05, p < 0.001).
Figure 5.
Mean ± SEM frequency of aggressive behavior directed at cage mates by dominant (open bars) and subordinate females (closed bars) during the no choice and choice conditions. Asterisk indicates that aggressive behavior in dominant females was significantly higher during week 2 compared with weeks 1 and 3 during both diet conditions.
Rates of harassment or aggression received from others was correspondingly lower during the choice (0.70 ± 0.29) compared to the no choice condition (1.35 ± 0.55; F 1, 8 = 8.95, p = 0.02). However, targets of this behavior were not influenced by diet condition (F 1, 8 = 3.85, p = 0.09), as subordinate animals were significantly more often the recipients of aggressive behavior (1.86 ± 0.53) than dominant females (0.19 ± 0.64; F 1, 8 = 19.92, p = 0.002). The increase in aggressive behavior initiated by dominant females during week 2 of each diet phase (Figure 5) was directed primarily at subordinate females, as rates of harassment varied significantly by week and status (F 2, 16 = 16.05, p < 0.001) with aggression received by subordinates higher during week 2 (2.67 ± 0.51) compared to weeks 1 (1.33 ± 0.50) and 3 (1.58 ± 0.67). Not surprisingly, in response to receiving more harassment, subordinate females emitted more submissive behaviors than dominant females (8.17 ± 1.37 vs. 0.72 ± 1.69; F 1, 8 = 39.92, p = 0.009). This pattern of behavior was not influenced by diet condition (F 1, 8 = 2.85, p = 0.13) or by diet and status interaction (F 1, 8 = 0.92, p = 0.37; data not shown).
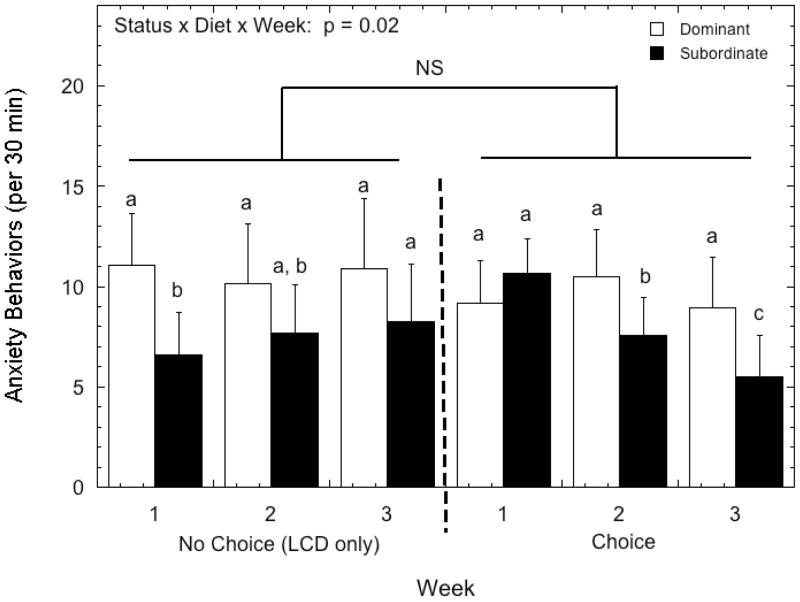
Overall rates of anxiety-like behaviors were not influenced by status (Figure 6; F 1, 8 = 0.69, p = 0.43) nor were they affected by diet condition (F 1, 8 = 0.39, p = 0.55). However, there was a significant status by week by diet interaction (F 2, 16 = 4.79, p = 0.023), as rates of anxiety-like behaviors decreased significantly across the 3 weeks of the diet choice condition in subordinate but not dominant females. In contrast, anxiety- like behaviors were stable in dominant animals but increased from week 1 to week 3 in subordinates during the LCD-only condition.
Figure 6.
Mean ±SEM of anxiety-like behavior by dominant (open bars) and subordinate females (closed bars) during both the no choice and choice conditions. Different letters indicate significant status differences in rates of anxiety (post-hoc test, p < 0.02). “NS” indicates the indicated comparison was not significant (p > 0.05).
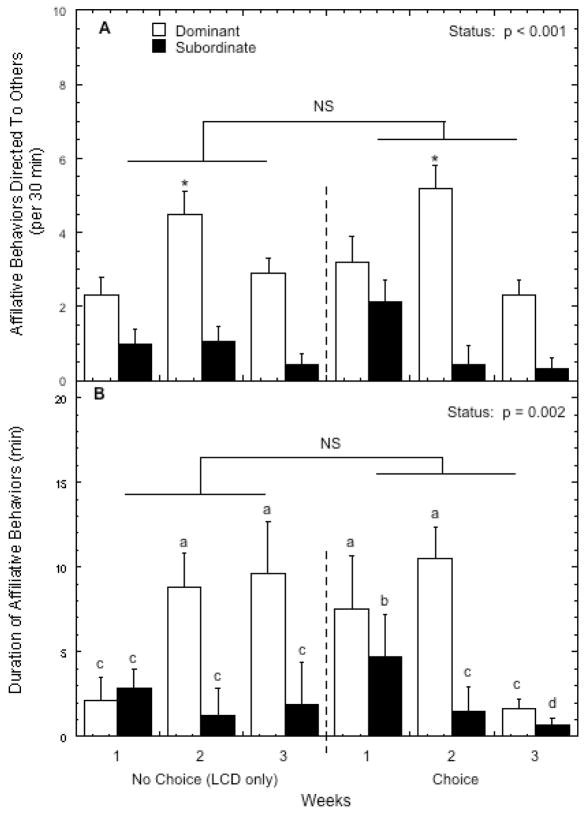
Regardless of diet condition, dominant females directed more affiliative behavior at group mates (F 1,8 = 54.14, p < 0.001) than did subordinate females (Figure 7A). Diet condition did not affect the frequency of affiliative behavior (F 1,8 = 0.93, p = 0.36) and there was not a significant interaction between diet and status (F 1,8 = 0.18, p = 0.68). The higher rates of affiliative behavior by dominant females were increased further during week 2 (F 2, 16 = 12.52, p = 0.001) and this pattern was not affected by diet availability (F 2, 16 = 0.74, p = 0.49). Furthermore, bouts of affiliation initiated by dominant females were significantly longer than those initiated by subordinates (Figure 7B; F 1,8 = 8.47, p = 0.02). While the time engaged in affiliation varied significantly by diet across the 3-weeks (F 2, 16 = 8.19, p = 0.004), this was further modified by status (F 2, 8 = 3.49, p = 0.055), as durations of affiliation increased in dominant but not subordinates during the no choice condition but decreased progressively during the choice phase in subordinate and dominant animals. Finally, dominant females were more often the recipients of affiliative behaviors (3.14 ± 0.42 vs. 1.22 ± 0.35 per 30 minutes; F 1,8 = 12.36, p = 0.008). The increased rates of affiliation initiated by dominant females during week 2 of each diet phase (Figure 7A) was directed significantly more at other dominant females (F 2, 16 = 11.32, p = 0.001) as affiliation received by dominant animals increased during week 2 (4.50 ± 0.63) compared to weeks 1 (2.46 ± 0.43) and week 3 (2.46 ± 0.38). These effects of status were not influenced by diet condition (data not shown; F 1,8 = 1.02, p = 0.34).
Figure 7.
Mean ±SEM of the frequency of affiliative behavior directed at cage mates (panel A) and the durations of these behaviors (panel B) by dominant (open bars) and subordinate females (closed bars) during both the no choice and choice conditions. Different letters indicate time points are significantly different (post-hoc test, p < 0.05). Asterisk indicates that affiliative behavior directed to others by dominant females was significantly higher during week 2 compared with weeks 1 and 3 during both diet conditions. “NS” indicates the indicated comparison was not significant (p > 0.05)
Discussion
Socially housed female rhesus monkeys preferred a palatable, high calorie diet to a standard low calorie monkey chow but this is particularly evident in subordinate animals as 3 week access to the diet progressed. Importantly, females classified as subordinate received significantly less affiliation and higher rates of aggression from conspecifics and emitted more submissive behavior. Importantly, subordinate females consumed significantly more calories from the HCD during the diet choice condition as well as more LCD during the no choice phase. The consumption of a HCD during the diet choice condition was associated with a flattening of the diurnal rhythm in cortisol and increased cortisol response to an acute social separation in all females. While aggressive behavior was significantly decreased in all females during the diet choice condition, the increased consumption of HCD diet by subordinate females was also associated with a progressive decline in anxiety-like behavior.
In the current study, all animals, regardless of social status, preferred the HCD to the LCD when the choice of diet was available. However, subordinate females consumed significantly more calories from this HCD than did the dominant females. These results corroborate our previous preliminary report in monkeys [41] and data from a number of rodent models suggesting that exposure to chronic stressors increases intake and preference for HCDs [43, 44, 73]. Data from humans also support the relation between stressor exposure and the ingestion of comfort foods [27–30]. Furthermore, during the choice condition, subordinate females consumed on average the same number of calories of the LCD as dominant monkeys consumed the HCD. The social status difference in calorie intake during the choice condition was also evident during the no choice phase, as subordinates consumed nearly double the calories from the LCD compared with the dominant females. However, subordinates nonetheless consumed significantly more calories during the choice compared to the no choice condition and calorie consumption increased progressively during each of the three-week phases. In contrast, dominant animals appeared to calorically regulate despite the availability of the HCD during the choice condition, as total calorie consumption was similar during the choice and no choice phases. These data imply that while calorie intake by dominant females is regulated by the interplay between satiety and orexigenic signals regardless of diet availability, food intake by subordinates is more than a satiety issue particularly when the opportunity to consume a HCD is present. Finally, subordinate females also consumed more calories than dominant animals during the nighttime hours from the HCD, which is similar to the association between night snacking and high levels of psychosocial distress seen in humans [74].
As noted above, subordinate females ate more calories than dominant animals during the no choice condition when only the LCD was available. This observation is inconsistent with the longstanding notion of the anorexic effects of stress in several different rodent models fed standard lab diets [7, 8, 13–15] including exposure to a predator [16] and social subordination [15, 17]. Indeed, previous studies of rhesus monkeys indicate subordinates consume less food [18, 19], have significantly lower body weights and are hypoleptinemic when fed the low fat, high fiber Purina diet used in this study [20]. On the other hand, studies with hamsters that also employ a social component as a part of the stress paradigm indicate stress increases consumption of laboratory chow [36, 37, 75]. Reconciling these discrepancies is difficult given differences in species as well as stressor type and duration. Nevertheless, previous diet history may also be an important consideration. In a preliminary analysis of the cohort of females used in the present study before they had any history of HCD exposure, subordinates indeed ate less of the Purina LCD than dominant monkeys [40]. Because this same cohort was used in the initial validation of the automated feeding system in which subordinates were observed to consume significantly more of both the high sugar or high fat-high sugar diets [41], it is possible that a prior history of HCD consumption changed the motivation of the subordinates to consume the LCD – implying that individuals attempting to restrict calorie intake by eating less palatable foods may still overeat. Thus, whether subordinates show reduced or excess intake of otherwise less calorically dense, laboratory chow may depend on previous access for more palatable diets. Nonetheless, this hypothesis is somewhat at odds with data from rodents, which indicate that following withdrawal of a preferred HCD, animals become hypophagic and more anxious when only a LCD is available but increase intake of the preferred food when it is re-introduced [46, 76, 77]. However, these rodent studies did not include a stress component and thus the data are not directly comparable. Clearly, additional studies are needed to assess the interaction of stress and diet history on emotional feeding.
The significantly greater intake of the HCD and total calories overall by subordinate monkeys occurred in the context of significantly higher rates of harassment from more dominant animals, a defining feature of social subordination in macaques [49]. Furthermore, subordinate animals less often initiated affiliative behavior and were less frequently the target of these prosocial behaviors. Indeed, when the initiation of aggression and affiliation by dominant females inexplicably increased during week 2 of each diet phase, the analyses showed that the aggression was most often directed at subordinates and the dominant females were affiliating with one another. However, these behavioral differences were not associated with social status differences in morning or diurnal cortisol or the cortisol response to a social separation. As noted previously, a dysregulation of the LHPA axis, assessed by a dexamethasone suppression test [41, 50, 52, 78] or ACTH challenge [55] is a characteristic of socially subordinate macaques. Other approaches to show status differences in cortisol may yield variable results [79–82]. Given the relation between a flattening of the diurnal cortisol rhythm and stress exposure in people [64–66], we predicted this too might be a characteristic of subordinate female monkeys. However, confirming a previous report [83], no differences in diurnal cortisol were observed between dominant and subordinate females. Furthermore, the acute social separation test was clearly a potent stressor for all animals, as it did not differentiate dominant from subordinate females. Other parameters of LHPA status, including assessments of glucocorticoid negative feedback tests, may provide additional biomarkers of psychosocial stress exposure experienced by subordinate rhesus monkey females.
One way that palatable, HCDs may provide relief from stress is by reducing LHPA reactivity. HCD intake in rodents dampens the response of the LHPA axis in response to an acute stressor [84, 85] as well as repeated restraint stress [43], an effect that may be dependent on diet choice [42]. Given the expectation that subordinate females would eat significantly more calories from the HCD, we predicted their cortisol response to the social separation test would be attenuated and diurnal cortisol rhythm normalized during the choice condition. However, neither hypothesis was supported. Indeed, the diurnal cortisol rhythm was flattened during the choice phase due to an elevation in early evening levels of cortisol, and the cortisol response to the social separation was enhanced in all females. These observations support other data that find consumption of calorically dense foods increases both basal and stress-induced LHPA activity [86–90] and are consistent with clinical studies showing a positive relation between central obesity and a hyperactive LHPA axis [91–93]. Taken together the data actually suggest that increased consumption of HCD may exacerbate LHPA dysregulation resulting from exposure to psychosocial stress that could further increase the motivation to eat palatable, calorically dense diets. Indeed, the most parsimonious explanation for the increased consumption of HCD in chronically stressed individuals, including our subordinate monkeys, is that consumption of these foods activate reward pathways that are otherwise compromised by stressor exposure. Activation of central CRF pathways decreases reward value of familiar stimuli and increases reward-seeking behavior [94–96]. Previous studies in macaques show that dopamine 2 receptor (D2R) binding density is significantly reduced in subordinate animals [57, 58]. Furthermore, calorically dense foods but not a standard diet or palatable food devoid of calories (saccharin) increases levels of dopamine in the nucleus accumbens [97–100], linking intake of these foods to stimulation of reward systems [100–104]. Furthermore, unrestricted consumption of a HCD itself reduces D2R binding [105], providing evidence for the hypothesis that the metabolic stress associated with eating these foods synergizes with psychosocial stressor exposure to further increase compulsive consumption of these diets.
Clear effects of diet choice and the opportunity to consume the HCD on socio-emotional behavior were evident. Most notably, however, social structure was not altered during the choice condition. Aggressive behavior directed towards others was decreased during the choice condition. Despite the decrease in frequency, subordinates were still most often the target of the behavior from more dominant cage mates. In addition, measures of anxiety progressively decreased during the 3-week choice condition in subordinate females in association with increasing consumption of the HCD. Data from rodent models using standard tests of anxiety that involve no social component show a palatable, high caloric diet is anxiolytic [47, 85, 90, 106]. Under these testing conditions, withdrawal of a highly preferred food is anxiogenic [46, 47]. However, our counterbalanced design did not allow us to fully examine that hypothesis. It is possible that the decrease in anxiety behaviors by subordinates during the choice phase was also in part due to them receiving less harassment from more dominant females. However, this decrease in anxiety could not be explained by a corresponding increase in affiliation. Indeed, time spent in affiliation decreased over the three weeks of the choice condition compared to the LCD only phase. We did not assess how other behaviors, specifically solitary behaviors or simple locomotor activity, were affected by diet choice. Importantly, our analysis cannot determine whether the reduced aggression observed during the choice condition was due to a decreased motivation of animals to aggress group mates or for animals, i.e., subordinates, to be less available targets of aggression by being less active. One might expect that increased consumption of a HCD would lead to more sedentary behavior [107, 108], reducing the probability of aggression.
In summary, the present study shows that when given a choice of diets subordinate female rhesus monkeys consume significantly more calories from a highly palatable high fat – high sugar diet than do dominant females. Furthermore, dominant females regulated caloric intake regardless of diet availability whereas subordinates consumed significantly more calories when the calorically dense diet was available. Availability of the HCD during the choice condition increased both basal and stress-induced activity of the LHPA axis in both dominant and subordinate animals, supporting the notion that consumption of a HCD is a metabolic stressor that may interact with the psychosocial stress of social subordination to further enhance intake of these diets. The intent of this study was not to define the metabolic consequences of these differences in food intake, although we report that the gain in weight for subordinates during the choice phase was significant. As this represents a ~1% increase, clearly this change is not clinically significant. Future studies, using a larger sample size with longer access to these diets will show how an obese phenotype emerges in subordinate animals and importantly how the structure of meals may change in response to diet availability [109]. Indeed, a limitation of the present study was the use of status categories rather than individual social status ranks, as resolution to detect differences may be lost when females rank 2 and 3 in the five member groups are placed in different categories. Nevertheless, the data support the notion that humans under chronic stress exposure increase the consumption of a preferred diet when a choice is available as a coping strategy to reduce perceived psychological stress [31, 110]. The present study shows that socially housed rhesus macaques represent a translational animal model of chronic psychosocial stress, as these individuals face social challenges on a daily basis. The use of this model can define the neurobiological mechanisms that lead to the comorbidity of stress and emotional feeding.
Research highlights.
Subordinate females eat more calories
Subordinate female monkeys eat more calories from a high caloric diet
Dominant females regulate calorie intake, regardless of diet
Eating a high caloric diet may elevate serum cortisol
Acknowledgments
The authors thank Jennifer Whitley, Marta Checchi, Shannon Moss, and Jeff Fisher for the expert technical assistance. This work was funded by NIH grants HD46501, training grants numbers 4425, RR024505 (MA), T32-MH073525 (VM), and K12 GM000680, and, in part, RR00165. Further supported was provided by the Center for Behavioral Neuroscience through the STC Program of the National Science Foundation IBN-9876754. The YNPRC is fully accredited by AAALAC, International.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 2.Schulkin J, Gold PW, McEwen BS. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–43. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 3.Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005 doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Holsboer F. Stress, hypercortisolism and corticosteroid receptors in depression: implications for therapy. J Affect Disord. 2001;62:77–91. doi: 10.1016/s0165-0327(00)00352-9. [DOI] [PubMed] [Google Scholar]
- 5.Lo Sauro C, Ravaldi C, Cabras PL, Faravelli C, Ricca V. Stress, hypothalamic-pituitary-adrenal axis and eating disorders. Neuropsychobiology. 2008;57:95–115. doi: 10.1159/000138912. [DOI] [PubMed] [Google Scholar]
- 6.Warne JP. Shaping the stress response: Interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol. 2009;300:137–46. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 7.Houshyar H, Manalo S, Dallman MF. Time-dependent alterations in mRNA expression of brain neuropeptides regulating energy balance and hypothalamo-pituitary-adrenal activity after withdrawal from intermittent morphine treatment. J Neurosci. 2004;24:9414–24. doi: 10.1523/JNEUROSCI.1641-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dallman M, Bhatnagar S. Chronic stress and energy balance: Role of the HPA axis. In: McEwen B, editor. Handbook of Physiology, Section 7: The Endocrine System. Oxford University Press; New York: 2001. [Google Scholar]
- 9.Richard D, Lin Q, Timofeeva E. The corticotropin-releasing factor family of peptides and CRF receptors: their roles in the regulation of energy balance. Eur J Pharmacol. 2002;440:189–97. doi: 10.1016/s0014-2999(02)01428-0. [DOI] [PubMed] [Google Scholar]
- 10.Heinrichs SC, Richard D. The role of corticotropin-releasing factor and urocortin in the modulation of ingestive behavior. Neuropeptides. 1999;33:350–9. doi: 10.1054/npep.1999.0047. [DOI] [PubMed] [Google Scholar]
- 11.Hotta M, Shibasaki T, Arai K, Demura H. Corticotropin-releasing factor receptor type 1 mediates emotional stress-induced inhibition of food intake and behavioral changes in rats. Brain Res. 1999;823:221–5. doi: 10.1016/s0006-8993(99)01177-4. [DOI] [PubMed] [Google Scholar]
- 12.Krahn DD, Gosnell BA, Levine AS, Morley JE. Behavioral effects of corticotropin-releasing factor: localization and characterization of central effects. Brain Res. 1988;443:63–9. doi: 10.1016/0006-8993(88)91598-3. [DOI] [PubMed] [Google Scholar]
- 13.Marti O, Marti J, Armario A. Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. Physiol Behav. 1994;55:747–53. doi: 10.1016/0031-9384(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 14.Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–8. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- 15.Gamaro GD, Manoli LP, Torres IL, Silveira R, Dalmaz C. Effects of chronic variate stress on feeding behavior and on monoamine levels in different rat brain structures. Neurochem Int. 2003;42:107–14. doi: 10.1016/s0197-0186(02)00080-3. [DOI] [PubMed] [Google Scholar]
- 16.Jochman KA, Newman SM, Kalin NH, Bakshi VP. Corticotropin-releasing factor-1 receptors in the basolateral amygdala mediate stress-induced anorexia. Behav Neurosci. 2005;119:1448–58. doi: 10.1037/0735-7044.119.6.1448. [DOI] [PubMed] [Google Scholar]
- 17.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, et al. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80:683–93. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Small MF. Body fat, rank, and nutritional status in a captive group of rhesus monkeys. International Journal of Primatology. 1981;2:91–95. [Google Scholar]
- 19.Belzung C, Anderson R. Social rank and responses to feeding competition in rhesus monkeys. Behavioral Processes. 1986;12:307–316. doi: 10.1016/0376-6357(86)90001-X. [DOI] [PubMed] [Google Scholar]
- 20.Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–19. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Licinio J, Wong ML, Gold PW. The hypothalamic-pituitary-adrenal axis in anorexia nervosa. Psychiatry Res. 1996;62:75–83. doi: 10.1016/0165-1781(96)02991-5. [DOI] [PubMed] [Google Scholar]
- 22.McElroy SL, Kotwal R, Malhotra S, Nelson EB, Keck PE, Nemeroff CB. Are mood disorders and obesity related? A review for the mental health professional. J Clin Psychiatry. 2004;65:634–51. doi: 10.4088/jcp.v65n0507. quiz 730. [DOI] [PubMed] [Google Scholar]
- 23.Benton T, Staab J, Evans DL. Medical co-morbidity in depressive disorders. Ann Clin Psychiatry. 2007;19:289–303. doi: 10.1080/10401230701653542. [DOI] [PubMed] [Google Scholar]
- 24.Werrij MQ, Mulkens S, Hospers HJ, Jansen A. Overweight and obesity: the significance of a depressed mood. Patient Educ Couns. 2006;62:126–31. doi: 10.1016/j.pec.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63:824–30. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldbacher EM, Matthews KA. Are psychological characteristics related to risk of the metabolic syndrome? A review of the literature. Ann Behav Med. 2007;34:240–52. doi: 10.1007/BF02874549. [DOI] [PubMed] [Google Scholar]
- 27.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosom Med. 2000;62:853–65. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 29.Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci. 2004;1032:208–10. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- 30.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–58. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Gibson EL. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiol Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 32.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge eating. Int J Eat Disord. 2003;34:183–97. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 33.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100:11696–701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–80. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 35.la Fleur SE, Akana SF, Manalo SL, Dallman MF. Interaction between corticosterone and insulin in obesity: regulation of lard intake and fat stores. Endocrinology. 2004;145:2174–85. doi: 10.1210/en.2003-1359. [DOI] [PubMed] [Google Scholar]
- 36.Foster MT, Solomon MB, Huhman KL, Bartness TJ. Social defeat increases food intake, body mass, and adiposity in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1284–93. doi: 10.1152/ajpregu.00437.2005. [DOI] [PubMed] [Google Scholar]
- 37.Solomon MB, Foster MT, Bartness TJ, Huhman KL. Social defeat and footshock increase body mass and adiposity in male Syrian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R283–90. doi: 10.1152/ajpregu.00330.2006. [DOI] [PubMed] [Google Scholar]
- 38.Meisel RL, Hays TC, Del Paine SN, Luttrell VR. Induction of obesity by group housing in female Syrian hamsters. Physiol Behav. 1990;47:815–7. doi: 10.1016/0031-9384(90)90002-l. [DOI] [PubMed] [Google Scholar]
- 39.Tamashiro KL, Nguyen MM, Ostrander MM, Gardner SR, Ma LY, Woods SC, et al. Social stress and recovery: implications for body weight and body composition. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1864–74. doi: 10.1152/ajpregu.00371.2007. [DOI] [PubMed] [Google Scholar]
- 40.Michopoulos V, Shepard KN, Arce M, Whitley J, Wilson ME. Society forthe Study of Ingestive Behavior. Portland OR: 2010. Food history and diet choice affect food intake in monkeys; p. 179. [Google Scholar]
- 41.Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiology & Behavior. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–9. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- 43.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 44.Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, et al. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin and corticosterone concentrations after restraint. Endocrinology. 2008 doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Minor TR, Saade S. Poststress glucose mitigates behavioral impairment in rats in the “learned helplessness” model of psychopathology. Biol Psychiatry. 1997;42:324–34. doi: 10.1016/S0006-3223(96)00467-2. [DOI] [PubMed] [Google Scholar]
- 46.Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009;34:38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–9. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 48.Teegarden SL, Nestler EJ, Bale TL. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiatry. 2008;64:941–50. doi: 10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60:459–72. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- 50.Kaplan JR, Adams MR, Clarkson TB, Koritnik DR. Psychosocial influences on female ‘protection’ among cynomolgus macaques. Atherosclerosis. 1984;53:283–95. doi: 10.1016/0021-9150(84)90129-1. [DOI] [PubMed] [Google Scholar]
- 51.Cohen S. Social status and susceptibility to respiratory infections. Ann N Y Acad Sci. 1999;896:246–53. doi: 10.1111/j.1749-6632.1999.tb08119.x. [DOI] [PubMed] [Google Scholar]
- 52.Wilson ME, Pazol K, Legendre A, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic - hypothalamic - pituitary - adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005:26. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]
- 53.Paiardini M, Hoffman J, Cervasi B, Ortiz AM, Stroud F, Silvestri G, et al. T-cell phenotypic and functional changes associated with social subordination and gene polymorphisms in the serotonin reuptake transporter in female rhesus monkeys. Brain Behav Immun. 2009;23:286–93. doi: 10.1016/j.bbi.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biological Psychiatry. 1997;41:871–82. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 55.Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biological Psychiatry. 1998;44:882–91. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- 56.Kaplan JR, Adams MR, Clarkson TB, Manuck SB, Shively CA, Williams JK. Psychosocial factors, sex differences, and atherosclerosis: lessons from animal models. Psychosomatic Medicine. 1996;58:598–611. doi: 10.1097/00006842-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, et al. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–74. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 58.Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, et al. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–3. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 59.Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair JB, et al. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 60.Michopoulos V, Berga SL, Kaplan JR, Wilson ME. Social subordination and polymorphisms in the gene encoding the serotonin transporter enhance estradiol inhibition of luteinizing hormone secretion in female rhesus monkeys. Biol Reprod. 2009;81:1154–63. doi: 10.1095/biolreprod.109.079038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain Behav Immun. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- 62.Shively CA, Register TC, Friedman DP, Morgan TM, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biol Psychol. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 63.Troisi A. Displacement activities as a behavioral measure of stress in nonhuman primates and human subjects. Stress. 2002;5:47–54. doi: 10.1080/102538902900012378. [DOI] [PubMed] [Google Scholar]
- 64.Van den Bergh BR, Van Calster B, Pinna Puissant S, Van Huffel S. Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: Associations with diurnal cortisol profiles. Horm Behav. 2008;54:253–7. doi: 10.1016/j.yhbeh.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 65.O’Connor DB, Hendrickx H, Dadd T, Elliman TD, Willis TA, Talbot D, et al. Cortisol awakening rise in middle-aged women in relation to psychological stress. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 66.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 67.Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 doi: 10.1007/s12020-009-9250-7. [DOI] [PubMed] [Google Scholar]
- 68.Schino G, Troisi A, Perretta G, Monaco V. Measuring anxiety in nonhuman primates: effect of lorazepam on macaque scratching. Pharmacol Biochem Behav. 1991;38:889–91. doi: 10.1016/0091-3057(91)90258-4. [DOI] [PubMed] [Google Scholar]
- 69.Troisi A, Schino G, D’Antoni M, Pandolfi N, Aureli F, D’Amato FR. Scratching as a behavioral index of anxiety in macaque mothers. Behav Neural Biol. 1991;56:307–13. doi: 10.1016/0163-1047(91)90469-7. [DOI] [PubMed] [Google Scholar]
- 70.Troisi A. Gender differences in vulnerability to social stress: a Darwinian perspective. Physiol Behav. 2001;73:443–9. doi: 10.1016/s0031-9384(01)00459-0. [DOI] [PubMed] [Google Scholar]
- 71.Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann N Y Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- 72.Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- 73.Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89:536–42. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 74.Colles SL, Dixon JB, O’Brien PE. Night eating syndrome and nocturnal snacking: association with obesity, binge eating and psychological distress. Int J Obes (Lond) 2007;31:1722–30. doi: 10.1038/sj.ijo.0803664. [DOI] [PubMed] [Google Scholar]
- 75.Bartness TJ. Photoperiod, sex, gonadal steroids, and housing density affect body fat in hamsters. Physiol Behav. 1996;60:517–29. doi: 10.1016/s0031-9384(96)80027-8. [DOI] [PubMed] [Google Scholar]
- 76.Pickering C, Alsio J, Hulting AL, Schioth HB. Withdrawal from free-choice high-fat high-sugar diet induces craving only in obesity-prone animals. Psychopharmacology (Berl) 2009;204:431–43. doi: 10.1007/s00213-009-1474-y. [DOI] [PubMed] [Google Scholar]
- 77.Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1066–76. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression, and brain dopamine in female cynomolgus monkeys. Ann N Y Acad Sci. 1997;807:574–7. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- 79.Gust DA, Gordon TP, Hambright MK, Wilson ME. Relationship between social factors and pituitary-adrenocortical activity in female rhesus monkeys (Macaca mulatta) Horm Behav. 1993;27:318–31. doi: 10.1006/hbeh.1993.1024. [DOI] [PubMed] [Google Scholar]
- 80.Stavisky RC, Adams MR, Watson SL, Kaplan JR. Dominance, cortisol, and behavior in small groups of female cynomolgus monkeys (Macaca fascicularis) Hormones & Behavior. 2001;39:232–8. doi: 10.1006/hbeh.2001.1650. [DOI] [PubMed] [Google Scholar]
- 81.Czoty PW, Gould RW, Nader MA. Relationship between social rank and cortisol and testosterone concentrations in male cynomolgus monkeys (Macaca fascicularis) J Neuroendocrinol. 2009;21:68–76. doi: 10.1111/j.1365-2826.2008.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sassenrath EN. Increased adrenal responsiveness to social stress in rhesus monkeys. Hormones & Behavior. 1970;1:283–298. [Google Scholar]
- 83.Collura LA, Hoffman JB, Wilson ME. Administration of human leptin differentially affects parameters of cortisol secretion in socially housed female rhesus monkeys. Endocrine. 2009 doi: 10.1007/s12020-009-9250-7. in press. [DOI] [PubMed] [Google Scholar]
- 84.Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272:R840–8. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- 85.Buwalda B, Blom WA, Koolhaas JM, van Dijk G. Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol Behav. 2001;73:371–7. doi: 10.1016/s0031-9384(01)00493-0. [DOI] [PubMed] [Google Scholar]
- 86.Kitraki E, Soulis G, Gerozissis K. Impaired neuroendocrine response to stress following a short-term fat-enriched diet. Neuroendocrinology. 2004;79:338–45. doi: 10.1159/000079665. [DOI] [PubMed] [Google Scholar]
- 87.Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol. 1997;273:E1168–77. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- 88.Legendre A, Harris RB. Exaggerated response to mild stress in rats fed high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1288–1294. doi: 10.1152/ajpregu.00234.2006. [DOI] [PubMed] [Google Scholar]
- 89.Legendre A, Papakonstantinou E, Roy MC, Richard D, Harris RB. Differences in response to corticotropin-releasing factor after short- and long-term consumption of a high-fat diet. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1076–85. doi: 10.1152/ajpregu.00592.2006. [DOI] [PubMed] [Google Scholar]
- 90.Soulis G, Kitraki E, Gerozissis K. Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol. 2005;25:869–80. doi: 10.1007/s10571-005-4943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vicennati V, Pasquali R. Abnormalities of the hypothalamic-pituitary-adrenal axis in nondepressed women with abdominal obesity and relations with insulin resistance: evidence for a central and a peripheral alteration. J Clin Endocrinol Metab. 2000;85:4093–8. doi: 10.1210/jcem.85.11.6946. [DOI] [PubMed] [Google Scholar]
- 92.Rosmond R, Dallman MF, Bjorntorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–9. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 93.Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, et al. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. J Clin Endocrinol Metab. 2002;87:166–75. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- 94.Macey DJ, Koob GF, Markou A. CRF and urocortin decreased brain stimulation reward in the rat: reversal by a CRF receptor antagonist. Brain Res. 2000;866:82–91. doi: 10.1016/s0006-8993(00)02229-0. [DOI] [PubMed] [Google Scholar]
- 95.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Izzo E, Sanna PP, Koob GF. Impairment of dopaminergic system function after chronic treatment with corticotropin-releasing factor. Pharmacol Biochem Behav. 2005;81:701–8. doi: 10.1016/j.pbb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 97.Blackburn JR, Phillips AG, Jakubovic A, Fibiger HC. Increased dopamine metabolism in the nucleus accumbens and striatum following consumption of a nutritive meal but not a palatable non-nutritive saccharin solution. Pharmacol Biochem Behav. 1986;25:1095–100. doi: 10.1016/0091-3057(86)90091-2. [DOI] [PubMed] [Google Scholar]
- 98.Marinelli M, Rudick CN, Hu XT, White FJ. Excitability of dopamine neurons: modulation and physiological consequences. CNS Neurol Disord Drug Targets. 2006;5:79–97. doi: 10.2174/187152706784111542. [DOI] [PubMed] [Google Scholar]
- 99.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–15. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- 100.Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–41. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- 101.Pelchat ML. Of human bondage: food craving, obsession, compulsion, and addiction. Physiol Behav. 2002;76:347–52. doi: 10.1016/s0031-9384(02)00757-6. [DOI] [PubMed] [Google Scholar]
- 102.Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–44. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 103.Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav. 1996;53:221–6. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 104.Martel P, Fantino M. Influence of the amount of food ingested on mesolimbic dopaminergic system activity: a microdialysis study. Pharmacol Biochem Behav. 1996;55:297–302. doi: 10.1016/s0091-3057(96)00087-1. [DOI] [PubMed] [Google Scholar]
- 105.Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–41. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alsio J, Roman E, Olszewski PK, Jonsson P, Fredriksson R, Levine AS, et al. Inverse association of high-fat diet preference and anxiety-like behavior: a putative role for urocortin 2. Genes Brain Behav. 2009;8:193–202. doi: 10.1111/j.1601-183X.2008.00464.x. [DOI] [PubMed] [Google Scholar]
- 107.Judge MK, Zhang J, Tumer N, Carter C, Daniels MJ, Scarpace PJ. Prolonged hyperphagia with high-fat feeding contributes to exacerbated weight gain in rats with adult-onset obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R773–80. doi: 10.1152/ajpregu.00727.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Basterfield L, Lumley LK, Mathers JC. Wheel running in female C57BL/6J mice: impact of oestrus and dietary fat and effects on sleep and body mass. Int J Obes (Lond) 2009;33:212–8. doi: 10.1038/ijo.2008.253. [DOI] [PubMed] [Google Scholar]
- 109.Zorrilla EP, Inoue K, Fekete EM, Tabarin A, Valdez GR, Koob GF. Measuring meals: structure of prandial food and water intake of rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1450–67. doi: 10.1152/ajpregu.00175.2004. [DOI] [PubMed] [Google Scholar]
- 110.Wallis DJ, Hetherington MM. Emotions and eating. Self-reported and experimentally induced changes in food intake under stress. Appetite. 2009;52:355–62. doi: 10.1016/j.appet.2008.11.007. [DOI] [PubMed] [Google Scholar]