Abstract
Several neuroendocrine signals of the hypothalamic-pituitary-adrenal (HPA) axis are released following exposure to stressful events. It has long been proposed that the signals in this cascade each act to modify ongoing and future behavior. In this study we investigated whether blocking glucocorticoid synthesis, corticotropin-releasing factor (CRF)-1 receptors, or CRF-2 receptors during social defeat would alter subsequent behavioral responses. We used a conditioned defeat model in Syrian hamsters in which social defeat results in a dramatic shift from territorial aggression to increased submissive and defensive behavior in future social encounters. We found that intracerebroventricular administration of anti-sauvagine-30, a CRF-2 receptor antagonist, prior to social defeat training reduced the acquisition of conditioned defeat. In contrast, the acquisition of conditioned defeat was not altered by the CRF-1 receptor antagonist CP-154,526 or the glucocorticoid synthesis inhibitor metyrapone. Our results suggest that CRF, and perhaps related neuropeptides such as urocortins, act at CRF-2 receptors to promote the development of defeat-induced changes in social behavior, whereas signaling at CRF-1 and glucocorticoid receptors plays a negligible role in this process.
Keywords: social defeat, conditioned defeat, stress, anxiety, corticotropin-releasing factor, CRF-2 receptor
1. Introduction
Exposure to stressful events is a key factor in the etiology of several mood and anxiety disorders [1,2]. Corticotropin-releasing factor (CRF) is a neuropeptide that synchronizes many of the neuroendocrine, autonomic, and behavioral responses to stress [3,4]. Upon exposure to a stressor, CRF is released from the paraventricular nucleus of the hypothalamus into the pituitary where it triggers the release of adrenocorticotropic hormone (ACTH). ACTH, in turn, travels through the blood stream to the adrenal cortex where it stimulates the release of glucocorticoids such as cortisol and corticosterone. Each of the neuroendocrine signals of this cascade is capable of acting in the brain to modulate behavioral changes that occur following stressful events.
Glucocorticoids feed back on the brain and affect gene transcription through activation of nuclear glucocorticoid and mineralocorticoid receptors. In addition to inhibiting the hypothalamic-pituitary-adrenal (HPA) axis, glucocorticoid feedback has been shown to modulate ongoing and future behavior. For example, administration of corticosterone increases aggressive behavior [5], whereas the glucocorticoid synthesis inhibitor metyrapone decreases aggressiveness [6]. However, corticosterone treatment does not always promote aggression. Mice injected with corticosterone prior to an encounter with a larger, more aggressive opponent display increased submission [7]. Similarly, corticosterone treatment prior to social defeat increases submissiveness in mice tested in future aggressive encounters [8]. In subsequent studies, Leshner and colleagues showed that ACTH also increases future submissive behavior, but this effect is due to ACTH’s trophic actions on glucocorticoids [8]. Altogether, these results suggest that glucocorticoids increase aggressiveness in naïve animals and increase submissiveness in subordinate animals, perhaps by enhancing the salience of social cues or by increasing the animal’s motivation to respond.
Several rodent studies have shown that the glucocorticoids released during stressful events enhance the acquisition and consolidation of associative memory [9–11]. In humans, cortisol administration has been reported to enhance recall of emotionally arousing pictures compared to neutral images [12]. Likewise, blocking glucocorticoid synthesis with the drug metyrapone impairs memory for aversive experiences such as footshock [13] or exposure to a video of a threatening conspecific [14]. The mechanisms of glucocorticoid action have been particularly well delineated by Roozendaal and colleagues using an inhibitory avoidance task. They have shown that stimulation of glucocorticoid receptors in the basolateral amygdala (BLA) enhances memory consolidation by modulating α1-adrenergic receptors which, in turn, facilitates the β-adrenergic-cAMP cascade [15]. These findings on stress-related memories contrast with classic genomic actions of glucocorticoids and provide an example of rapid, non-genomic glucocorticoid action at membrane-bound receptors.
In addition to CRF’s critical role in initiating the neuroendocrine response to a stressor, CRF and other stress-related neuropeptides such as urocortins act in non-hypothalamic brain regions to modulate behavioral responses to aversive and emotional events. For example, post-training injection of a non-selective CRF receptor antagonist into the BLA has been shown to impair memory consolidation in an inhibitory avoidance task [16]. The actions of CRF and the urocortins are mediated by CRF-1 and CRF-2 receptors, and the effects of these ligands depend in part on the type of receptor activated. Activation of CRF-1 receptors in both the dorsal hippocampus and BLA have been shown to enhance conditioned fear [17,18]. In contrast, the effects of CRF-2 receptor activation appear to be brain region-dependent. Pharmacological activation of CRF-2 receptors in the lateral septum impairs fear conditioning [17], whereas CRF-2 receptor activation in other brain regions enhances stress-induced changes in behavior. For example, pre-training injection of a CRF-2 receptor antagonist into the dorsal hippocampus prevents the acquisition of stress-enhanced fear conditioning [19]. Similarly, pre-training injection of a CRF-2 receptor antagonist into the dorsal raphe nucleus prevents the acquisition of learned helplessness [20].
The goal of the current study was to determine which neuroendocrine signals of the HPA axis modulate the acquisition of stress-induced changes in behavior using a social defeat model in Syrian hamsters called conditioned defeat. Conditioned defeat is characterized by a defeat-induced switch from species-typical territorial aggression to heightened submissive and defensive behavior in future social encounters. Social defeat in hamsters activates the HPA axis [21,22], although it is unclear which neuroendocrine signals, if any, modulate the acquisition of conditioned defeat. Because CRF, urocortins, and glucocorticoids are each capable of modulating experience-dependent changes in behavior that occur following stressful events, we hypothesized that blocking glucocorticoid synthesis, blocking CRF-1 receptors, and blocking CRF-2 receptors would reduce the acquisition of conditioned defeat.
2. Methods
2.1. General procedures
2.1.1. Animals
Male Syrian hamsters (Mesocricetus auratus) were purchased from Charles River Laboratories (Wilmington, MA). Subjects were three to four months old and weighed 120–140 g at the start of the study. They were individually housed for 10–14 days prior to testing to allow them to scent mark their territory. They were also handled daily to habituate them to the stress of human contact during the experimental procedures. Older hamsters (>6 months) that weighed 160–180 g were housed individually and used as resident aggressors for social defeat. Younger hamsters (~2 months) that weighed 90–110 g were group-housed and used as non-aggressive intruders for conditioned defeat testing. All animals were housed in polycarbonate cages (20 X 40 X 20 cm) with corncob bedding, cotton nesting materials, and wire mesh tops. All animals were housed in a temperature-controlled colony room (20 ± 2 °C) and maintained on a 14:10 hr light-dark cycle. Food and water were available ad libitum. All procedures were approved by the Georgia State University Animal Care and Use Committee and are in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.1.2. Conditioned defeat protocol
Our conditioned defeat protocol has been extensively described elsewhere [23] and is briefly described here. Prior to each experiment, hamsters were matched by weight and randomly assigned to groups. We performed social defeat and subsequent behavioral testing under red light during the first 3 hr of the dark phase of the light-dark cycle. Social defeat consisted of a single 15-min exposure to a resident aggressor in the aggressor’s home-cage. To equalize the duration of social defeat, we started the 15-min exposure at the first attack initiated by the resident aggressor, which occurred within the first 30 s of all encounters. To determine whether subjects received comparable social defeats we recorded all social defeats and quantified the number of attacks subjects received, the duration of aggression subjects received, and the duration of submissive and defensive behavior subjects displayed. Two subjects were removed from the study because of minor wounding during social defeat and were treated by a veterinarian.
Conditioned defeat testing occurred 24 h after social defeat. Testing consisted of a 5-min exposure to a non-aggressive intruder in the subject’s home cage. We recorded the total duration of four classes of behavior during the 5-min test: (a) social (attend, approach, investigate, sniff, nose touch, and flank mark); (b) nonsocial (locomotion, exploration, self-groom, nest build, feed, and sleep); (c) submissive and defensive (flee, avoid, tail up, upright and side defense, full submissive posture, stretch-attend, head flag, and attempt to escape from cage); and (d) aggressive (upright and side offense, chase, and attack including bite). Training and testing sessions were videotaped and later scored by observers blind to the experimental conditions using behavioral analysis software (The Observer, Noldus Information Technology, Wageningen, Netherlands). Inter-observer reliability on the duration of submissive and defensive behavior was 92%. A reduction in conditioned defeat was indicated by a statistically significant decrease in the duration of submissive and defensive behavior during testing.
2.1.3. Surgical procedures
Hamsters were anesthetized with sodium pentobartitol (90 mg/kg) and stereotaxically implanted with a 4 mm, 26-gauge guide cannula aimed at the lateral ventricle. Lambda and bregma were leveled prior to guide cannula implantation. The stereotaxic coordinates were 0.9 mm anterior to bregma, 1.4 mm lateral to bregma, and 3.3 mm below dura. The guide cannula was aimed 1.1 mm dorsal to the lateral ventricle to avoid puncturing it, and the final depth was reached only when a 33-gauge injection needle with a 2.2 mm projection was inserted. After surgery, dummy stylets were placed in the guide cannula to help prevent clogging. All animals were given 10–14 days to recover from surgery. At the end of the experiment, hamsters were given a lethal dose of sodium pentobarbital and infused with 0.5 µl of India ink to verify injection placements. Brains were removed and stored in 10% buffered formalin. Later, brains were sliced with a razor blade along the guide cannula track and examined for ink in the lateral ventricle. Only hamsters with ink inside the lateral ventricle were included in statistical analysis.
2.1.4. Statistical analysis
Behavioral data from social defeat training and conditioned defeat testing are shown as group means ± SEM. Data were analyzed using separate one-way between-subjects ANOVAs or T-tests, and Tukey tests were used for pairwise comparisons. All statistical tests were two-tailed and the alpha level was 0.05.
2.2. Experimental procedures
2.2.1. Metyrapone and cortisol levels
In this experiment we verified that metyrapone [2-methyl-1,2-di-3pyridyl-1-propanone (Sigma)], a glucocorticoid synthesis inhibitor, would prevent a defeat-induced increase in circulating cortisol. We specifically tested cortisol levels because it, rather than corticosterone, is the primary glucocorticoid in Syrian hamsters. We injected 18 animals with 0.2 ml subcutaneous (s.c) metyrapone (50 mg/kg or 100 mg/kg) or vehicle 90-min prior to a 15-min social defeat. The doses of metyrapone and the delay between injection and social defeat were adapted from previous research [24]. Metyrapone was dissolved in polyethylene glycol and diluted with saline to reach a final concentration of 40% polyethylene glycol. Immediately following social defeat, hamsters were rapidly decapitated and trunk blood was collected for cortisol radioimmunoassay (RIA). Trunk blood was transferred into heparinized tubes kept on ice, centrifuged at 4°C and 3500 rpm for 20 min, and the plasma was stored at −20°C until assay. The cortisol assay was performed by the Endocrine Core Laboratory, Yerkes Primate Research Center of Emory University (Atlanta, GA), using an RIA kit produced by Diagnostic Systems Laboratories (Webster, TX). All samples were run in duplicate in a single assay. The normal range for this assay is 0.5 – 60 µg/dl in a 25µl sample. Intra-assay variation was 4.9%.
2.2.2. Metyrapone and conditioned defeat
In this experiment we tested the prediction that systemic administration of metyrapone would reduce the acquisition of conditioned defeat. Nineteen adult male hamsters were given a 0.2 ml injection of metyrapone (50 mg/kg; s.c.) or vehicle 90-min prior to a 15-min social defeat. We used 50 mg/kg of metyrapone because it was the minimum effective dose validated in the previous experiment described above. Twenty-four hours after social defeat, animals were tested for conditioned defeat behavior.
2.2.3. CRF receptor antagonists and conditioned defeat
In this experiment we hypothesized that blockade of CRF-1 and CRF-2 receptors would reduce the acquisition of conditioned defeat. To test this hypothesis we used three separate cohorts of animals and injected a non-selective CRF receptor antagonist (D-Phe CRF(12–41), Bachem), a selective CRF-2 receptor antagonist (anti-sauvagine-30, Polypeptide Laboratories), and a selective CRF-1 receptor antagonist (CP-154,526, courtesy of Pfizer, Groton, CT) into the lateral ventricle. Drug doses were as follows: D-Phe CRF (0 µg or 25 µg in 3 µl saline); anti-sauvagine-30 (0 µg, 10 µg or 20 µg in 3 µl saline); and CP-154,526 (0 µg, 10 µg, 20 µg, or 40 µg in 3 µl saline and 15% DMSO). Doses of D-Phe CRF, anti-sauvagine-30, and CP-154,526 were selected on the basis of previous research [25–27]. All drugs were infused into the lateral ventricle 30 min prior to social defeat. We performed infusions by hand with a 5 µl Hamilton syringe connected to a 33-gauge needle via polyethylene tubing at a rate of 3 µl per min. The needle remained in place for an additional 1 min to allow diffusion of the drug solution. Drug or vehicle was kept separate from the water in the tubing by a 0.5 µl air bubble. Movement of the air bubble down the tubing was taken as evidence of a successful injection. The dummy stylet was replaced before social defeat. All animals were tested for conditioned defeat as described above.
3. Results
3.1. Blocking glucocorticoid synthesis and the acquisition of conditioned defeat
3.1.1. Blocking glucocorticoid synthesis with metyrapone
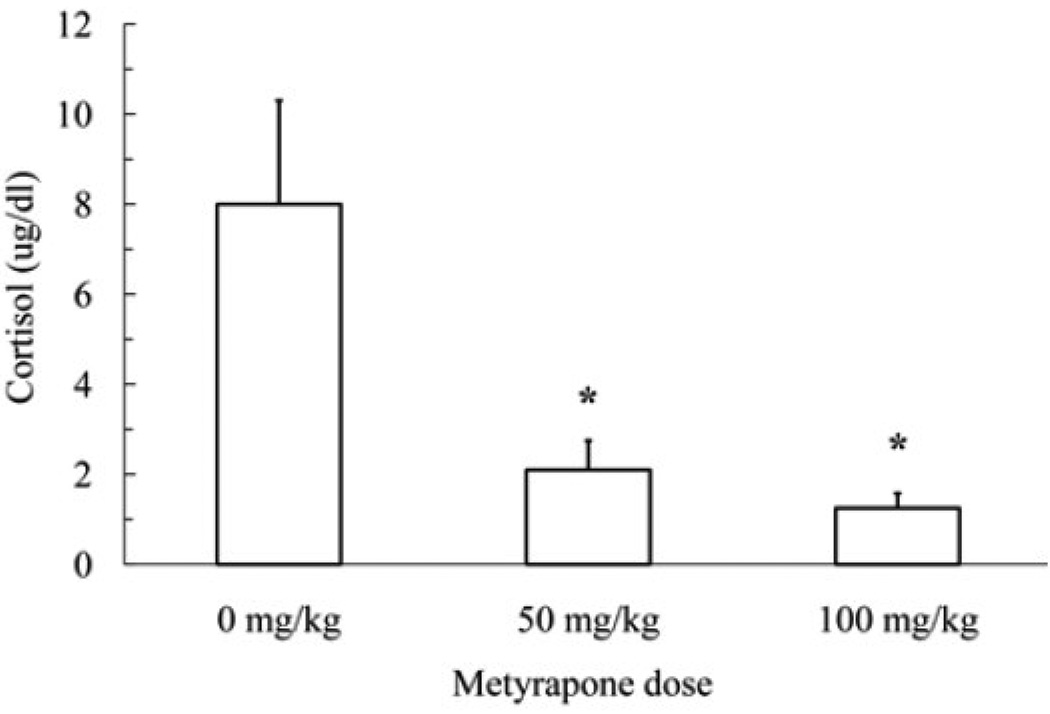
Metyrapone treatment blocked the elevated cortisol levels exhibited after social defeat (F(2,15) = 40.5, p < 0.001) (Figure 1). Post-hoc analysis revealed that the 50 and 100 mg/kg doses were both significantly different from vehicle but were not significantly different from each other (p < .05).
Figure 1.
Cortisol levels (mean ± SD) are shown for hamsters that received an injection of metyrapone (50 mg/kg, N = 6; 100 mg/kg, N = 6) or vehicle (0 mg/kg, N = 6) prior to a 15-min social defeat. The data indicate that metyrapone treatment blocks defeat-induced increases in cortisol. The asterisks indicate a significant difference compared to vehicle controls (p < .05).
3.1.2. Conditioned defeat and blocking glucocorticoid synthesis
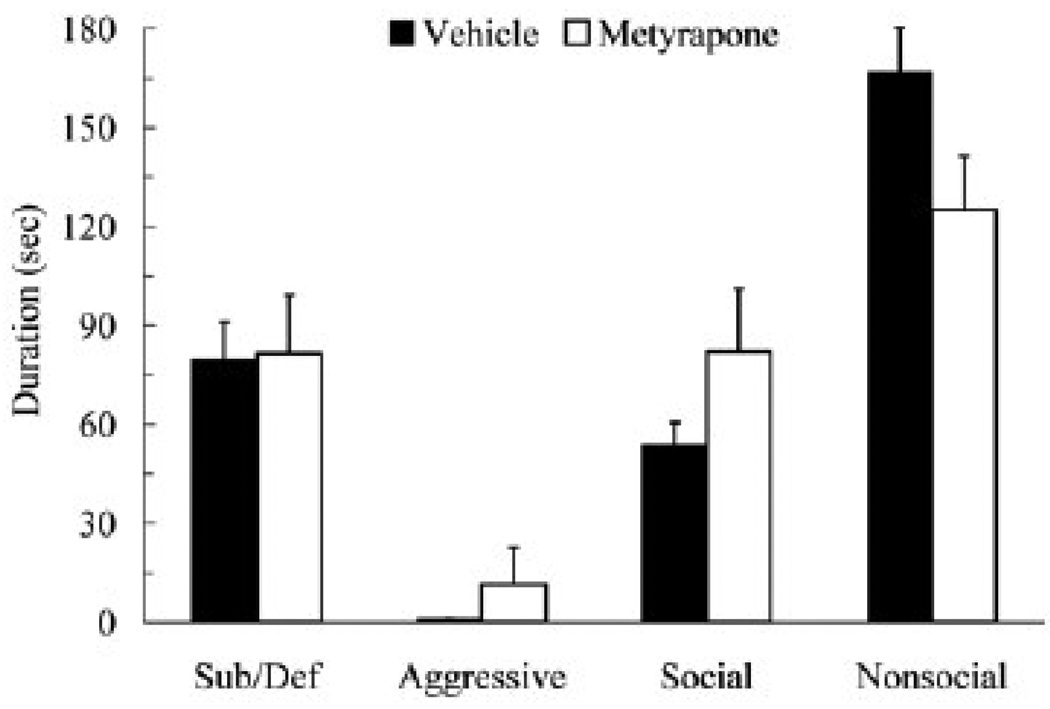
In a subsequent conditioned defeat experiment, we used the lowest effective dose of metyrapone only (50 mg/kg). When given prior to social defeat, metyrapone did not significantly alter the duration of submissive and defensive behavior displayed at testing (t(17) = 0.03, p > 0.05) (Figure 2). Also, metyrapone did not significantly alter the duration of aggressive, social, or nonsocial behavior during testing (p > 0.05). To account for possible variation in social defeat experience, we measured the strength of social defeat and the response of subjects to social defeat. Vehicle-treated and metyrapone-treated animals did not significantly differ in the number of attacks received, in the duration of aggression received, or in the duration of submissive and defensive behavior produced during social defeat (p > 0.05; Table 1).
Figure 2.
Durations (mean ± SEM) of submissive and defensive (sub/def), aggressive, social, and nonsocial behavior are shown for defeated hamsters during a 5-min test with a non-aggressive opponent. Animals received vehicle (N = 9) or metyrapone (50 mg/kg, N = 10) 90 min prior to social defeat. Metyrapone-treated animals did not significantly differ from vehicle controls (p > 0.05).
Table 1.
Effects of drug treatment on social defeat experience. We measured social defeat strength by recording the frequency of attacks initiated by resident aggressors and the total duration of aggression subjects received. We measured response to social defeat by recording the total duration of submissive and defensive (sub/def) behavior subjects produced. Drug treatments did not significantly alter any social defeat measure. All data are group means ± SEM.
| Attacks (frequency) |
Aggression (sec) |
Sub/Def (sec) |
|
|---|---|---|---|
| Metyrapone treatment | |||
| Vehicle | 12.8 ± 1.2 | 427.7 ± 64.4 | 619.3 ± 23.9 |
| 50 mg/kg | 14.7 ± 1.5 | 367.2 ± 49.6 | 611.3 ± 31.4 |
| α-level | p > 0.05 | p > 0.05 | p > 0.05 |
| D-Phe CRF treatment | |||
| Vehicle | 12.8 ± 1.2 | 398.9 ± 27.1 | 619.4 ± 26.5 |
| 25 µg | 13.3 ± 1.3 | 376.2 ± 28.1 | 633.2 ± 34.1 |
| α-level | p > 0.05 | p > 0.05 | p > 0.05 |
| Anti-sauvagine-30 treatment | |||
| Vehicle | 14.5 ± 1.5 | 420.6 ± 26.1 | 638.7 ± 30.2 |
| 10 µg | 14.1 ± 1.4 | 392.6 ± 55.6 | 654.3 ± 35.3 |
| 20 µg | 13.9 ± 1.4 | 414.0 ± 51.1 | 625.2 ± 29.8 |
| α-level | p > 0.05 | p > 0.05 | p > 0.05 |
| CP-154,526 treatment | |||
| Vehicle | 13.9 ± 1.2 | 386.0 ± 37.8 | 601.7 ± 22.5 |
| 10 µg | 16.1 ± 1.3 | 395.2 ± 29.9 | 636.3 ± 21.7 |
| 20 µg | 13.6 ± 1.3 | 375.5 ± 35.7 | 613.8 ± 35.9 |
| 40 µg | 15.1 ± 1.1 | 368.6 ± 31.7 | 594.6 ± 22.5 |
| α-level | p > 0.05 | p > 0.05 | p > 0.05 |
3.2. CRF receptor blockade and the acquisition of conditioned defeat
3.2.1. Conditioned defeat and non-selective CRF receptor blockade
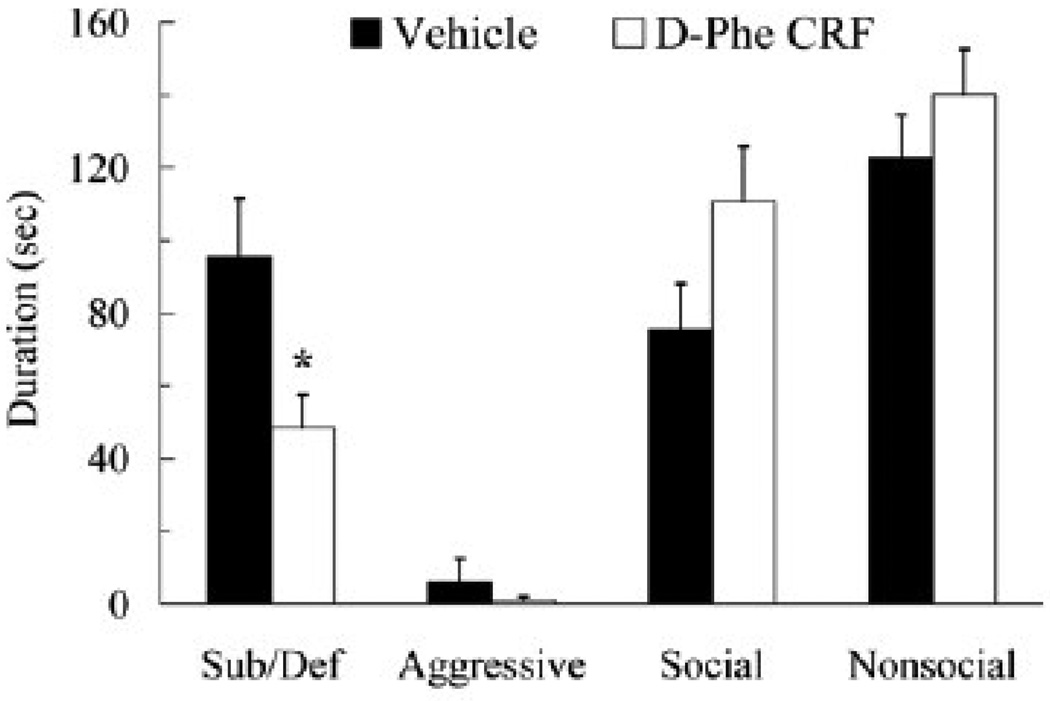
Injection of D-Phe CRF into the lateral ventricle prior to social defeat reduced conditioned defeat behavior at testing (Figure 3). Specifically, D-Phe CRF-treated animals displayed less submissive and defensive behavior at testing than did vehicle controls (t(22) = 2.54, p = .019). D-Phe CRF-treated animals did not significantly differ from controls in the duration of aggressive, social, or nonsocial behavior during testing (p > .05). Reduction in the acquisition of conditioned defeat was not due to systematic variation in social defeat experience. D-Phe CRF animals and vehicle controls did not significantly differ in the number of attack received, the total duration of aggression received, or the total duration of submissive and defensive behavior produced (p > 0.05; Table 1).
Figure 3.
Durations (mean ± SEM) of submissive and defensive (sub/def), aggressive, social, and nonsocial behavior are shown for defeated hamsters during a 5-min test with a non-aggressive opponent. Animals received an injection of vehicle (N = 12) or D-Phe CRF (25 µg, N = 12) into the lateral ventricle 30 min prior to social defeat. The asterisk indicates a significant difference compared to vehicle controls (p < 0.05).
3.2.2. Conditioned defeat and selective CRF-2 receptor blockade
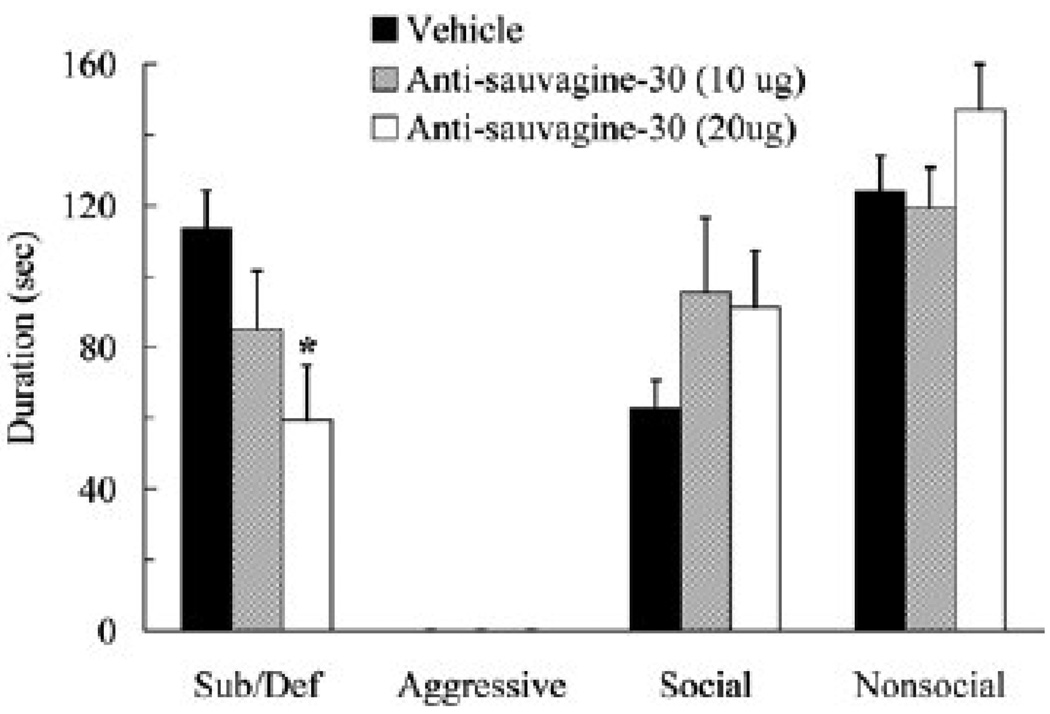
Animals that received 20 µg of anti-sauvagine-30 into the lateral ventricle prior to social defeat showed reduced conditioned defeat 24 hours later (Figure 4). Specifically, they exhibited significantly less submissive and defensive behavior during conditioned defeat testing than did vehicle controls (F(2,28) = 3.67, p = .038; Tukey, p = .030). In contrast, they did not significantly differ from vehicle controls in the duration of aggressive, social, or nonsocial behavior (p > .05). Variation in social defeat experience could not account for the effect of anti-sauvagine-30 treatment on the acquisition of conditioned defeat. Anti-sauvagine-30 treatment did not alter the number of attacks subjects received, the total duration of aggression subjects received, or the amount of submissive and defensive behavior subjects produced (p > 0.05; Table 1).
Figure 4.
Durations (mean ± SEM) of submissive and defensive (sub/def), aggressive, social, and nonsocial behavior are shown for defeated hamsters during a 5-min test with a non-aggressive opponent. Animals received an injection of vehicle (N = 11) or anti-sauvagine-30 (10 µg, N = 10; 20 µg, N = 10) into the lateral ventricle 30 min prior to social defeat. The asterisk indicates a significant difference compared to vehicle controls (p < 0.05).
3.2.3. Conditioned defeat and selective CRF-1 receptor blockade
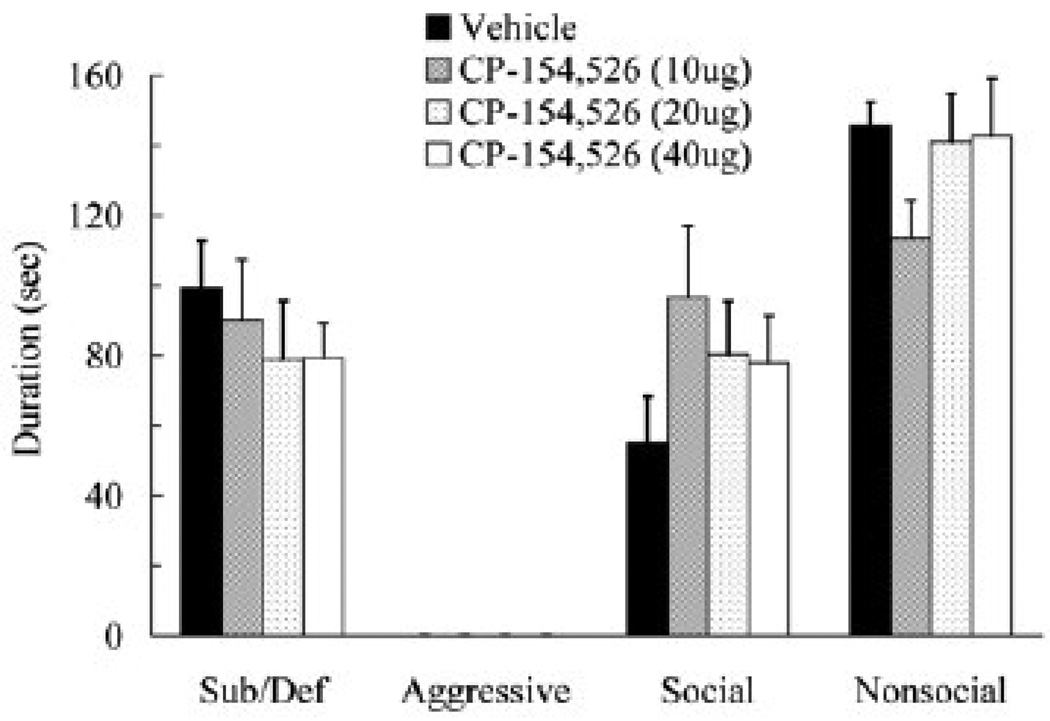
Injection of CP-154,526 into the lateral ventricle prior to social defeat did not reduce conditioned defeat behavior during testing (Figure 5). Specifically, CP-154,526 did not produce a significant decrease in submissive and defensive behavior during conditioned defeat testing (F(3,39) = 0.49, p > .05). Likewise, CP-154,526-treated animals did not significantly differ from vehicle controls in the duration of aggressive, social, or nonsocial behavior (p > .05). CP-154,526 treatment did not alter the number of attacks subjects received, the total duration of aggression subjects received, or the amount of submissive and defensive behavior subjects produced during defeat training (p > 0.05; Table 1).
Figure 5.
Durations (mean ± SEM) of submissive and defensive (sub/def), aggressive, social, and nonsocial behavior are shown for defeated hamsters during a 5-min test with a non-aggressive opponent. Animals received an injection of vehicle (N = 12) or CP-154,526 (10 µg, N = 10; 20 µg, N = 10; 40 µg, N = 11) into the lateral ventricle 30 min prior to social defeat. CP-154,526-treated animals did not significantly differ from vehicle controls (p > 0.05).
3.2.4. Anatomical controls
Several animals received an injection outside the lateral ventricle and into either the lateral septum or caudate nucleus and these animals were analyzed as anatomical controls. To increase our sample size, we pooled vehicle-treated animals from each CRF antagonist experiment, and we pooled drug-treated animals from the multiple doses of each drug. We found that none of the CRF antagonists reduced the acquisition of conditioned defeat in anatomical controls. Specifically, vehicle animals showed 107.4 sec (±16.4, N = 8) of submissive and defensive behavior, D-Phe CRF animals showed 135.9 sec (±24.3, N = 2), anti-sauvagine-30 animals showed 126.8 sec (±21.5, N = 6), and CP-154,526 animals showed 115.8 sec (±19.3, N = 3) during conditioned defeat testing (F(3,15) = 0.31, p > .05). Because activation of CRF-2 receptors in the lateral septum is known to modulate stress-related behavior, we separately analyzed anatomical controls that received anti-sauvagine-30 injection into the lateral septum. These animals did not shown impaired acquisition of conditioned defeat (105.2 ± 26.2 sec of submissive and defensive behavior, N = 4).
4. Discussion
We found that blocking glucocorticoid synthesis with metyrapone prior to social defeat failed to reduce the display of submissive and defensive behavior at conditioned defeat testing. Also, blocking glucocorticoid synthesis did not alter submissiveness during social defeat. In contrast, we found that non-selective pharmacological blockade of CRF receptors prior to social defeat reduced submissive and defensive behavior during testing. This effect was mimicked by a selective CRF-2 receptor antagonist but not by a selective CRF-1 receptor antagonist. Taken together, our results suggest that CRF and related neuropeptides such as urocortins act on CRF-2 receptors to promote the development of conditioned defeat, whereas glucocorticoid feedback and signaling at CRF-1 receptors play a negligible role in this behavioral response.
Our results are in contrast to previous research on glucocorticoids and stress-induced changes in behavior. Others have found that post-training glucocorticoid treatment in rats enhances memory consolidation for aversive experiences [10,11], whereas blocking glucocorticoid synthesis impairs it [13,24]. In our study, drug treatments were administered prior to social defeat training so that we could target the acquisition of conditioned defeat. Pre-training drug treatments are common in Pavlovian fear conditioning, learned helplessness, and other models of stress-induced changes in behavior. However, pre-training drug treatments do not exclude the possibility that drugs might alter fear, anxiety, or attentional processes during memory acquisition. It seems unlikely that pre-training drug administration accounts for the failure of metyrapone to alter the formation of conditioned defeat, partly because pre-training glucocorticoid treatment enhances the acquisition of other types of stress-induced changes in behavior [9]. Also, our metyrapone results are consistent with our previous research that indicates a limited role for cortisol in the development of conditioned defeat [25]. Also, cortisol appears insufficient to alter conditioned defeat behavior in female Syrian hamsters. We have shown that female hamsters exhibit robust increases in plasma ACTH and cortisol following social defeat, yet they display very little conditioned defeat behavior [23].
Our results are consistent with the overall effects of CRF and related neuropeptides on stress-induced changes in behavior. Intracerebroventricular administration of urocortin, which is an endogenous ligand for both CRF-1 and CRF-2 receptors [28], has been shown to facilitate both the acquisition and consolidation of passive avoidance learning [29]. Although our results suggest that blocking CRF receptors reduces the acquisition of conditioned defeat, facilitating the acquisition of conditioned defeat by activating CRF receptors has been more difficult to demonstrate. Our lab found that intracerebroventricular administration of a non-selective CRF receptor agonist prior to social defeat did not enhance the acquisition of conditioned defeat [30]. Our data on CRF agonists and antagonists together suggest that activation of CRF receptors is necessary but not sufficient for the acquisition of conditioned defeat. It seems likely that non-CRF mechanisms also play an important role in the acquisition of conditioned defeat and that signaling at CRF receptors alone does not provide a sufficient neurochemical representation of a social defeat experience.
Both CRF-1 and CRF-2 receptors have been shown to modulate behavioral changes that occur following stressful events. Activation of CRF-1 receptors in the hippocampus and BLA appear to enhance the formation of memories for aversive events [17,18,31,32]. In our study, intracerebroventricular administration of a CRF-1 receptor antagonist failed to alter the acquisition of conditioned defeat. On possible explanation for our results is that the drug did not travel to its site of action in sufficient concentration. One potential site of action for CRF ligands is the dorsal raphe nucleus [33,34]. We have previously shown that injection of a non-selective CRF receptor antagonist into the dorsal raphe nucleus reduces the acquisition of conditioned defeat in hamsters, although a selective CRF-2 receptor antagonist does not [35]. These results leave open a possible role for CRF-1 receptors in the dorsal raphe nucleus, although CP-154,526 may not have reached this brain region in our study.
The activation of CRF-2 receptors has been shown to both enhance and impair stress-induced changes in behavior. Activation of CRF-2 receptors in the lateral septum impairs the acquisition of conditioned fear, perhaps because CRF-2 receptor activation in the lateral septum enhances anxiety [17,36,37]. In contrast, activation of CRF-2 receptors in brain regions outside the lateral septum enhances the formation of stress-induced changes in behavior. For example, injection of a CRF-2 receptor antagonist into the dorsal hippocampus of mice prevents stress-enhanced fear conditioning [19]. Also, injection of a CRF-2 receptor antagonist into the dorsal raphe nucleus of rats reduces the acquisition of learned helplessness [20].
Candidate brain regions for mediating the effect of CRF-2 receptor blockade on the acquisition of conditioned defeat include the BLA, hippocampus, medial amygdala, bed nucleus of the stria terminalis (BNST), and lateral septum. The BLA is a critical neural substrate for the plastic changes that underlie the acquisition of conditioned defeat [38–40]. In rats and mice, CRF-1 receptors in the BLA modulate the formation of stress-induced behavioral changes [18,32]. It is unclear whether BLA CRF-2 receptors can modulate similar processes in hamsters. We have shown that neural transmission in both the hippocampus and medial amygdala contributes to the acquisition of conditioned defeat, likely via their respective connections with the BLA [39,41]. Interestingly, CRF-2 receptors in both brain regions may contribute to behavioral changes that occur following stressful events. For example, stress-enhanced fear conditioning is disrupted by CRF-2 receptor blockade in the hippocampus [19]. Also, social defeat activates medial amygdala cells that express CRF-2 receptor mRNA [42]. We have previously shown that pharmacological blockade of CRF-2 receptors in the BNST prior to testing reduces the expression of conditioned defeat [26]. A role for BNST CRF-2 receptors in the acquisition of conditioned defeat appears unlikely because temporarily inactivating the BNST with muscimol fails to alter the acquisition of conditioned defeat [43]. The lateral septum is also an unlikely candidate partly because CRF-2 receptor activation in the lateral septum impairs the acquisition of conditioned fear [17], whereas our results suggest that CRF-2 receptor activation enhances the acquisition of conditioned defeat. Also, our anatomical control data suggest that CRF-2 receptors in the lateral septum did not mediate the effect of intracerebroventricular anti-sauvagine-30 injection.
The present findings improve our understanding of the neurochemical signals that modulate experience-dependent changes in behavior that occur in hamsters following stressful events. Our results indicate that CRF-2 receptors are an important component of the neural circuitry controlling the acquisition of conditioned defeat, whereas CRF-1 receptors and glucocorticoid feedback are not. Our results do not support long held assumptions that glucocorticoid feedback consistently functions to modulate ongoing and future behavior [7]. Rather they suggest that activation of CRF-2 receptors, by peptides such as urocortin 2 [44] and urocortin 3 [45], modulates behavioral responses to stressful events.
Research Highlights
-
➢
Blocking glucocorticoid synthesis does not alter the formation of conditioned defeat.
-
➢
Blocking CRF-2, but not CRF-1, receptors reduces the formation of conditioned defeat.
-
➢
Centrally acting CRF-related neuropeptides are more critical for modulating defeat-induced changes in behavior in hamsters than are glucocorticoids.
Acknowledgments
We thank Alisa Norvelle for her expert technical assistance. This research was supported by National Institutes of Health (NIH) grants MH62044 to Kim Huhman and F32 MH72085 to Matthew Cooper. Also, this research is based upon work supported in part by The Center for Behavioral Neuroscience, a National Science Foundation (NSF) Science and Technology Center program under agreement No. IBN-9876754.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. Journal of Endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 3.Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 4.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848:141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 5.Haller J, Albert I, Makara GB. Acute behavioural effects of corticosterone lack specificity but show marked context-dependency. J Neuroendocrinol. 1997;9:515–518. doi: 10.1046/j.1365-2826.1997.00603.x. [DOI] [PubMed] [Google Scholar]
- 6.Mikics E, Kruk MR, Haller J. Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology. 2004;29:618–635. doi: 10.1016/S0306-4530(03)00090-8. [DOI] [PubMed] [Google Scholar]
- 7.Leshner AI, Politch JA. Hormonal control of submissiveness in mice: irrelevance of the androgens and relevance of the pituitary-adrenal hormones. Physiol Behav. 1979;22:531–534. doi: 10.1016/0031-9384(79)90021-0. [DOI] [PubMed] [Google Scholar]
- 8.Leshner AI. The interaction of experience and neuroendocrine factors in determining behavioral adaptations to aggression. Progress in Brain Research. 1980;53:427–438. doi: 10.1016/S0079-6123(08)60081-3. [DOI] [PubMed] [Google Scholar]
- 9.Beylin AV, Shors TJ. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm Behav. 2003;43:124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roozendaal B, de Quervain DJ, Ferry B, Setlow B, McGaugh JL. Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation. J Neurosci. 2001;21:2518–2525. doi: 10.1523/JNEUROSCI.21-07-02518.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol. Learn. Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan TW, Lovallo WR. Enhanced memory for emotional material following stress-level cortisol treatment in humans. Psychoneuroendocrinology. 2001;26:307–317. doi: 10.1016/s0306-4530(00)00058-5. [DOI] [PubMed] [Google Scholar]
- 13.Liu L, Tsuji M, Takeda H, Takada K, Matsumiya T. Adrenocortical suppression blocks the enhancement of memory storage produced by exposure to psychological stress in rats. Brain Res. 1999;821:134–140. doi: 10.1016/s0006-8993(99)01085-9. [DOI] [PubMed] [Google Scholar]
- 14.Yang EJ, Wilczynski W. Interaction effects of corticosterone and experience on aggressive behavior in the green anole lizard. Horm Behav. 2003;44:281–292. doi: 10.1016/s0018-506x(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 15.Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor--cAMP/cAMP/PKA system in influencing memory consolidation. Eur. J. Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- 16.Roozendaal B, Brunson KL, Holloway BL, McGaugh JL, Baram TZ. Involvement of stress-released corticotropin-releasing hormone in the basolateral amygdala in regulating memory consolidation. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13908–13913. doi: 10.1073/pnas.212504599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999;19:5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007;150:818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sananbenesi F, Fischer A, Schrick C, Spiess J, Radulovic J. Mitogen-activated protein kinase signaling in the hippocampus and its modulation by corticotropin-releasing factor receptor 2: a possible link between stress and fear memory. J Neurosci. 2003;23:11436–11443. doi: 10.1523/JNEUROSCI.23-36-11436.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, Watkins LR, Maier SF. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–1025. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huhman KL, Bunnell BN, Mougey EH, Meyerhoff JL. Effects of social conflict on POMC-derived peptides and glucocorticoids in male golden hamsters. Physiol Behav. 1990;47:949–956. doi: 10.1016/0031-9384(90)90023-w. [DOI] [PubMed] [Google Scholar]
- 22.Huhman KL, Moore TO, Ferris CF, Mougey EH, Meyerhoff JL. Acute and repeated exposure to social conflict in male golden hamsters: increases in plasma POMC-peptides and cortisol and decreases in plasma testosterone. Horm Behav. 1991;25:206–216. doi: 10.1016/0018-506x(91)90051-i. [DOI] [PubMed] [Google Scholar]
- 23.Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- 25.Jasnow AM, Banks MC, Owens EC, Huhman KL. Differential effects of two corticotropin-releasing factor antagonists on conditioned defeat in male Syrian hamsters (Mesocricetus auratus) Brain Res. 1999;846:122–128. doi: 10.1016/s0006-8993(99)02007-7. [DOI] [PubMed] [Google Scholar]
- 26.Cooper MA, Huhman KL. Corticotropin-releasing factor type II (CRF2) receptors in the bed nucleus of the stria terminalis modulate conditioned defeat in Syrian hamsters (Mesocricetus auratus) Behav Neurosci. 2005;119:1042–1051. doi: 10.1037/0735-7044.119.4.1042. [DOI] [PubMed] [Google Scholar]
- 27.Nijsen MJ, Croiset G, Stam R, Bruijnzeel A, Diamant M, de Wied D, Wiegant VM. The role of the CRH type 1 receptor in autonomic responses to corticotropin- releasing hormone in the rat. Neuropsychopharmacology. 2000;22:388–399. doi: 10.1016/S0893-133X(99)00126-8. [DOI] [PubMed] [Google Scholar]
- 28.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 29.Telegdy G, Tiricz H, Adamik A. Involvement of neurotransmitters in urocortin-induced passive avoidance learning in mice. Brain Res Bull. 2005;67:242–247. doi: 10.1016/j.brainresbull.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Faruzzi AN. Ph.D. dissertation. Atlanta, USA: Georgia State University; 2005. Corticotropin releasing factor receptors and agonistic behavior in Syrian hamsters. [Google Scholar]
- 31.Zorrilla EP, Schulteis G, Ormsby A, Klaassen A, Ling N, McCarthy JR, Koob GF, De Souza EB. Urocortin shares the memory modulating effects of corticotropin-releasing factor (CRF): mediation by CRF1 receptors. Brain Res. 2002;952:200–210. doi: 10.1016/s0006-8993(02)03345-0. [DOI] [PubMed] [Google Scholar]
- 32.Robison CL, Meyerhoff JL, Saviolakis GA, Chen WK, Rice KC, Lumley LA. A CRH1 antagonist into the amygdala of mice prevents defeat-induced defensive behavior. Ann N Y Acad Sci. 2004;1032:324–327. doi: 10.1196/annals.1314.052. [DOI] [PubMed] [Google Scholar]
- 33.Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- 34.Lukkes J, Vuong S, Scholl J, Oliver H, Forster G. Corticotropin-releasing factor receptor antagonism within the dorsal raphe nucleus reduces social anxiety-like behavior after early-life social isolation. J Neurosci. 2009;29:9955–9960. doi: 10.1523/JNEUROSCI.0854-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper MA, Huhman KL. Corticotropin-releasing factor receptors in the dorsal raphe nucleus modulate social behavior in Syrian hamsters. Psychopharmacology (Berl) 2007;194:297–307. doi: 10.1007/s00213-007-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bakshi VP, Smith-Roe S, Newman SM, Grigoriadis DE, Kalin NH. Reduction of stress-induced behavior by antagonism of corticotropin- releasing hormone 2 (CRH2) receptors in lateral septum or CRH1 receptors in amygdala. J.Neurosci. 2002;22:2926–2935. doi: 10.1523/JNEUROSCI.22-07-02926.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Todorovic C, Radulovic J, Jahn O, Radulovic M, Sherrin T, Hippel C, Spiess J. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. Eur. J. Neurosci. 2007;25:3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- 38.Jasnow AM, Shi C, Israel JE, Davis M, Huhman KL. Memory of social defeat is facilitated by cAMP response element-binding protein overexpression in the amygdala. Behav Neurosci. 2005;119:1125–1130. doi: 10.1037/0735-7044.119.4.1125. [DOI] [PubMed] [Google Scholar]
- 39.Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn. Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jasnow AM, Cooper MA, Huhman KL. N-methyl-D-aspartate receptors in the amygdala are necessary for the acquisition and expression of conditioned defeat. Neuroscience. 2004;123:625–634. doi: 10.1016/j.neuroscience.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 41.Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn. Mem. 2010;17:109–116. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav Brain Res. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc.Natl.Acad.Sci.U.S.A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc.Natl.Acad.Sci.U.S.A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]