Abstract
MHC class I molecules can be engineered as single chain trimers (SCT) that sequentially incorporate all three subunits of the fully assembled proteins, namely peptide, β2m, and heavy chain. SCTs have been made with many different MHC-peptide complexes and are used as novel diagnostic and therapeutic reagents as well as probes for diverse biological questions. Here we review the recent and diverse applications of SCT. These applications include new approaches to enumerate disease-related T cells, DNA vaccines, eliciting responses to preassembled MHC-peptide complexes, and unique probes of lymphocyte development and activation. Future applications of SCTs will be driven by their further engineering and the ever expanding identification of disease-related peptides using chemical, genetic and computational approaches.
RATIONALE FOR SINGLE CHAIN TRIMER (SCTs) CONSTRUCTION
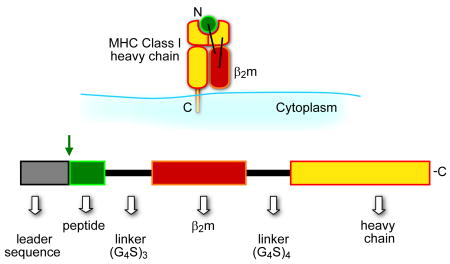
Self major histocompatibility complex 1 (MHCI) molecules consisting of a heavy chain and β2 microglobulin (β2m) bind peptides from pathogen-infected or transformed cells and present them on the cell surface to CD8 T cells for immune surveilance1. The genetic hallmark of the MHCI heavy chains is their multigenic and polymorphic nature, conferring the ability of an individual to bind a plethora of different peptides. More specifically, humans and mice express 4–6 different class I heavy chains each of which is polymorphic i.e. has multiple allelic forms. Furthermore, each different MHCI allele binds tens of thousands of different peptides (typically 8–10 amino acids) into its membrane distal binding groove situated between two anti-parallel helices. MHC molecules do not discriminate self from non-self peptides and thus an antigenic peptide must be processed efficiently and have a sufficiently high MHC binding affinity and/or abundance to compete with an extensive pool of endogenous (non-antigenic) peptides. Thus antigenicity for T cell responses to disease-related peptides can frequently be enhanced by improving processing, abundance or MHC binding. For example, several defined tumor-associated antigens have been found to be poor MHCI binders thus limiting effectiveness in eliciting CD8 T cell immunity2,3. To obviate the need for antigenic peptides to be processed and compete for MHC binding, we and others have engineered fully assembled class I molecules or SCTs 4–6. The SCT format consists of a single polypeptide comprised of an antigenic peptide followed by a flexible linker that connects the C terminus of the peptide to the N terminus of β2m, and another flexible linker that connects the C terminus of β2m with the N terminus of the heavy chain (Box 1, Fig. 1). This format evolved from a series of previously reported constructs with linker attached peptides to MHC class I or class II subunits7–12. In this review article we discuss features of SCTs applicable for their use as vaccines, diagnostics and probes of lymphocyte biology.
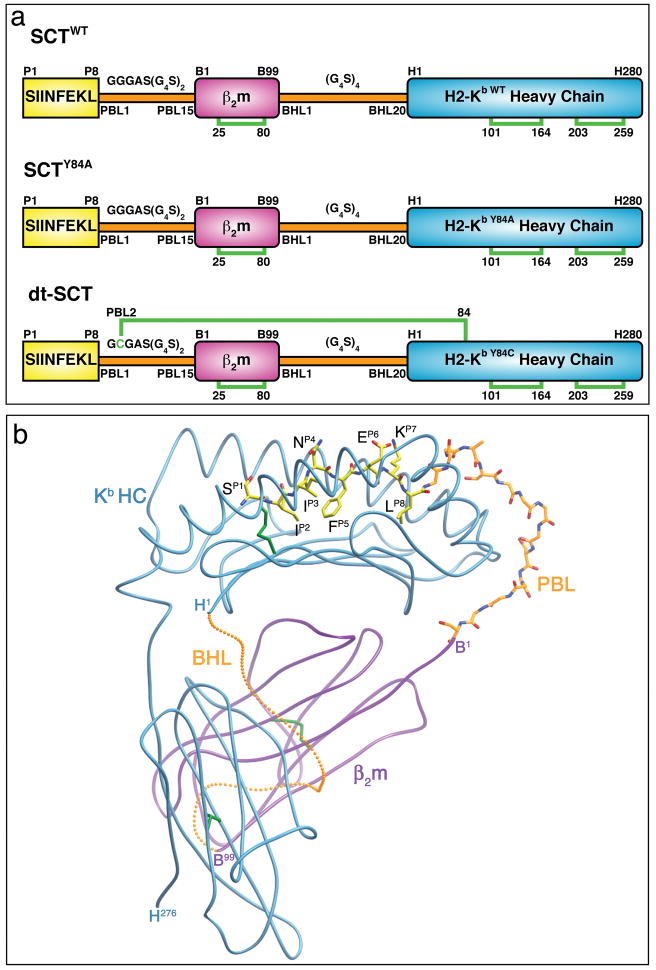
Figure 1. SCT design and structural features.
(a) The original SCT design incorporated all three pMHC components into a single polypeptide chain by sequentially attaching OVA peptide (OVAp) (yellow) to β2m (magenta) to HC (cyan) with flexible linkers (orange). Two new generations of SCTs improve peptide binding within the SCT. The SCTY84A variant improves accommodation of the first linker, whereas the dtSCT variant contains a disulfide trap to staple peptide into the MHC ligand binding cleft. Disulfide bonds bridging the indicated cysteine residues are represented as green brackets. (b) The crystal structure of SCTWT shows that SCTs adopt the same ectodomain structure as native pMHCI. This is represented in the ribbon diagram of SCTWT, where OVAp and PBL are rendered as ball and stick models and colored as follows: OVAp and PBL carbon atoms, yellow and orange, respectively; nitrogen atoms, blue; oxygen atoms, red. The protein is oriented with the OVAp N terminus on the left and the membrane-proximal α3 domain at the bottom. A possible conformation for the BHL is represented and rendered as small orange balls. PBL: peptide-β2m linker, BHL: β2m-heavy-chain linker. Figure adapted from Mitaksov et al (14).
SCT DESIGN
The SCT format has been used extensively for generating different mouse and human peptide-MHCI (pMHCI) complexes which have been used either as i) soluble recombinant proteins expressed in bacteria or insect cells or ii) cellular membrane-associated proteins expressed in mammalian cells by transfection or transduction13. SCTs have several remarkable properties that are listed in Text Box 1 and are discussed below in the context of specific SCT applications. Most importantly, SCT adopt the same ectodomain structure as native pMHCI as shown by their crystal structure (Fig. 1) and are equivalently recognized by diverse TCRs14. It remains somewhat surprising that the SCT format works so well. The reason is that the SCT linker extending from the C terminus of the peptide to β2m would be expected to disrupt the highly conserved H bonding network anchoring the C terminus of the peptide in the heavy chain F pocket. As shown in Figure 2a the F pocket of the MHCI peptide binding groove accommodates the C terminal peptide residue that is prominent in the anchoring of all peptides to their respective MHCI heavy chain. Disruption of the F pocket peptide binding in the SCT is compensated for by the fact that the SCT format allows rebinding of the linker-attached peptide to the heavy chain14,15. Indeed, this rebinding is likely the reason why successful SCT construction requires inclusion of β2m, i.e. inclusion of β2m in the SCT facilitates peptide rebinding due to the cooperative nature of their binding16. In any case, nascent SCTs appear to spontaneously assemble in the ER lumen in a manner independent of peptide processing in the cytosol and chaperone-assisted peptide loading complex in the ER lumen (see Text Box 2). In addition, surface SCTs are extraordinarily refractory to the binding of competitor (non-linker attached) peptides5. Thus SCTs truly display a preassembled and stable phenotype, and recent structural engineering now allows for the peptides to essentially be “stapled” into the MHCI groove by disulfide traps.
TEXT BOX 1. Components and properties of SCTs.
Intact covalent structure in cells. Western blot analyses showed SCT components in live cells remain intact with native folding as determined using conformation-dependent mAbs.
Rapid and chaperone (TAP/tapasin/calreticulin)-independent expression. This feature also renders SCT resistant to several viral immune evasion strategies.
Extended cell surface half-life as determined by surface decay of SCT expression after Brefeldin A treatment. In these comparisons surface SCT were appreciably more stable on the cell surface than native class I loaded with endogenous peptides.
Universal construction. SCT constructs have been made with several different human, rat and mouse heavy chains, β2m and different antigenic peptides.
-
Preservation of CD8 T cell detection.
T cells generated to native class I-peptide detect cells expressing SCT
T cells generated to SCTs detect cells expressing native class I-peptide
Staining reagents made with recombinant SCTs detect the same T cells as conventional straining reagents made with the same components
Resistant to displacement by high-affinity competitor peptide. SCTs expressed on cells were 10,000 fold more resistant to competitor peptide binding than class I loaded with endogenous peptides. In these assays the binding of competitor peptides was monitored using T cells that are highly sensitive to low levels of peptide binding.
-
High immunogenicity
APCs expressing SCT elicit robust CD8 T cell responses in vitro.
plasmid DNA encoding SCT elicit robust CD8 T cell responses in vivo.
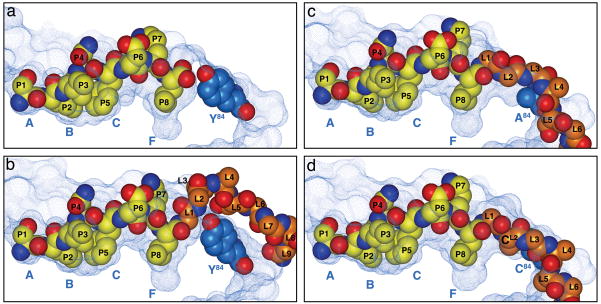
Figure 2. Linker accommodation in three generations of SCT.
The panels in this figure illustrate native MHCI-OVAp binding and three generations of SCTs: SCTWT, SCTY84A and dtSCT. In the native pMHCI, the HC residue Tyr84 is a steric impediment to C terminal peptide extensions. Thus in the SCTWT, the linker extending from the C terminus of the peptide must bulge above Tyr84, which impairs peptide anchoring to F pocket residues in the HC ligand binding cleft (15). In the SCTY84A, the Ala84 substitution allows for better linker accommodation. In dtSCT, the dual substitution of Cys residues in position 84 of the HC and the second linker position (L2) introduces a disulfide trap. This trap essentially staples the peptide into the MHC groove to increase thermal stability and render the complex refractory to peptide exchange. The solvent-accessible surfaces of the peptide-binding grooves are displayed as blue dotted surfaces and the peptide (P1-P8) and first nine residues (SCTwt; L1-L9) or six residues (SCTY84A and dtSCT; L1-L6) of PBL are represented as SPK models. The heavy chain pocket locations (A – F) are indicated. Figure adapted from Mitaksov et al (14).
TEXT BOX 2. Processing and MHCI binding of antigenic peptides.
Proteins from which antigenic peptides are derived undergo proteolysis by the proteasome in the cytosol.
Peptides 8–12 amino acids length are efficiently transported from the cytosol into the ER lumen by TAP, a heterodimeric, ATP-dependent pump.
Further proteolytic trimming of peptides can occur within the ER lumen by aminopeptidases.
Peptides typically of 8–10 amino acid length bind to MHCI heavy chains preassembled with β2m.
Peptide binding to heavy chain/β2m heterodimers occurs most often when they are physically associated with the peptide loading complex or PLC.
The PLC consists of TAP, calreticulin, ERp57 and tapasin, and these proteins act cooperatively to facilitate the binding of peptides to MHCI molecules.
Which peptides bind to which MHCI molecules is determined by the architecture of their different peptide binding grooves as determined by polymorphic residues.
After a peptide binds to MHCI, the complex dissociates from the PLC and transits via the Golgi to the cell surface.
DNA VACCINES
Vaccination with naked plasmid DNA is an attractive approach to elicit specific immunity to cancers or pathogens17. The basic approach of DNA vaccination (outlined in Text Box 3) is attractive due to the fact that it is relatively safe, cost effective and has the potential for sustained antigen delivery within cells. In contrast to using virus to deliver antigen, DNA vaccines do not elicit anti-vector immune responses, and are therefore more suitable for multiple administrations. Furthermore, in contrast to using recombinant proteins or peptides, plasmid DNA encoding antigen is relatively stable in cells and thus may be more effective at eliciting immunological memory. Although data from DNA vaccination studies is highly encouraging17, potency remains an important issue. Indeed, inefficient epitope processing or MHCI loading after DNA vaccination can be major impediments for CD8 T cell activation, thus inspiring SCTs to be tested as DNA vaccines. There have been several recent papers showing plasmid DNA encoding an SCT can elicit robust CD8 T cell responses in mouse models18–27. And the SCTs tested as vaccines have incorporated several different tumor or pathogen epitopes. Furthermore, these studies demonstrate the flexibility of the SCT platform by combining it with other strategies to enhance DNA vaccine potency. For example, SCTs were co-expressed with an anti-apoptotic protein to prolong DC survival or with a universal CD4 helper epitope to stimulate Th2 help23,25,27.
Text Box 3. DNA vaccine priming of CD8 T cells.
DNA sequence encoding antigen is inserted enzymatically into the cloning site of a mammalian expression plasmid
Plasmid DNA is purified and typically delivered to the skin of the host by either gene gun (coated on gold particles) or by electrophoration.
Plasmid DNA is taken up by various cell types including DCs.
Using host cellular components, the plasmid DNA enters the nucleus and plasmid-encoded proteins are expressed.
For CD8 T cell immune responses, plasmid encoded antigen is i) processed in the cytosol, ii) binds MHCI molecules in the ER lumen and iii) presented at the cell surface by MHCI to CD8 T cells.
Presentation to CD8 T cells can occur directly by plasmid synthesizing DCs, or indirectly by cross presentation if the plasmid encoded antigen is synthesized in a non-DC cell type and subsequently taken up by DCs.
Recent findings of SCT vaccine efficacy raise the important question of whether they are more advantageous than other strategies to elicit CD8 T cell immunity. This question was addressed using a mouse model of cervical cancer20. In this study, plasmid DNA encoding an SCT incorporating a mouse MHCI molecule bound by a peptide derived from the E6 oncoprotein of HPV elicited a strong CD8 T cell response and protective immunity against lethal tumor challenge. By contrast, plasmid DNA encoding the entire E6 protein had little effect. In another relevant study, it was shown that an SCT vaccine was more immunogenic than an ER-targeted minigene encoding the antigenic peptide24. These demonstrations of SCT superiority were attributed to the preprocessed and/or preloaded nature of the SCT resulting in stable antigen display. Based on this speculation, it is likely that the advantage of SCT will be most pronounced when incorporating antigenic peptides that are inefficiently processed or are poor MHC binders. To maximize SCT vaccine efficacy, it will be important to understand the mechanism by which antigen is presented. Although still controversial, two different models have been proposed for the general mechanism of antigen presentation after DNA vaccination. DNA could be taken up by a DC and the antigen directly presented to CD8 T cells28. In a direct presentation model, it is easy to envisage the SCT advantage. Alternatively, DNA could be taken up by a non-DC and the antigenic peptide processed from the SCT and cross presented by a DC29. In cross presentation models the SCT could be stabilizing the antigenic peptide and/or promoting DC uptake and expression. An attractive mechanism by which this might be happening is by membrane exchange, known as trogocytosis30. Basic research applications of SCTs have a great potential to help resolve the mechanism by which antigen is presented after DNA vaccination; resolving this mechanism has remarkable promise for improving the effectiveness of DNA vaccines. For example, several recent studies have highlighted the importance of the CD8+ DC subset for crosspriming CD8 T cells31–33. Thus future strategies to specifically target SCT expression to specific APC subsets may be a highly effective strategy to elicit robust responses after DNA vaccination.
MODULATORS OF LYMPHOCYTE ACTIVATION
In addition to DNA vaccines, other SCT-based therapies to modulate immune responses are worth discussion. For example, SCTs with self MHCI molecules bound to known disease-related epitopes could be introduced into autologous DC ex vivo and reintroduced into patients to prime CD8 T cell responses. Alternatively, antigen-presenting cells (APCs) expressing self MHCI molecules that contain disease-related peptides could be used for the ex vivo expansion of patients CD8 T cells for adoptive therapies34. The attractiveness of these approaches is that APCs expressing SCT are potent stimulators of CD8 T cells in vitro and SCT-stimulated CD8 T cells are capable of killing tumor cells or pathogen-infected cells 20,25. These, like other SCT approaches, will greatly benefit from the ongoing discovery of new disease-related epitopes identified using chemical, genetic, and computational approaches. Two published SCT-based strategies are also noteworthy. An SCT of HLA-A2 bound to a highly immunogenic peptide was linked to an antibody single chain variable domain specific for a tumor-associated antigen35. Tumor cells expressing this antigen were coated with this bifunctional recombinant protein and were lysed by CD8 T cells specific for the SCT. This therapeutic approach has the potential to eliminate tumors in vivo that have down regulated MHCI expression. In a second novel immunotherapeutic approach an SCT was designed to block human NK cell detection of pig xenografts36,37. More specifically, an SCT was constructed that incorporated human HLA-E, a non-classical MHC molecule, bound to a MHCI signal peptide. This SCT was then expressed on porcine cells and was shown to inhibit NK cell killing via the CD94/NKG2A inhibitory receptor. The above examples attest to the diverse translational opportunities of novel SCT-based immunotherapies that warrant further exploration.
MULTIMERIC LYMPHOCYTE STAINING REAGENTS and DISULFIDE TRAPS
Conceptually, perhaps the most straightforward application of SCTs is for making better multivalent reagents for visualizing and enumerating T cells or NK cells. Traditionally such reagents are tetramers that are formed with E. coli-expressed soluble MHC chains refolded in vitro with synthetic peptides38. A biotinylation site is included on the C terminus of the MHC chain for tetramerization to provide increased avidity and a streptavidin moiety is used with various fluorescent tags for visualization. Since tetramers specifically bind TCRs, they are widely used for enumeration of antigen-specific CD8 T cells or CD4 T cells. In addition the same reagents can be used to visualize NK cells that bear receptors with disparate binding to different class I alleles. Tetramers can also be used preparatively to isolate and expand lymphocytes in vitro for analyses or for use in adoptive transfer to assess in vivo function. Importantly, however, certain tetramers or other multivalent MHC/peptide staining reagents can have problems related to peptide dissociation, and the SCT format has the potential to stabilize weak binding peptides39. To establish the feasibility of this approach, tetramers and dimeric Ig fusion proteins have been made with SCTs and were shown to reliably stain CD8 T cells in an MHC/peptide-specific manner4,14,15,40. However, the best strategy for securing peptide comes from our recent re-engineering of the SCT.
To better accommodate C terminal peptide extensions and to strategically introduce a disulfide bond to trap peptide in the MHCI peptide binding platform, we employed structure-based approaches14,39,41. To define the structural impediment of C terminal peptide anchoring in the SCT, we compared the MHC-peptide structures of class I with class II, since class II molecules tolerate peptides with C-terminal extensions and class I molecules do not. These comparisons revealed that the invariant tyrosine at position 84 of all mouse and human heavy chains is most prominent in preventing C terminal extensions (see Fig 2). Thus Tyr84 was a predicted impediment to linker accommodation in the SCT. Indeed our crystal structure of an SCT revealed that Tyr84 precluded optimal linker accommodation by forcing a near 90 degree turn in the protein main chain at the first linker position (Fig 2). Thus to improve linker accommodation, Tyr84 of the SCT was mutated to alanine. Functional comparisons showed that the SCT with the alanine substitution had improved thermal stability, and crystallographic comparisons showed that the alanine substitution allowed relaxation of the linker that connects peptide with β2m14,15. As mentioned above, the mechanism by which SCTs are refractory to competitive (non-linker attached) peptide binding is the rapid rebinding of the linker-attached peptide after dissociation14,15. This mechanism explains why SCT made with high affinity peptides such as Kb/OVAp (SIINFEKL) are highly resistant to competitor peptide binding, whereas SCTs with low affinity peptides are displaceable at very high concentrations of competitor peptide39,41. To more absolutely secure peptide binding in the SCT, we engineered a disulfide bond to trap the peptide into the MHCI molecule and prevent peptide dissociation or exchange. Based again on structural comparisons, the trap was introduced with a tyrosine to cysteine substitution at heavy chain position 84 and a glycine to cysteine substitution at the second position of the peptide-β2m linker (Fig 2). Chemical analysis verified disulfide formation and improved thermal stability, whereas competitive binding experiments verified complete exclusion of non-linker attached peptides14,39,41. Furthermore, structural studies confirmed disulfide bridge formation and revealed how the trap facilitates better linker accommodation and F pocket peptide accomodation (Fig 2)14. SCTs with this design were designated dtSCT and they have been formed with several different mouse and human MHCI/peptide complexes.
Using disulfide traps to secure peptide binding to MHC could be advantageous for all of the SCT applications discussed in this review. However, a clear propitious application of disulfide traps is their use as T cell staining reagents. Not only will the inclusion of traps mitigate problems of peptide dissociation, they also obviate the need to use the SCT format to make soluble, recombinant proteins. More specifically, we have successfully incorporated a disulfide trap into a recombinant mouse pMHCI complex without SCT linkers by introducing cysteines into the recombinant heavy chain at position 84 and the second linker residue connecting the peptide with β2m14. It is also noteworthy that a similarly placed trap has been engineered into single chain class II molecules and expressed as soluble recombinant protein in insect cells42. Not only do these recombinant class II molecules with traps make effective tetramers, but by fixing peptide binding in a specific binding register, they also have the potential to resolve binding ambiguities that can occur43,44. Interestingly, binding of peptides to alternative MHC registers may induce unique autoantigens implicated in disease45,46.
BASIC PROBES FOR LYMPHOCYTE ACTIVATION AND DEVELOPMENT
Concurrent with their applied applications, SCTs have been used as novel and insightful probes to investigate diverse areas of lymphocyte biology. For example, they have been used to address the importance of the immunological synapse in lymphocyte activation47. SCTs with various C-terminal elongations were used to demonstrate the importance of pMHC size in TCR triggering48,49. More specifically, elongated SCTs led to less efficient entry of the TCR co-receptor CD3 into the immune synapse resulting in poor signaling. Immune synapse formation also appears to result in antigen receptors on the surface of lymphocytes actively capturing fragments of the plasma membrane of APCs. This general phenomenon of membrane transfer has been visualized in several different cellular interactions and is called trogocytosis30. However, the functional importance of trogocytosis in antigen- specific activation of T cells has not been established. Again the stable, preassembled nature of the SCT makes it an effective probe for pMHC transfer. For example, using an SCT approach it was shown that different states of in vitro T cell activation (i.e. cytolysis, IFNγ production or fratricide) correlate with different levels of pMHC trogocytosis50. Interestingly, DC subsets also appear to differ in their ability to induce trogocytosis, perhaps helping to explain their disparate immune functions51. A future challenge is to assess the physiologic importance of trogocytosis for in vivo T cell activation.
The receptors on T cells and NK cells that detect MHCI complexes not only determine activation status, but also play prominent roles in the development of these cells.52,53 More specifically, inhibitory NK cell receptors were recently shown to interact with self-MHC molecules during development to help NK cells acquire functional competence. This mechanism of self recognition (termed licensing) had been difficult to study due to the multiplicity of inhibitory NK receptors detecting different MHCI polymorphic gene products. A reductionist approach to probe the mechanism of NK cell licensing was afforded by making a B6 congenic mouse expressing an SCT transgene and no other MHCI molecules. Importantly, in this SCT mouse strain, the singly expressed MHCI molecule is recognized by a single NK cell receptor. This SCT mouse, in combination with supporting approaches, showed that NK cells are licensed by inhibitory receptors that engage self MHC molecules during development52. Although this licensing mechanism resembles positive selection in T cells, NK receptors that recognize MHCI, unlike TCRs, are inhibitory and germ line encoded. SCT mice have also provided insight into the mechanism of CD8 T cell selection and their development of MHC and peptide specificity. For example, CD8 T cells from SCT transgenic mice were found to be highly MHC specific demonstrating that the development of MHC restriction results from positive selection and not negative selection on diverse pMHCI complexes. Furthermore, the role of peptide in positive selection of CD8 T cells was assessed by comparing the peptide specificity of the CD8 T cell repertoire that developed in two different SCT mouse strains, one expressing Kb bound by the OVA peptide, another Kb bound by the VSV peptide 54. This study thus provided definitive evidence that positive selection is largely responsible for both MHC and peptide specificity54. By contrast, using a similar approach, CD4 T cells developing in the presence of a single pMHCII complex were found to be crossreactive on other pMHCII complexes reflecting a lack of negative selection on other pMHCII complexes10,55,56. It is possible that these findings reflect fundamental differences in CD8 vs. CD4 T cell development, or more likely that both positive and negative selection contribute to pMHC specificity. It should be noted that both the MHCI and MHCII single chains used for these studies are highly effective at excluding the binding of contaminating peptides. However, absolute assurance that selection in these mice is solely based on a single pMHC will benefit from future generations of single chain constructs, like ones containing disulfide traps. Furthermore, future SCT transgenic mice can be engineered to determine which endogenous peptides are important for selection as well as when, where and how much pMHC expression is required.
Concluding remarks
The unique properties and versatility of the SCT format portend an increasing diversity of future applications. For example, future SCT applications could test the functional consequences of presenting closely related peptides by the same MHCI molecule. More specifically, SCT approaches could provide in vivo comparisons of CD8 T cell responses to peptide agonists vs. antagonists. Alternatively, vaccine applications could test the importance of immunodominant vs. subdominant determinants in pathogen protection, including protection against related viruses. It will be particularly informative for in vivo studies to study cell-type specific expression of SCT as well as to control levels and timing of expression. As we move forward, definition of when, where and how much SCT to express, will improve their applications as vaccines as well as probes for distinct in vivo pathways of antigen presentation.
Acknowledgments
The authors would like to thank NIH, MRCE for Biodefense and Emerging Infectious Disease Research and the UK Medical Research Council for financial support and Dr. Vesselin Mitaksov for the original creation of Figures 1 and 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 2.Chapatte L, et al. Processing of tumor-associated antigen by the proteasomes of dendritic cells controls in vivo T-cell responses. Cancer Res. 2006;66:5461–5468. doi: 10.1158/0008-5472.CAN-05-4310. [DOI] [PubMed] [Google Scholar]
- 3.Houghton AN, Guevara-Patino JA. Immune recognition of self in immunity against cancer. J Clin Invest. 2004;114:468–471. doi: 10.1172/JCI22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greten TF, et al. Peptide-beta2-microglobulin-MHC fusion molecules bind antigen-specific T cells and can be used for multivalent MHC-Ig complexes. J Immunol Methods. 2002;271:125–135. doi: 10.1016/s0022-1759(02)00346-0. [DOI] [PubMed] [Google Scholar]
- 5.Yu YY, et al. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J Immunol. 2002;168:3145–3149. doi: 10.4049/jimmunol.168.7.3145. [DOI] [PubMed] [Google Scholar]
- 6.Vest HN, et al. Phage display of peptide/major histocompatibility class I complexes. Eur J Immunol. 2001;31:32–38. doi: 10.1002/1521-4141(200101)31:1<32::aid-immu32>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Mage MG, et al. A recombinant, soluble, single-chain class I major histocompatibility complex molecule with biological activity. Proc Natl Acad Sci U S A. 1992;89:10658–10662. doi: 10.1073/pnas.89.22.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mottez E, et al. Cells expressing a major histocompatibility complex class I molecule with a single covalently bound peptide are highly immunogenic. J Exp Med. 1995;181:493–502. doi: 10.1084/jem.181.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toshitani K, et al. Expression of a single-chain HLA class I molecule in a human cell line: presentation of exogenous peptide and processed antigen to cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1996;93:236–240. doi: 10.1073/pnas.93.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ignatowicz L, et al. The repertoire of T cells shaped by a single MHC/peptide ligand. Cell. 1996;84:521–529. doi: 10.1016/s0092-8674(00)81028-4. [DOI] [PubMed] [Google Scholar]
- 11.Uger RA, Barber BH. Creating CTL targets with epitope-linked beta 2-microglobulin constructs. J Immunol. 1998;160:1598–1605. [PubMed] [Google Scholar]
- 12.White J, et al. Soluble class I MHC with beta2-microglobulin covalently linked peptides: specific binding to a T cell hybridoma. J Immunol. 1999;162:2671–2676. [PubMed] [Google Scholar]
- 13.Hansen T, et al. Preparation of stable single-chain trimers engineered with peptide, beta2 microglobulin, and MHC heavy chain. Curr Protoc Immunol. 2009;Chapter 17(Unit 17) doi: 10.1002/0471142735.im1705s87. [DOI] [PubMed] [Google Scholar]
- 14.Mitaksov V, et al. Structural Engineering of pMHC Reagents for T Cell Vaccines and Diagnostics. Chem Biol. 2007;14:909–922. doi: 10.1016/j.chembiol.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lybarger L, et al. Enhanced immune presentation of a single-chain major histocompatibility complex class I molecule engineered to optimize linkage of a C-terminally extended peptide. J Biol Chem. 2003;278:27105–27111. doi: 10.1074/jbc.M303716200. [DOI] [PubMed] [Google Scholar]
- 16.Otten GR, et al. Peptide and beta 2-microglobulin regulation of cell surface MHC class I conformation and expression. J Immunol. 1992;148:3723–3732. [PubMed] [Google Scholar]
- 17.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Primeau T, et al. Applications of major histocompatibility complex class I molecules expressed as single chains. Immunol Res. 2005;32:109–122. doi: 10.1385/ir:32:1-3:109. [DOI] [PubMed] [Google Scholar]
- 19.Jaramillo A, et al. Recognition of HLA-A2-restricted mammaglobin-A-derived epitopes. Breast Cancer Res Treat. 2004;88:29–41. doi: 10.1007/s10549-004-8918-1. [DOI] [PubMed] [Google Scholar]
- 20.Huang CH, et al. Cancer immunotherapy using a DNA vaccine encoding a single-chain trimer of MHC class I linked to an HPV-16 E6 immunodominant CTL epitope. Gene Ther. 2005;12:1180–1186. doi: 10.1038/sj.gt.3302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung CF, et al. A DNA vaccine encoding a single-chain trimer of HLA-A2 linked to human mesothelin peptide generates anti-tumor effects against human mesothelin-expressing tumors. Vaccine. 2007;25:127–135. doi: 10.1016/j.vaccine.2006.06.087. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, et al. Hepatitis B virus core antigen epitopes presented by HLA-A2 single-chain trimers induce functional epitope-specific CD8+ T-cell responses in HLA-A2.1/Kb transgenic mice. Immunology. 2007;121:105–112. doi: 10.1111/j.1365-2567.2007.02543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang B, et al. Intradermal administration of DNA vaccines combining a strategy to bypass antigen processing with a strategy to prolong dendritic cell survival enhances DNA vaccine potency. Vaccine. 2007;25:7824–7831. doi: 10.1016/j.vaccine.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmowski MJ, et al. A single-chain H-2Db molecule presenting an influenza virus nucleoprotein epitope shows enhanced ability at stimulating CD8+ T cell responses in vivo. J Immunol. 2009;182:4565–4571. doi: 10.4049/jimmunol.0803893. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, et al. Single-Chain HLA-A2 MHC Trimers That Incorporate an Immundominant Peptide Elicit Protective T Cell Immunity against Lethal West Nile Virus Infection. J Immunol. 2010 doi: 10.4049/jimmunol.0903955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li L, et al. Engineering superior DNA vaccines: MHC class I single chain trimers bypass antigen processing and enhance the immune response to low affinity antigens. Vaccine. 2010;28:1911–1918. doi: 10.1016/j.vaccine.2009.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, et al. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life and intracellular targeting strategies with a strategy to boost CD4+ T cell. Hum Gene Ther. 2007;18:1129–1139. doi: 10.1089/hum.2007.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Porgador A, et al. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J Exp Med. 1998;188:1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauterbach H, et al. Insufficient APC capacities of dendritic cells in gene gun-mediated DNA vaccination. J Immunol. 2006;176:4600–4607. doi: 10.4049/jimmunol.176.8.4600. [DOI] [PubMed] [Google Scholar]
- 30.Davis DM. Intercellular transfer of cell-surface proteins is common and can affect many stages of an immune response. Nat Rev Immunol. 2007;7:238–243. doi: 10.1038/nri2020. [DOI] [PubMed] [Google Scholar]
- 31.Allan RS, et al. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 32.Hildner K, et al. Batf3 deficiency reveals a critical role for CD8alpha+ dendritic cells in cytotoxic T cell immunity. Science. 2008;322:1097–1100. doi: 10.1126/science.1164206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belz GT, et al. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obermann S, et al. Peptide-beta2-microglobulin-major histocompatibility complex expressing cells are potent antigen-presenting cells that can generate specific T cells. Immunology. 2007;122:90–97. doi: 10.1111/j.1365-2567.2007.02616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oved K, et al. Antibody-mediated targeting of human single-chain class I MHC with covalently linked peptides induces efficient killing of tumor cells by tumor or viral-specific cytotoxic T lymphocytes. Cancer Immunol Immunother. 2005;54:867–879. doi: 10.1007/s00262-005-0666-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crew MD, et al. An HLA-E single chain trimer inhibits human NK cell reactivity towards porcine cells. Mol Immunol. 2005;42:1205–1214. doi: 10.1016/j.molimm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Lilienfeld BG, et al. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation. 2007;14:126–134. doi: 10.1111/j.1399-3089.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- 38.Altman JD. Flow cytometry applications of MHC tetramers. Methods Cell Biol. 2004;75:433–452. doi: 10.1016/s0091-679x(04)75017-7. [DOI] [PubMed] [Google Scholar]
- 39.Truscott SM, et al. Disulfide bond engineering to trap peptides in the MHC class I binding groove. J Immunol. 2007;178:6280–6289. doi: 10.4049/jimmunol.178.10.6280. [DOI] [PubMed] [Google Scholar]
- 40.Ohashi T, et al. Activation and detection of HTLV-I Tax-specific CTLs by epitope expressing single-chain trimers of MHC Class I in a rat model. Retrovirology. 2008;5:90. doi: 10.1186/1742-4690-5-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truscott SM, et al. Human major histocompatibility complex (MHC) class I molecules with disulfide traps secure disease-related antigenic peptides and exclude competitor peptides. J Biol Chem. 2008;283:7480–7490. doi: 10.1074/jbc.M709935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jordan KR, et al. Peptide vaccines prevent tumor growth by activating T cells that respond to native tumor antigens. Proc Natl Acad Sci U S A. 2010;107:4652–4657. doi: 10.1073/pnas.0914879107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levisetti MG, et al. The insulin-specific T cells of nonobese diabetic mice recognize a weak MHC-binding segment in more than one form. J Immunol. 2007;178:6051–6057. doi: 10.4049/jimmunol.178.10.6051. [DOI] [PubMed] [Google Scholar]
- 44.Landais E, et al. New design of MHC class II tetramers to accommodate fundamental principles of antigen presentation. J Immunol. 2009;183:7949–7957. doi: 10.4049/jimmunol.0902493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stadinski BD, et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohan JF, et al. Unique autoreactive T cells recognize insulin peptides generated within the islets of Langerhans in autoimmune diabetes. Nat Immunol. 2010;11:350–354. doi: 10.1038/ni.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 48.Choudhuri K, et al. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 49.Choudhuri K, et al. Peptide-major histocompatibility complex dimensions control proximal kinase-phosphatase balance during T cell activation. J Biol Chem. 2009;284:26096–26105. doi: 10.1074/jbc.M109.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hudrisier D, et al. T cell activation correlates with an increased proportion of antigen among the materials acquired from target cells. Eur J Immunol. 2005;35:2284–2294. doi: 10.1002/eji.200526266. [DOI] [PubMed] [Google Scholar]
- 51.Smyth LA, et al. The relative efficiency of acquisition of MHC:peptide complexes and cross-presentation depends on dendritic cell type. J Immunol. 2008;181:3212–3220. doi: 10.4049/jimmunol.181.5.3212. [DOI] [PubMed] [Google Scholar]
- 52.Kim S, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez NC, et al. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang B, et al. A single peptide-MHC complex positively selects a diverse and specific CD8 T cell repertoire. Science. 2009;326:871–874. doi: 10.1126/science.1177627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huseby ES, et al. Negative selection imparts peptide specificity to the mature T cell repertoire. Proc Natl Acad Sci U S A. 2003;100:11565–11570. doi: 10.1073/pnas.1934636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huseby ES, et al. How the T cell repertoire becomes peptide and MHC specific. Cell. 2005;122:247–260. doi: 10.1016/j.cell.2005.05.013. [DOI] [PubMed] [Google Scholar]