Abstract
Along with its ability to directly regulate gene expression, estradiol influences cell signaling and brain functions via rapid, membrane-initiated events. In the female rat striatum, estradiol activates membrane-localized estrogen receptors to influence synaptic neurotransmission, calcium channel activity, and behaviors related to motor control. Yet, the mechanism by which estradiol acts to rapidly affect striatal physiology has remained elusive. Here we find that membrane estrogen receptors couple to the metabotropic glutamate receptors mGluR5 and mGluR3, providing the framework to understand how membrane estrogen receptors affect striatal function. Using CREB phosphorylation as a downstream measure of ER/mGluR activation, membrane-localized ERα activates mGluR5 signaling to mediate MAPK-dependent CREB phosphorylation. Further, ERα and ERβ activate mGluR3 to attenuate L-type calcium channel-dependent CREB signaling. Interestingly, while this fundamental mechanism of ER/mGluR signaling was initially characterized in hippocampal neurons, estrogen receptors in striatal neurons are paired with a different set of mGluRs, resulting in the potential to functionally isolate membrane-initiated estrogen signaling across brain regions, via use of specific mGluR modulators. These results provide both a mechanism for the rapid actions of estrogens within the female striatum, as well as demonstrate that estrogen receptors can interact with a more diverse set of surface membrane receptors than previously recognized.
Keywords: estradiol, metabotropic glutamate receptor, CREB, MAPK, L-type calcium channel, striatum
For well over 25 years, estradiol has been known to directly influence the physiology of the female rat striatum. For instance, estrogens have been shown to enhance dopamine release (Becker, 1990; Xiao and Becker, 1998; Xiao et al., 2003), inhibit GABAergic neurotransmission (Hu et al., 2006), and attenuate L-type calcium channel currents (Mermelstein et al., 1996). These actions of estradiol are thought to affect striatal-mediated behaviors. For example, estradiol acting within the female striatum can enhance sensorimotor control (Becker et al., 1987). Similar effects are believed to occur in women, where estrogen signaling has been implicated in affecting fine motor control, as well as alleviating the symptoms of Parkinson’s disease (Hampson and Kimura, 1988; Hampson, 1990; Mayeux et al., 1992; Sherwin, 1997; Saunders-Pullman et al., 1999).
Estradiol appears to act directly on striatal neurons, with observable changes found in various preparations within seconds of hormone administration. Furthermore, membrane impermeable analogs of estradiol are effective in mimicking the actions of the hormone (Mermelstein et al., 1996; Xiao and Becker, 1998). Within striatum, Becker and colleagues recently demonstrated that activation of classical estrogen receptors localized to the neuronal membrane affected locomotor control and GABA release (Schultz et al., 2009). Thus, similar to what has been hypothesized in other brain regions (Vasudevan and Pfaff, 2007), the actions of estradiol within the striatum are presumed to be due to membrane-localized estrogen receptors. The mechanism by which these estrogen receptors affect cell function has yet to be identified.
In female hippocampal neurons, we have recently characterized two distinct estrogen-sensitive signaling pathways that also rely upon membrane localized estrogen receptors (ERs). The first pathway involves estrogen receptor α (ERα) activation of mGluR1a, leading to mitogen-activated protein kinase (MAPK)-dependent CREB phosphorylation. The second pathway involves ERα and estrogen receptor β (ERβ) activation of mGluR2, resulting in an inhibition of L-type calcium channel currents, and a resulting decrement of L-type calcium channel-dependent CREB phosphorylation (Boulware et al., 2005). The two pathways are functionally segregated within neurons by different caveolin-comprised caveolae (Luoma et al., 2008). The first pathway relies upon caveolin-1 (CAV1) clustering of ERα to mGluR1a. The second pathway involves caveolin-3 (CAV3)-dependent clustering of ERα/ERβ to mGluR2 (Boulware et al., 2007). While initially characterized in hippocampal neurons, ER/mGluR interactions have been implicated throughout the nervous system. Examples include regulation of hypothalamic function, influencing sexual receptivity and progesterone synthesis within glia, as well as altering spinal cord neurotransmission (Chaban et al., 2007; Dewing et al., 2007; Kuo et al., 2009).
The functional coupling of ERs to mGluRs potentially provides a unifying mechanism for the many actions of estradiol on striatal physiology and behavior, as activation of mGluRs will have many effects upon neuronal function both dependent and independent of CREB (Wang et al., 2004; Micevych and Mermelstein, 2009). With this in mind, we sought to determine whether in striatal neurons, membrane-localized ERs are also functionally coupled to mGluRs. Conceptually, we found several parallels in estrogen signaling between striatal and hippocampal neurons. However, the specifics of estrogen activation of intracellular signaling are in fact unique. Striatal neurons utilize different mGluRs than the other brain regions previously characterized. Specifically, estradiol administration led to activation of mGluR5 and mGluR3, although striatal neurons do express both mGluR1a and mGluR2. And thus while these results further demonstrate that membrane ERs can affect G protein-coupled receptor (GPCR) signaling across different brain regions, the interactions between ERs and mGluRs are more complex than previously realized.
EXPERIMENTAL PROCEDURES
Cell culture
Striatal neurons were cultured from female 1-2 day old rat pups as previously described (Mermelstein et al., 2000), using a protocol approved by the Animal Care and Use Committee at the University of Minnesota. Chemicals were purchased from Sigma (St. Louis, MO) unless stated otherwise. Following decapitation, the striatum of 4-6 animals were isolated following removal in ice-cold modified Hank’s Balanced Salt Solution containing 20% fetal bovine serum (FBS; Hyclone, Logan, UT), and (in mM): 4.2 NaHCO3, and 1 HEPES, pH 7.35, 300 mOsm. The tissue was then washed and digested for 5 min in a Trypsin solution (Type XI; 10 mg/ml) containing (in mM) 137 NaCl, 5 KCl, 7 Na2HPO4, 25 HEPES, and DNase (1500U), pH 7.2, 300 mOsm. Following additional washes, tissue was dissociated and pelleted twice by centrifugation (180g for 10 min) to remove contaminants. Cells were then plated (~1×105 viable cells/well) onto 10 mm glass coverslips (treated with Matrigel to promote adherence; BD Biosciences, San Jose, CA), and incubated for 15 min at room temperature. Two milliliters of Minimum Essential Media (MEM; Invitrogen, Carlsbad, CA) containing (in mM): 28 glucose, 2.4 NaHCO3, 0.0013 transferrin (Calbiochem, La Jolla, CA), 2 glutamine, 0.0042 insulin with 1% B-27 supplement (Invitrogen) and 10% FBS, pH 7.35, 300 mOsm, were added to each coverslip. In order to inhibit glial growth, 1 ml of media was replaced with a solution containing 4μM cytosine 1-β-D-arabinofuranoside and 5% FBS twenty-four hours after plating. Seventy-two hours later, 1 ml of media was replaced with modified MEM solution containing 5% FBS. Gentamicin (2μg/ml; Invitrogen) was added to all media solutions to eliminate bacterial growth.
Drugs
The drugs utilized were as follows: 17β-estradiol (17βE, 1nM); (RS)-3,5- 4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT, 1nM; Tocris); 2,3-bis(4-Hydroxyphenyl)-propionitrile (DPN, 10nM; Tocris); 2-Methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP, 5μM; Tocris). 7a,17b-[9[4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol (ICI 182,780) (100 nM, Tocris Bioscience, Ellisville, MO), 17β-estradiol, 17-hemisuccinate-BSA (EBSA) (1 nM, Steraloids, Newport, RI), (1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U0126) (10 μM, Calbiochem, San Diego, CA), (S)-(+)-a-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385) (100 μM, Tocris Bioscience), 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) (5 μM, Tocris Bioscience), (RS)-2-chloro-5-hydroxyphenylglycine (CHPG) (1 mM, Tocris Bioscience), (RS)-3,5-dihydroxyphenylglycine (DHPG) (50 μM, Tocris Bioscience), (2S)-2-amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid (LY341495) (100 nM, Tocris Bioscience). EBSA was filtered to remove free estrogens as described previously (Boulware and Mermelstein, 2009).
Immunocytochemistry
The immunocytochemistry protocols followed those previously described (Mermelstein et al., 2001; Boulware et al., 2005). For experiments utilizing DNA vector expression, cells were transfected the previous day using a standard calcium-phosphate method (Deisseroth et al., 1998; Weick et al., 2003). Briefly, cultured striatal neurons (9 d.i.v.) were incubated in a Tyrode’s solution at RT for 2 hr. Cell stimulations (and drug exposure durations before fixation) were carried out as follows: vehicle (5 min); estradiol (5 min); 20mM K+ (3 min); estradiol and 20mM K+ (5 min estradiol alone followed by 3 min estradiol in 20 mM K+). When utilizing mGluR agonists, similar time courses were followed and all inhibitors/antagonists were applied 30 min prior to stimulation.
Cells were fixed for 20 min following stimulation using ice-cold 4% paraformaldehyde (Electron Microscopy Sciences, Ft. Washington, PA) in PBS containing 4 mM EGTA. Following three PBS washes, cells were permeabilized via a 5 min incubation in a 0.1% Triton X-100 (VWR Scientific, West Chester, PA) solution. After three more washes, cells were blocked at 37°C for 30 min in PBS containing 1% BSA and 2% goat serum (Jackson ImmunoResearch, West Grove, PA). Cells were then incubated for 1 h at 37°C in a block solution containing a monoclonal antibody directed against serine 133 phosphorylated CREB (pCREB, clone 634-2, 1:1000, Millipore, Billerica, MA) and, to identify individual cell morphology, a polyclonal antibody against microtubule-associated protein 2 (MAP2, 1:1000 Calbiochem). After the 1 h incubation, cells were washed three times with PBS and incubated for 1 h at 37°C in a block solution containing FITC- and either CY5- or rhodamine RedX-conjugated secondary antibodies for visualization of MAP2 and pCREB respectively (Jackson ImmunoResearch). After washing excess secondary antibody, cells were mounted using the antiquenching and mounting medium Citifluor (Ted Pella, Redding, CA). Nuclear fluorescent intensities for pCREB (n~25 cells/group) were acquired using either a Yokogawa spinning-disc confocal system mounted to an Olympus IX-70 inverted microscope and attached to an 12-bit digital camera (Hamamatsu Photonics, Hamamatsu City, Japan), or a Leica TCS SPE point-scanning confocal utilizing 12-bit photomultipliers. Data acquired from the Yokogawa system were quantified using Metamorph (version 6.0) software (Universal Imaging, Downington, PA). Data acquired from the Leica system were quantified with the Leica Application Advanced Fluorescence Suite.
The confocal excitation and detection settings (i.e. laser intensity, image acquisition time, etc.) for each experiment were determined using coverslips stimulated with 20 mM K+. Inter-coverslip variability was accounted for by subjecting two coverslips to each treatment. Data was acquired from coverslips in a random order. On average, a single experimental group was comprised of data obtained from between 28 and 31neurons (absolute minimum across all experiments n=24; maximum n=36). For image acquisition, neurons were selected randomly using MAP2 fluorescence, allowing the experimenter to remain blind to pCREB intensities. Images were captured through the approximate midline of each cell. During data analysis, the MAP2 staining was utilized to draw a region of interest (ROI) outlining the nucleus of each neuron. The ROI was then transferred to the pCREB image and average fluorescence intensities within the nucleus were recorded. For all experiments images were background subtracted. Each experiment was performed at least 3 times to verify results.
siRNA Transfection and Real-time PCR
All siRNA reagents were obtained from Dharmacon (Lafayette, CO) unless stated otherwise. Cultured striatal neurons were co-transfected 7 d.i.v. with ON-TARGETplus SMARTpool siRNAs for either rat CAV1, CAV3, mGluR2, mGluR3 or control siRNAs against no known gene target, in addition to the siGLO transfection indicator. Transfection protocols followed the manufacturer’s instructions, except cells were incubated with 250 μl of the transfection mixture and incubated at 37°C for 5 hours. Following transfection, cells were washed once with DMEM (Gibco) before being placed back into their original media. Based on siGLO fluorescence, > 90% of the cultured cells took up the siRNA. To assay relative changes in mRNA abundance, half of the transfected cultures were processed 24 hours following transfection using quantitative PCR (qPCR). The remaining cultures were used in the immunocytochemistry experiments.
For qPCR, cDNA generation was first performed using standard methods. To stabilize mRNA, transfected cultured neurons (8 d.i.v.) were first placed in RNA later (Qiagen, Valencia, CA). RNA was then isolated using a standard kit (RNAeasy Mini kit, Qiagen) followed by the reverse transcription of the mRNA into cDNA (QuantiTect, Qiagen). The cDNA was amplified using the DyNAmo HS SYBR Green master mix (New England Biolabs, Ipswich, MA). All qPCR reactions were performed and analyzed using the DNA Engine Opticon 2 (Bio-Rad Laboratories, Hercules, CA) and standardized to β-actin. The critical cycle threshold was set at 25 standard deviations above baseline. PCR reactions for individual cDNA samples were performed in triplicate and overall experiments were repeated at least twice. The thermal cycling program included an initial denaturing step at 95°C for 15 min followed by 45 cycles consisting of a 10 sec denaturing step at 94°C, annealing for 30 sec at 60°C (56°C for CAV1) and extension for 30 sec at 72°C. Following each extension, fluorescent intensity was measured at 75°C.
The upper and lower primer sequences for CAV1 (GenBank accession number NM_031556) were 5′-GCA GTT GTA CCG TGC ATC AAG AG-3′ (nucleotides 385-407) and 5′-CGG ATA TTG CTG AAT ATC TTG CC-3′ (nucleotides 490-512), yielding a predicted product size of 127 bp. The primer sequences for CAV3 (GenBank accession number NM_019155) were 5′-GGA GAT AGA CTT GGT GAA CAG AGA-3′ (nucleotides 60-83) and 5′-CAG GGC CAG TGG AAC ACC-3′ (nucleotides 241-258), yielding a predicted product of size 198 bp. The primer sequences for mGluR2 (GenBank accession number NM_001105711) were 5′-GTG GTG ACA TTG CGC TGT AA-3′ (nucleotides 2146-2166) and 5′-GCG ATG AGG AGC ACA TTG TA-3′ (nucleotides 2199-2219), yielding a predicted product of size 73 bp. The primer sequences for mGluR3 (GenBank accession number NM_001105712) were 5′- GAC AGC AGC AAC TAT GAG C-3′ (nucleotides 1138-1157) and 5′- CAA TGC TGT CTG CTC CTT TAT-3′ (nucleotides 1339-1360), yielding a predicted product of size 222 bp. The primers used for β-actin were (GenBank accession number NM_03144) 5′-AGG CCC CTC TGA ACC CTA AG-3′ (nucleotides 120-139) and 5′-CCA GAG GCA TAC AGG GAC AAC-3′ (nucleotides 217-238), yielding a predicted product size of 118 bp. PCR products were sequenced for verification of product identity. For each experiment, siRNAs reduced the targeted mRNA abundance by at least 70%.
Immunohistochemistry
Experiments were performed on individually housed, ovariectomized rats, ~12 weeks of age. Ovariectomy occurred two weeks prior to the immunohistochemistry studies. Food and water were available ad libitum. Animals were maintained on a 14-h light, 10-h dark cycle with lights on at 0600 h and off at 1800 h. All protocols were approved by the Animal Care and Use Committee at the University of Minnesota.
In order to maintain accurate timings of hormone/drug treatments, each immunohistochemistry run (n=5 runs) was comprised of one animal/condition. To accustom the animals to experimenter handling and repeated injections, subjects were injected daily for two days with saline (0.3 ml, s.c.) prior to the day of experimentation. On the day of testing, all animals received three injections. The first injection contained MPEP (10mg/kg, i.p. in 0.1M PBS) or vehicle only. Twenty minutes later, animals were administered estradiol (10μg, s.c. in ethyl oleate) or vehicle. Fifteen minutes following the second injection, animals were sacrificed by administering pentobarbital (200 mg/kg, i.p.) and intracardially perfused with saline followed by 4% paraformaldehyde. To minimize blood coagulation, 10,000 U of Heparin (Henry Schein, Melville, NY) was injected into the left ventricle prior to perfusion). Brains were then removed, post-fixed one hour at 4°C and incubated in a 30% sucrose solution in 0.1M PBS for 4 days at 4°C. For each brain, eight, 40μm coronal sections through the striatum were collected using a freezing sliding microtome. Data was collected and analyzed from both hemispheres.
Immunohistochemistry methods used a free-floating, peroxidase-based technique using gentle agitation during all incubations. Sections were washed four times with 0.05M Tris, 0.9% NaCl pH 7.6 (TBS) and blocked for 30 min with 3% H2O2 and 10% methanol. Sections were washed an additional four times with TBS and incubated for 1 h in a block solution containing 10% normal horse serum in TBS with 0.3% Triton X-100 (TBS-TX). Sections were incubated for ~40 h at 4°C in a 2% normal horse serum, TBS-TX solution (pH 7.4) at 4°C containing a monoclonal antibody directed against serine 133 phosphorylated CREB (pCREB, clone 634-2, 1:1000, Millipore). After incubation, sections were washed four times in TBS-TX and then incubated for 90 min at room temp in a 2% horse serum, TBS-TX solution containing biotinylated anti-mouse IgG (H+L) (1:100, Vector Laboratories, Inc., Burlingame, CA). Sections were washed twice in TBS-TX and twice in TBS. Vectastain ABC Elite reagent, avidin-biotin-horseradish peroxidase complex, (Vector Laboratories, Inc.) was prepared according to manufacturer’s directions (1:50 A and 1:50 B in TBS). Sections were incubated in ABC Elite reagent for 1 h at room temp and washed four times with TBS. Labeling was visualized with a solution of 0.05% ammonium nickel sulfate, 0.00625% 3,3′diaminobenzidine tetrahydrochloride dihydrate, and 0.01275% H2O2 in TBS. Once stained nuclei were visible, sections were washed twice with TBS and mounted on ProbeOn Plus Slides (Fisher Scientific, Waltham, MA). Sections on slides were dehydrated with 70%, 95% and 100% ethanol, cleared in methyl salicylate, and mounted with DPX mounting media (Fluka, part of Sigma-Aldrich). Of note, the omission of primary antibody resulted in a complete absence of immunoreactivity.
Data acquisition was performed using equipment housed in the Biomedical Imaging Processing Laboratory Core (BIPL) at the University of Minnesota. Brightfield images were captured using a 20x/0.75 NA plan-apochromat objective on a Zeiss Axiovert 2 Upright Microscope coupled to a 12-bit color CCD SPOT camera (Diagnostic Instruments, Inc., Sterling Heights, MI). Images were taken from both sides of each brain section; 12-16 images were collected per brain region. Analysis of images was performed with ImageJ. Images were inverted, five ROIs spanning the striatum were drawn to measure background, and the mean gray value and standard deviation were calculated. Threshold was set at 10 standard deviations above background. The integrated density of all particles above that threshold was calculated for each image.
Statistics
Immunocytochemistry and immunohistochemistry experiments were analyzed using ANOVAs (F values) and Bonferroni’s Multiple Comparison post-hoc tests. Statistical differences between all treatment groups are depicted within each figure as different alphabetical characters. Probability values less than 0.05 were considered a priori as significant. Data are presented as mean±SEM.
RESULTS
Estradiol-Mediated CREB Phosphorylation in Striatal Neurons
While rapid estradiol-induced CREB phosphorylation has been described in various rodent brain regions, one notable exception is the female rat striatum. Through activation of membrane-localized estrogen receptors, the female rat striatum has been shown to exhibit various other responses following direct estradiol administration. If these membrane estrogen receptors were also functionally coupled to CREB signaling, we would be able to use our immunocytochemistry technique to identify the signaling pathway(s) through which membrane estrogen receptors signal in this brain region. As a direct test of this hypothesis, we find that a five minute exposure to estradiol (1nM) regulates CREB phosphorylation in cultured female striatal neurons (Figure 1A,B). Striatal cultures generated from male rats were unresponsive to estradiol at various concentrations, when tested up to 100 times the effective concentration observed in female-derived cultures (data not shown).
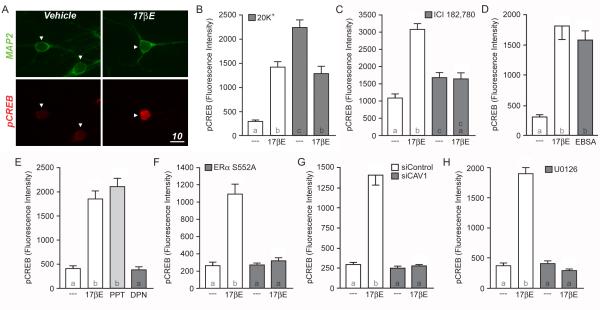
Figure 1.
Estradiol-induced CREB phosphorylation in female striatal neurons. (A) Confocal images of cultured neurons immunolabeled with Microtubule Associated Protein 2 (MAP2; green) and CREB, phosphorylated at serine 133 (pCREB; red). In comparison to vehicle controls, striatal neurons treated with estradiol (17βE; 1 nM) for five minutes exhibit increased staining for pCREB (scale bar, 10 μm). Arrowheads indicate nuclei of neurons. (B) Quantification of pCREB immunolabeling following estradiol and/or 20 mM K+ stimulation (F = 38.65; letters within the bars indicate statistically different groups). While estradiol alone increased CREB phosphorylation (open bars), estradiol attenuated the rise in CREB phosphorylation mediated by a three-minute depolarization with 20 mM K+ (filled bars). (C) Pretreatment of striatal cultures with the estrogen receptor antagonist, ICI 182,780 (ICI; 100 nM), eliminated estradiol-induced CREB phosphorylation (F = 31.26). (D) The membrane-impermeable estrogen analog, estradiol conjugated to bovine serum albumen (EBSA) mimicked the actions of estradiol (F = 29.46). (E) Activation of ERα with the receptor agonist PPT (1 nM) mimicked the actions of estradiol, whereas the ERβ-specific agonist DPN (10 nM) was without effect (F = 60.08). (F-H) The actions of estradiol on CREB phosphorylation could be blocked by inhibiting the trafficking of ERα to the surface membrane via overexpression of EGFP-S522A (F; F = 41.28), disruption of caveolin-1 (CAV1) expression by means of siRNA knockdown (G; F = 41.28) or inhibition of MAPK signaling by pretreating the cultures with the MEK inhibitor U0126 (10 μM) (H; F = 159.9).
Estradiol in the absence of any additional stimulation will increase CREB phosphorylation. However, estradiol can also attenuate L-type calcium channel-mediated CREB phosphorylation. L-type calcium channel-mediated CREB phosphorylation can be induced within our culture preparation by using a three minute 20mM K+ depolarization (for details regarding the privileged role of L-type calcium channels in signaling to CREB see (Mermelstein et al., 2000; Weick et al., 2003; Zhang et al., 2005)). As shown in Figure 1B, estradiol not only increases CREB phosphorylation in striatal neurons when applied alone (open bars), but will also attenuate L-type calcium channel-mediated CREB phosphorylation when administered during a 20mM K+ depolarization (filled bars). Of note, for the remainder of these studies, the bidirectional responses to estradiol were examined separately, with the first series of experiments focusing on estradiol-induced CREB activation (Figures 1-3), followed by estradiol attenuation of L-type calcium channel-mediated CREB phosphorylation (Figure 4).
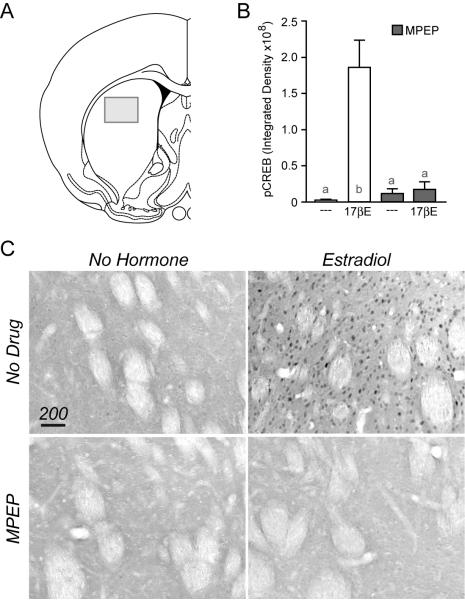
Figure 3.
Estradiol induces CREB phosphorylation via mGluR5 in rat striatum. (A) Illustration depicting the approximate location within the striatum in which pCREB staining was quantified. (B) CREB phosphorylation was significantly increased 15 minutes following an injection (s.c.) of estradiol when compared to vehicle controls. This effect of estradiol was blocked by the mGluR5 antagonist MPEP, injected (i.p.) 20 min prior to hormone exposure (F = 9.37). (C) Example immunohistochemical images of pCREB in striatal tissue.
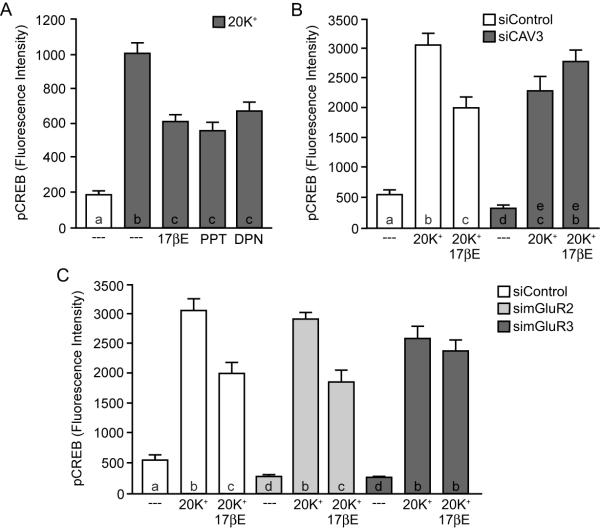
Figure 4.
Estradiol attenuation of L-type calcium channel-dependent CREB phosphorylation in striatal neurons is dependent on mGluR3. (A) Activation of either ERα or ERβ with PPT or DPN mimicked the actions of estradiol on attenuating L-type calcium channel-dependent CREB phosphorylation (F = 43.69). (B) Knockdown of caveolin-3 (CAV3) expression eliminated estradiol regulation of L-type calcium channel signaling (F = 49.86). (C) In striatal neurons, disruption of mGluR3, but not mGluR2 expression blocked the actions of estradiol on L-type calcium channel-mediated CREB phosphorylation (F = 54.93).
The rapid effects of estradiol that impact striatal physiology have been attributed to activation of membrane-localized estrogen receptors (Schultz et al., 2009). The next series of experiments support this hypothesis. The estrogen receptor antagonist ICI 182,780 (100nM) was found to block estradiol-induced CREB phosphorylation (Figure 1C). Of note, ICI 182,780 raised baseline CREB phosphorylation, albeit not nearly to the extent of estradiol (the underlying mechanism for this finding is unknown). The membrane-impermeable estrogen analog, estrogen conjugated to bovine serum albumin (EBSA; filtered to remove any potential free estrogens) mimicked the effect of estradiol on CREB activation (Figure 1D). In addition, through use of estrogen receptor specific agonists, estradiol-mediated CREB phosphorylation was attributed to activation of ERα, as PPT (1nM) induced CREB phosphorylation, whereas the ERβ agonist DPN (10nM) was without effect (Figure 1E). To verify membrane-localized ERα was responsible for estradiol-induced CREB phosphorylation, striatal neurons were transfected with DNA encoding for either EGFP or EGFP tethered to ERα containing a single serine to alanine point mutation at residue 552 (ERα S552A). Previous work has shown that this point mutation blocks the trafficking of all ERα to the surface membrane (Razandi et al., 2002; Boulware et al., 2007). In neurons transfected with ERα S552A, estradiol-induced CREB phosphorylation was absent (Figure 1F, filled bars) while the control EGFP transfected neurons exhibited typical estrogen responsiveness (open bars).
In addition to receptor trafficking to the surface membrane, ERα-mediated CREB phosphorylation is also dependent on the functional expression of the scaffolding protein caveolin-1 (CAV1). Introduction of small-interfering RNAs (siRNAs) targeted against CAV1 eliminated estradiol-induced CREB phosphorylation in striatal neurons (Figure 1G). The control siRNAs (siControl) was without effect. Additionally, estradiol-induced CREB phosphorylation was dependent upon activation of MAPK signaling, as inhibition of MEK by U0126 (10μM) eliminated the response (Figure 1H).
ERα Acts through mGluR5 to induce Striatal CREB Phosphorylation
In other nervous tissue, the key step by which membrane ERα signals to the nucleus to affect CREB phosphorylation is through interactions with metabotropic glutamate receptors (mGluRs). In hippocampal and arcuate neurons, estradiol through ERα activates mGluR1a signaling, leading to MAPK-dependent CREB phosphorylation (Boulware et al., 2005; Dewing et al., 2007). This response is blocked by mGluR1a antagonists. Thus, it was surprising to find that application of the mGluR1a antagonist LY367385 (100μM) failed to inhibit estradiol-induced CREB phosphorylation in striatal neurons (Figure 2A). These data required us to reevaluate our hypothesis of estrogen action. Because of the numerous parallels between estradiol-mediated CREB phosphorylation in striatal and hippocampal neurons, we hypothesized that two distinct, but related mechanisms were responsible when making comparisons across these brain regions. There are two group I mGluRs, mGluR1a and mGluR5, both of which are coupled to Gq signaling and are capable of activating MAPK. We therefore tested the hypothesis that in striatal neurons, ERα acts through mGluR5, rather than mGluR1a, to stimulate CREB phosphorylation.
Figure 2.
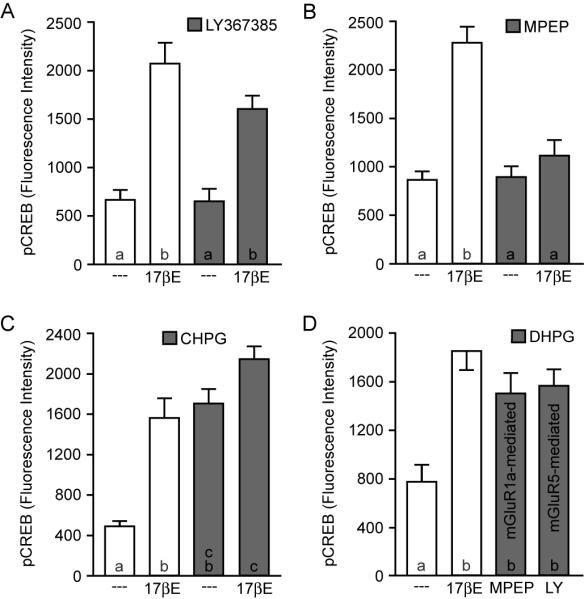
Estradiol-mediated CREB phosphorylation occurs via selective activation of mGluR5. (A-B) In contrast to cells from hippocampus and other brain regions, estradiol-induced CREB phosphorylation in striatal neurons is not blocked by the mGluR1a antagonist LY367385 (100 μM) (F = 21.56). Rather, estradiol-mediated CREB phosphorylation in striatal neurons is eliminated by the mGluR5 antagonist MPEP (5 μM) (F = 24.67). (C) The mGluR5 agonist CHPG (1 mM) increased CREB phosphorylation similar to estradiol, and occluded the effect of the steroid (F = 24.93). (D) Application of the pan-specific group I mGluR agonist DHPG (50 μM) in the presence of either a mGluR5 (MPEP) or a mGluR1a (LY367385) specific antagonist resulted in an increase in CREB phosphorylation, suggesting that while ERα activates mGluR5 in striatal neurons, both group I mGluRs are functionally linked to CREB signaling in this system.
Consistent with this revised hypothesis, in neurons treated with the mGluR5 antagonist MPEP (5μM), estradiol failed to induce CREB phosphorylation (Figure 2B). Furthermore, the mGluR5 agonist CHPG (1mM) mimicked the actions of estradiol. Application of CHPG and estradiol together did not significantly increase CREB phosphorylation in comparison to CHPG alone (Figure 2C). These data suggest that in striatal neurons, ERα acts through mGluR5 to elicit CREB responsiveness. While it was initially surprising to find estradiol-mediated CREB phosphorylation to be dependent upon activation of mGluR5 in striatal neurons when in other brain regions the effect was found to be mediated by mGluR1a, one possible explanation could be that striatal neurons do not express mGluR1a that are functionally coupled to CREB. To test this, striatal neurons were treated with the pan-specific group I agonist DHPG (50μM) in the presence of either an mGluR5 or mGluR1a antagonist. As shown in Figure 2D, activation of either mGluR1a or mGluR5 alone resulted in a significant increase in CREB phosphorylation, thus rejecting the notion that mGluR1a signaling to CREB is absent in striatal neurons. The mechanism regarding the pairing of ERα with specific group I mGluRs between various brain regions, and the physiological relevance of this selection process remain unknown (see Discussion).
The primary culture system has proved invaluable for isolating multiple estrogen-regulated signaling pathways. Our next experiment was designed to verify that the striatal data generated in vitro is in fact representative of in vivo signaling. Ovariectomized rats were given an acute injection of estradiol (10 μg s.c.) 15 min before their brains were collected for immunohistochemistry. Preserved brains were processed for the detection of phosphorylated CREB. Estradiol was found to significantly increase CREB phosphorylation within the striatum in comparison to vehicle controls (Figure 3). Furthermore, antagonism of mGluR5 with MPEP (10 mg/kg i.p., given 20 min prior to estradiol) blocked the actions of the hormone. MPEP alone was without effect. These data are consistent with the immunocytochemistry experiments, indicating estradiol-dependent CREB phosphorylation in female striatal neurons is dependent on activation of mGluR5.
ERα/β Act through mGluR3 to reduce L-type Calcium Channel-mediated CREB Phosphorylation in Striatal Neurons
Upon finding that estradiol-mediated CREB phosphorylation in female striatal neurons is dependent on activation of mGluR5, we next sought to outline the signaling mechanism by which estradiol attenuated L-type calcium channel signaling in this brain region. In striatal neurons, estradiol attenuation of L-type calcium channel-mediated CREB phosphorylation could be mimicked through activation of either ERα (with PPT) or ERβ (with DPN) (Figure 4A). Additionally, ERα/ERβ mediated attenuation of L-type calcium channel-dependent CREB phosphorylation required the expression of caveolin-3 (CAV3) (Figure 4B). Interestingly, siRNA knockdown of mGluR3 (but not mGluR2) eliminated the effect of estradiol on L-type calcium channel-mediated CREB phosphorylation (Figure 4C). This is in contrast to hippocampal neurons, where estradiol attenuation of L-type calcium channel signaling is dependent on mGluR2.
DISCUSSION
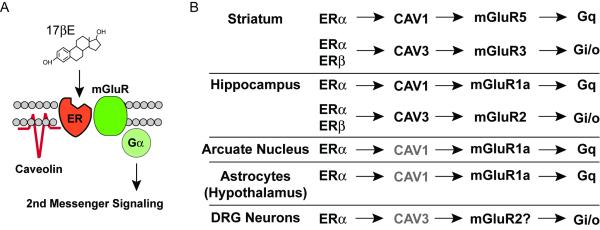
In striatal neurons, estradiol-mediated CREB phosphorylation occurs via ERα activation of mGluR5. In addition, membrane-localized ERα and ERβ activate mGluR3 to attenuate L-type calcium-channel dependent CREB signaling. ER activation of mGluR5 and mGluR3 is dependent upon functional expression of CAV1 and CAV3, respectively (Figure 5). These data provide an underlying framework to understand how estradiol acts within striatal neurons. In addition, striatal neurons display a heretofore unique pairing of ERs with mGluRs not observed in other brain regions (i.e. hippocampus, arcuate nucleus, hypothalamic astrocytes and DRG neurons). The additional complexity regarding ER/mGluR pairing cannot be attributed to differential expression of mGluRs between neuronal subpopulations, but most likely has functional connotations not yet fully appreciated.
Figure 5.
Brain region specificity in estradiol-induced activation of mGluRs. (A) General depiction of the proposed mechanism by which caveolin proteins act to functionally isolate distinct estrogen receptors and mGluRs, leading to activation of specific second messenger signaling cascades. (B) In striatal neurons, ERα is functionally coupled to mGluR5 via CAV1. In addition, a separate pool of ERα and ERβ are coupled to mGluR3 via expression of CAV3. This is in contrast to neurons of the hippocampus, arcuate nucleus and DRG, as well as astrocytes of the hypothalamus in which estrogen receptors appear principally coupled to mGluR1a and/or mGluR2. Grayed text indicates preparations in which the caveolin protein responsible for ER coupling to mGluRs was not determined.
From a historical perspective, estrogen acting within the striatum had originally been met with great skepticism. Original steroid binding studies led to the conclusion that there were little to no ERs in the rat striatum (Pfaff, 1973; Stumpf and Sar, 1976). Furthermore, the reported effects of estradiol acting within this brain region could not be reconciled with a traditional action of steroid hormone receptors (i.e. binding to DNA to regulate transcription). However, the recent realization that ERα and ERβ can be trafficked to the surface membrane to regulate second messenger signaling (Levin, 1999; Micevych and Dominguez, 2009), along with the detection of striatal ERs through the use of more sensitive and sophisticated detection techniques (Kuppers and Beyer, 1999; Mitra et al., 2003), have provided a model framework in which to understand estrogen action within this brain region. What is unique about this brain region though, is the relative distribution of ER protein between the cytosol/nucleus and surface membrane. In other cell types studied, it has been estimated that the vast majority of ER is nuclear, with only a small fraction trafficked to the surface. In contrast, ERs in the striatum appear to be principally localized to the membrane surface (Schultz et al., 2009). Thus, the striatum is uniquely poised to be affected by estradiol almost exclusively through membrane-initiated events.
The membrane-initiated effects of estradiol upon neuronal function have been well studied. Although there are multiple mechanisms by which estradiol is thought to induce these effects, our work has focused mainly on membrane ER activation of mGluRs. To date, studies in our lab and others have demonstrated that ER coupling to mGluR is relevant in various cells within the nervous system, including the hippocampus, hypothalamus and dorsal root ganglia. Rapid actions of estradiol in these regions play a role in regulation of activity-dependent transcription factors, progesterone synthesis in astrocytes and regulation of ATP-induced calcium release, (Boulware et al., 2005 ; Chaban et al., 2007; Kuo et al., 2009). These widespread effects suggest that estradiol activation of mGluR signaling could be involved in a diverse set of behaviors, including learning and memory, sexual receptivity, and nociception (Micevych and Mermelstein, 2008). In support of this hypothesis, ER/mGluR signaling has already been shown essential for the full display of rat lordosis (Dewing et al., 2007). That said, further studies are needed to elucidate the mechanism by which ERs activate mGluRs. However, ERs do not activate mGluRs through alterations in glutamatergic transmission, as ER/mGluR signaling is still observed following inhibition of neurotransmission, removal of exogenous glutamate, and even in preparations lacking neurotransmission (Boulware et al., 2005; Kuo et al., 2009). Notably, our striatal cultures lack glutamatergic neurotransmission, providing additional evidence of an alternative method by which ERs activate mGluRs. We hypothesize a transactivation mechanism by which ERs activate mGluRs following estradiol stimulation via a direct protein-protein interaction (Dewing et al., 2007).
In the rat striatum, estradiol has been shown to influence dopamine release, dopamine turnover, GABA neurotransmission and calcium channel activity (Di Paolo et al., 1985; Becker, 1990; Mermelstein et al., 1996; Xiao and Becker, 1998; Chaban et al., 2003; Xiao et al., 2003; Hu et al., 2006). Yet, the mechanism(s) by which estradiol affects striatal function has remained a mystery for over 25 years (Van Hartesveldt and Joyce, 1986). Interestingly, activation of mGluR signaling in striatum is thought to be involved in many of these same processes as estrogen (Luscher and Huber; Olive, 2009). Thus, the identification of ER/mGluR signaling in this brain region may provide a single unifying mechanism for what were previously thought to be distinct phenomena. That is, ER activation of mGluR signaling in the striatum can potentially account for the numerous actions this steroid hormone has been demonstrated to have in this brain region.
Measuring alterations in CREB phosphorylation following ER activation has been a useful assay in which to delineate the intracellular signaling pathways affected by membrane estrogen receptors. That said, this methodology is unable to identify the specifics of the ER/mGluR interaction. One question that remains unanswered is whether all, or just a subset of mGluRs, are functionally linked to ERs. This is partially because we do not know the percentage of mGluRs that are required for maximal signaling to CREB. The intrinsic amplification process of second messenger signaling is just one limiting factor. Additionally, the spatial localization of mGluRs within a single neuron will affect its physiological impact, such as its ability to signal to CREB. Hence targeting of ERs to specific mGluRs (if in fact only a subpopulation of mGluRs were linked to ERs) would alter the biological function of estrogens.
Another unanswered question relates to the exact positioning of ERs in/associated with the membrane. Lacking motifs of traditional transmembrane proteins, ERs have sometimes been portrayed as interacting with cell surface receptors when associated with the cytosolic side of the membrane. However, this position cannot account for the findings that (1) membrane impermeable estrogen analogs activate these receptors (e.g. (Vasudevan et al., 2005)), (2) surface protein labeling techniques are able to tag membrane ERs (e.g. (Gorosito et al., 2008; Bondar et al., 2009)), and (3) ER antibodies can identify membrane ERs in non permeabilized cells (e.g. (Pappas et al., 1995)). Thus, the ER is often depicted with part of the protein exposed to the extracellular surface (e.g. (Vasudevan and Pfaff, 2007; Levin, 2009)). That said, future work will be needed to determine the exact means by which membrane-localized ERs functionally interact with surface proteins.
Divergent functions in neurons are often localized to discrete functional regions. Subcellular compartmentalization of various signaling and effector proteins has been best demonstrated in transsynaptic signaling. For example, PDZ domain-containing scaffolding proteins have been studied in great detail, elucidating their significance in the localization and clustering of proper neurotransmitter receptors, downstream second messengers, and cytoskeletal proteins (Hata et al., 1998; Augustine et al., 2003). In the both the hippocampus and striatum, we have demonstrated the separation of Gq-coupled group I mGluRs and Gi/o-coupled group II mGluRs, imparted by their localization within distinct caveolae. These results imply the distinct localization of ERα and ERα/ERβ to CAV1 and CAV3 containing caveolae, respectively. This raises the question whether there is a functional advantage of having different estrogen receptors localized to discrete signaling complexes. Of potential relevance are the findings that estrogens can be synthesized and released in several brain regions (Wehrenberg et al., 2001; Rune and Frotscher, 2005; Mukai et al., 2006b; Mukai et al., 2006a; Zhou et al., 2007). Based upon these data, it has been suggested that estrogens may play physiological roles similar to neurotransmitters (Balthazart and Ball, 2006). Thus, whereas ovarian estradiol reaching the brain would most likely activate both mGluR signaling pathways simultaneously, locally synthesized estradiol could potentially activate one pathway over another. This could potentially parallel glutamate neurotransmission, such that either locally released estradiol or glutamate would result in the activation of same downstream signaling pathway. Increased temporal and spatial control over estradiol-sensitive signaling by the synthesis and release of the steroid in brain is an exciting possibility that deserves future study.
The molecular events regulated by striatal estrogen signaling translate to measurable changes in behavior. As an example, intrastriatal administration of estradiol to ovariectomized female rats results in enhanced sensorimotor performance (Becker et al., 1987). These findings are not limited to animal studies, as estrogens also appear to enhance sensorimotor control in women various striatal processes in women (Hampson and Kimura, 1988; Hampson, 1990). In addition, sex differences have been reported in Parkinson’s disease. Incidence and prevalence are lower in women, while the age of onset is later (Haaxma et al., 2007). Once the disease has progressed to the clinical phase, women score higher on motor tests (Saunders-Pullman et al., 1999). In postmenopausal women, estrogen treatment lowers the severity of symptoms and improves some memory impairment (Mayeux et al., 1992; Sherwin, 1997; Saunders-Pullman et al., 1999).
One of the most interesting aspects of these novel actions of estrogens is the apparent sex difference across multiple brain regions, even though expression of ERs is not particularly sexually dimorphic (McEwen, 2001). In fact, we see no sex difference in ERα or ERβ expression between male- and female-derived cultures (data not shown). Thus, based on these data, we hypothesize that male- derived neurons lack estrogen signaling to CREB, not due to a deficiency in ER expression, but rather the lack of estrogen receptor trafficking to the membrane in these cells.
An important aspect of our studies in characterizing estrogen action in striatum is the verification of culture data within the intact brain. The primary culture system provides exquisite control of the preparation, allowing for the clear identification of signaling pathways. Using cultured hippocampal neurons we first delineated ERα activation of mGluR1. The physiological significance of this finding came when mGluR1 antagonism in the arcuate nucleus was found to block estradiol-mediated μ-opioid receptor internalization in the medial preoptic nucleus and attenuate rat lordosis behavior (Dewing et al., 2007). Upon finding ERα activates mGluR5 in striatal cultures, we turned to immunohistochemistry techniques to demonstrate its potential significance in vivo (see Figure 3). While no means definitive, finding that antagonism of mGluR5 leads to an inhibition of estradiol-induced CREB phosphorylation in striatum strongly corroborates the in vitro studies. The lack of specific group II mGluR antagonists has limited our ability to verify our culture data in vivo, but multiple preparations have observed estradiol inhibition of L-type calcium channels (Mermelstein et al., 1996; Chaban et al., 2003) that have been attributed to activation of group II mGluRs (Boulware et al., 2005; Chaban et al., 2007).
Our observation of brain region specificity in ER/mGluR pairing suggests increased diversity regarding the mechanism of membrane estrogen action. Membrane ERs have also been shown to transactivate various receptor tyrosine kinases (Singh et al., 1999). In addition, novel membrane estrogen receptors have been characterized outside of the striatum, capable of triggering intracellular signaling events without coupling to a partner. This category of novel estrogen receptor includes estrogen acting at the membrane receptor known as ER-X (Toran-Allerand et al., 2002) and activation of an estradiol- and STX-sensitive GPCR in hypothalamus (Qiu et al., 2008). It is readily apparent that over the last ten years, our knowledge regarding estrogen action inside and outside of the nervous system has greatly expanded.
CONCLUSIONS
In sum, we have found that membrane estrogen receptors can pair with multiple mGluRs across various regions of the brain. Thus, in the future, the test for ER/mGluR interactions within the nervous system will also require a more expansive use of receptor antagonists than previously believed. Identifying which mGluRs are functionally coupled to ERs in a variety of cells will be critically important in understanding estrogen action in brain, and possibly to differentially influence various estradiol-sensitive processes.
Acknowledgements
This work was supported by NIH grant NS41302 to PGM, and training grants MH073129 to DG-S and DA07234 to MIB. The authors would like to thank Dr. E. Levin for EGFP-S522A ERα.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–249. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Becker JB. Direct effect of 17 beta-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- Becker JB, Snyder PJ, Miller MM, Westgate SA, Jenuwine MJ. The influence of estrous cycle and intrastriatal estradiol on sensorimotor performance in the female rat. Pharmacol Biochem Behav. 1987;27:53–59. doi: 10.1016/0091-3057(87)90476-x. [DOI] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Mermelstein PG. Membrane estrogen receptors activate metabotropic glutamate receptors to influence nervous system physiology. Steroids. 2009;74:608–613. doi: 10.1016/j.steroids.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- Chaban VV, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates ATP-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat DRG neurons. Society for Neuroscience; San Diego, CA: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T, Rouillard C, Bedard P. 17 beta-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E. Estrogen-related variations in human spatial and articulatory-motor skills. Psychoneuroendocrinology. 1990;15:97–111. doi: 10.1016/0306-4530(90)90018-5. [DOI] [PubMed] [Google Scholar]
- Hampson E, Kimura D. Reciprocal effects of hormonal fluctuations on human motor and perceptual-spatial skills. Behav Neurosci. 1988;102:456–459. doi: 10.1037//0735-7044.102.3.456. [DOI] [PubMed] [Google Scholar]
- Hata Y, Nakanishi H, Takai Y. Synaptic PDZ domain-containing proteins. Neurosci Res. 1998;32:1–7. doi: 10.1016/s0168-0102(98)00069-8. [DOI] [PubMed] [Google Scholar]
- Hu M, Watson CJ, Kennedy RT, Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009;150:1369–1376. doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers E, Beyer C. Expression of estrogen receptor-alpha and beta mRNA in the developing and adult mouse striatum. Neurosci Lett. 1999;276:95–98. doi: 10.1016/s0304-3940(99)00815-0. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cellular Functions of the Plasma Membrane Estrogen Receptor. Trends Endocrinol Metab. 1999;10:374–377. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Levin ER. Plasma membrane estrogen receptors. Trends Endocrinol Metab. 2009;20:477–482. doi: 10.1016/j.tem.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoma JI, Boulware MI, Mermelstein PG. Caveolin proteins and estrogen signaling in the brain. Mol Cell Endocrinol. 2008;290:8–13. doi: 10.1016/j.mce.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Denaro J, Hemenegildo N, Marder K, Tang MX, Cote LJ, Stern Y. A population-based investigation of Parkinson’s disease with and without dementia. Relationship to age and gender. Arch Neurol. 1992;49:492–497. doi: 10.1001/archneur.1992.00530290076015. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micevych PE, Mermelstein PG. Membrane estrogen receptors acting in the central nervous system. Immun, Endoc & Metab Agents in Med Chem. 2009;9:180–190. [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Ogiue-Ikeda M, Murakami G, Hojo Y, Ishii H, Kimoto T, Kawato S. Local neurosteroid production in the hippocampus: influence on synaptic plasticity of memory. Neuroendocrinology. 2006a;84:255–263. doi: 10.1159/000097747. [DOI] [PubMed] [Google Scholar]
- Mukai H, Takata N, Ishii HT, Tanabe N, Hojo Y, Furukawa A, Kimoto T, Kawato S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: synaptocrinology. Neuroscience. 2006b;138:757–764. doi: 10.1016/j.neuroscience.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–989. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. Faseb J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. Journal of Comparative Neurology. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Qiu J, Ronnekleiv OK, Kelly MJ. Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids. 2008;73:985–991. doi: 10.1016/j.steroids.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol. 2002;16:100–115. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- Rune GM, Frotscher M. Neurosteroid synthesis in the hippocampus: role in synaptic plasticity. Neuroscience. 2005;136:833–842. doi: 10.1016/j.neuroscience.2005.03.056. [DOI] [PubMed] [Google Scholar]
- Saunders-Pullman R, Gordon-Elliott J, Parides M, Fahn S, Saunders HR, Bressman S. The effect of estrogen replacement on early Parkinson’s disease. Neurology. 1999;52:1417–1421. doi: 10.1212/wnl.52.7.1417. [DOI] [PubMed] [Google Scholar]
- Schultz KN, von Esenwein SA, Hu M, Bennett AL, Kennedy RT, Musatov S, Toran-Allerand CD, Kaplitt MG, Young LJ, Becker JB. Viral vector-mediated overexpression of estrogen receptor-alpha in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29:1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen effects on cognition in menopausal women. Neurology. 1997;48:S21–26. doi: 10.1212/wnl.48.5_suppl_7.21s. [DOI] [PubMed] [Google Scholar]
- Singh M, Setalo G, Jr., Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf WE, Sar M. Steroid hormone target sites in the brain: the differential distribution of estrogin, progestin, androgen and glucocorticosteroid. J Steroid Biochem. 1976;7:1163–1170. doi: 10.1016/0022-4731(76)90050-9. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr., Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hartesveldt C, Joyce JN. Effects of estrogen on the basal ganglia. Neurosci Biobehav Rev. 1986;10:1–14. doi: 10.1016/0149-7634(86)90029-1. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Pfaff DW. Membrane-initiated actions of estrogens in neuroendocrinology: emerging principles. Endocr Rev. 2007;28:1–19. doi: 10.1210/er.2005-0021. [DOI] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff D. Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids. 2005;70:388–396. doi: 10.1016/j.steroids.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Tang Q, Parelkar NK, Liu Z, Samdani S, Choe ES, Yang L, Mao L. Glutamate signaling to Ras-MAPK in striatal neurons: mechanisms for inducible gene expression and plasticity. Mol Neurobiol. 2004;29:1–14. doi: 10.1385/MN:29:1:01. [DOI] [PubMed] [Google Scholar]
- Wehrenberg U, Prange-Kiel J, Rune GM. Steroidogenic factor-1 expression in marmoset and rat hippocampus: co-localization with StAR and aromatase. J Neurochem. 2001;76:1879–1886. doi: 10.1046/j.1471-4159.2001.00207.x. [DOI] [PubMed] [Google Scholar]
- Weick JP, Groth RD, Isaksen AL, Mermelstein PG. Interactions with PDZ proteins are required for L-type calcium channels to activate cAMP response element-binding protein-dependent gene expression. J Neurosci. 2003;23:3446–3456. doi: 10.1523/JNEUROSCI.23-08-03446.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse. 1998;29:379–391. doi: 10.1002/(SICI)1098-2396(199808)29:4<379::AID-SYN10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Xiao L, Jackson LR, Becker JB. The effect of estradiol in the striatum is blocked by ICI 182,780 but not tamoxifen: pharmacological and behavioral evidence. Neuroendocrinology. 2003;77:239–245. doi: 10.1159/000070279. [DOI] [PubMed] [Google Scholar]
- Zhang H, Maximov A, Fu Y, Xu F, Tang TS, Tkatch T, Surmeier DJ, Bezprozvanny I. Association of CaV1.3 L-type calcium channels with Shank. J Neurosci. 2005;25:1037–1049. doi: 10.1523/JNEUROSCI.4554-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lehan N, Wehrenberg U, Disteldorf E, von Lossow R, Mares U, Jarry H, Rune GM. Neuroprotection by estradiol: a role of aromatase against spine synapse loss after blockade of GABA(A) receptors. Exp Neurol. 2007;203:72–81. doi: 10.1016/j.expneurol.2006.07.020. [DOI] [PubMed] [Google Scholar]