Abstract
Early life adversity or parental neglect is linked to the development of a number of psychiatric illnesses, including major depression and substance use disorder. These two disorders are often comorbid and characterized by anhedonia, defined as the reduced ability to experience pleasure or reward. The aim of the present study was to determine the effects of neonatal maternal separation in Long Evans rats, a model of early life stress, on anhedonia under baseline conditions and in response to drug and stress exposure during adulthood. Three hours of daily maternal separation from postnatal day 1 to 14 led to marked decreases in arched-back nursing, licking, and grooming of pups by their dams. In adulthood, brain reward function was assessed using intracranial self-stimulation of the lateral hypothalamus. Decreased current thresholds derived from this procedure are interpreted as reward-enhancing effects, whereas elevations in thresholds are an operational measure of anhedonia. Maternally separated rats did not exhibit anhedonia under baseline conditions compared with non-handled controls but exhibited a greater reward-enhancing effect of acute amphetamine administration. Acute social defeat produced anhedonia in non-handled controls, but not in maternally separated rats. Conversely, control rats habituated to 7 days of repeated social defeat, whereas maternally separated rats developed an increased anhedonic response to the repeated stressor. One week after termination of stress exposure, maternally separated rats still exhibited an increased reward-enhancing effect of acute amphetamine administration compared with non-handled controls, regardless of prior social defeat experience. These data indicate that early life stress increases the reward-enhancing properties of amphetamine, protects against the anhedonic effects of acute stress exposure, and exacerbates the anhedonic response to repeated stress. Thus, early life stress may increase an individual’s vulnerability to depressive or addictive disorders when confronted with stress or drug challenge in adulthood.
Keywords: Early life stress, Maternal care, Anhedonia, Depression, ICSS, Psychostimulant
High comorbidity exists between affective disorders, such as major depressive disorder (MDD), and substance use disorders (Kessler et al., 2005), which may be attributable to common etiological conditions, such as stress (Kosten et al., 1998). Specifically, exposure to stressful life events is believed to precipitate depressive episodes (Charney and Manji, 2004) and increase craving for drugs of abuse (Sinha, 2008). Furthermore, dysregulation of reward function and altered reward sensitivity comprise a pathophysiological state that is common to both disorders. For example, one of the key features of MDD is anhedonia, or decreased interest or pleasure in rewarding events (American Psychiatric Association, 1994). Substance use disorders are characterized by heightened reward sensitivity during drug intake, as well as anhedonia during drug withdrawal (Leventhal et al., 2008). However, not all individuals develop MDD when confronted with stressful episodes, nor do they develop substance use disorders when experimenting with drugs of abuse.
Considerable epidemiological evidence suggests that exposure to negative early life events increases the vulnerability to a number of psychiatric illnesses in adulthood (Holmes and Robins, 1987; Holmes and Robins, 1988; Brown and Anderson, 1991; Feehan et al., 1991; Arellano, 1996; Russek and Schwartz, 1996; Canetti et al., 1997; Russek and Schwartz, 1997). Common adverse early life events include exposure to trauma or abuse from early childhood to adolescence, and these types of events are often investigated in epidemiological studies. Additionally, physical or emotional neglect during the early postnatal period may also confer vulnerability to psychiatric illnesses, including MDD and substance use disorders (Bifulco et al., 1991; Dobkin et al., 1997). While a direct link between early life stress and anhedonia has not been made, psychiatric illnesses such as MDD and substances use disorders that are associated with exposure to early life stress often include anhedonia as a core symptom. This form of early life adversity has been investigated extensively in rodent models, most often using the maternal separation procedure. However, the demonstration of anhedonia in a rodent model of early life stress remains challenging.
A number of possibilities may explain the discrepancy between clinical and preclinical findings on early life stress and anhedonia. First, most preclinical assessments of anhedonia after maternal separation occur under baseline conditions (i.e., not in response to any challenge; (Matthews et al., 1996; Shalev and Kafkafi, 2002; Ruedi-Bettschen et al., 2005; Ruedi-Bettschen et al., 2006). Early life adversity is purported to increase the vulnerability to psychiatric illness, although this vulnerability would indicate that some trigger is required to manifest the symptoms of the illness. Thus, understanding the effects of maternal separation on both baseline signs of anhedonia in adulthood and hedonic capacity in response to challenges (e.g., stress or drugs of abuse) is important. Second, unknown in most studies assessing behavioral responses to maternal separation is whether the separation procedure produces the desired maternal behaviors that are critical for the neurophysiological changes induced by this early life stressor. Specifically, the lack of maternal care toward the offspring is suggested to be a critical factor for determining behavioral and physiological deficits in adulthood (Champagne et al., 2003). High levels of maternal care, as measured by increased licking and grooming of the pups, results in a decreased neurophysiological response to stress in adulthood, similar to rats that were exposed to brief (e.g., 15 min) handling as neonates. Thus, it can be presumed that longer periods of neonatal maternal separation (e.g., 3 hr) would result in decreased levels of maternal care and an increased neurophysiological stress response in offspring. This interpretation is only partially true, as maternal separation has been shown to increase licking, grooming, and arched-back nursing compared to non-handled controls (Macri et al., 2004). Thus, it would be informative to determine whether any behavioral manifestation in adulthood is the direct result of changes in maternal care or some other factor associated with the maternal separation procedure, such as those responsible for changes in physical development.
The aims of the present study were to determine whether exposure to early life stress decreases the presence of maternal behaviors during the neonatal period and produces changes in brain reward function under baseline conditions and in response to both stress and drug challenges in adulthood. Brain reward function was assessed using intracranial self-stimulation (ICSS) of the posterior lateral hypothalamus, a sensitive procedure that can be used to quantify changes in hedonic capacity in response to both reward-enhancing (e.g., drugs of abuse) and reward-diminishing (e.g., stress) stimuli in the same subjects. That is, a rewarding stimulus, such as amphetamine treatment, is expected to lower the current that maintains ICSS responding presumably by facilitating activity in the brain circuits that carries the ICSS reward signal. Conversely, a stimulus that inhibits activity in these reward pathways would lead to the requirement of increased current intensities before the stimulus is perceived as rewarding. Decreased “interest or pleasure in rewarding stimuli”, including ICSS, is an operational definition of anhedonia analogous to the definition of anhedonia in clinical populations (American Psychiatric Association, 1994). An advantage of the ICSS procedure is that it does not require food or water deprivation, which is often necessary for and limits the interpretation of studies involving palatable food rewards. Specifically, in the studies presented here, we assessed the effects of neonatal maternal separation in rats on the presence of maternal behaviors during early postnatal life. During adulthood, we assessed hedonic capacity under baseline conditions and in response to acute and repeated social defeat stress and amphetamine administration using the ICSS procedure.
MATERIALS AND METHODS
Subjects
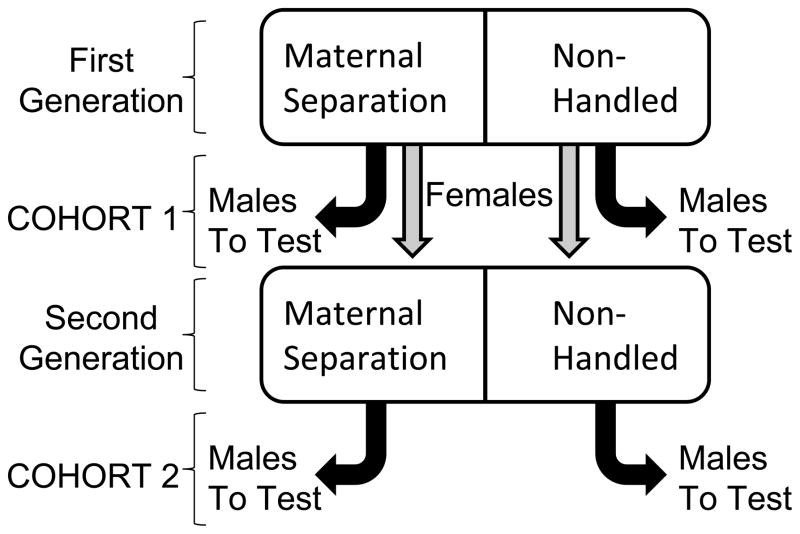
Two separate cohorts of maternally separated rats were used in this study. Maternal separation procedures were identical in both cohorts. In the first cohort, 30 timed-pregnant Long Evans rats (Charles River Laboratories, Raleigh, NC) arrived at the vivarium on gestational day 13 and were housed individually in Plexiglas cages with food and water available ad libitum. Maternal separation was conducted in 15 litters, while the other 15 litters remained undisturbed (see below). Male offspring from each litter comprised the first cohort of experimental rats. Female offspring from each litter were housed in pairs until 12 weeks of age. At this time point, one female from each litter was housed with a naive, age-matched, male Long Evans rat, which was later removed from the cage upon verification of pregnancy. Eighteen of the 30 females became pregnant and gave birth. Of these 18 females, nine had been exposed to maternal separation as neonates; their pups were also assigned to the maternal separation group. The other nine females served as non-handled controls as neonates; their pups were assigned to the non-handled control group. Male offspring from these litters comprised the second cohort of maternally separated and control rats (Fig. 1). All rats were maintained in a climate-controlled colony room at 21°C on a 12 h/12 h reverse light/dark cycle (lights off from 06:00–18:00). For social defeat experiments, separate male and female Long Evans rats were housed in pairs for a period of at least one breeding cycle before initiation of the experiments. The male rats from these pairs served as the aggressors in the social defeat experiments. All procedures were conducted in accordance with the guidelines from the National Institutes of Health and the Association for the Assessment and Accreditation of Laboratory Animal Care and were approved by the university’s Institutional Animal Care and Use Committee.
Figure 1.
Diagram depicting maternal separation groups and experimental design.
Scoring of Maternal Behaviors
The behavior of each dam was recorded during daily 60 min sessions beginning at 16:00 each day for the first 14 days postpartum, prior to the initiation of the maternal separation procedure. Maternal behaviors were recorded during this time period because the frequency of these behaviors is highest toward the end of the dark period and because the maternally separated rats will have been left undisturbed for the previous 20 h (Champagne et al., 2003). Behaviors included licking and grooming the pups, arched-back nursing, blanket nursing (i.e., the dam lies over the pups), passive nursing (i.e., the dam lies on her side or back while the pups nurse), and no contact with any of the pups. During each 60 min session, behaviors were scored once every 4 min for each dam for a total of 15 observations per litter per day.
Maternal Separation
Maternal separation procedures were identical for the two cohorts of rats. Pups were born on postnatal day 0 (PD0), and maternal separation was conducted from PD1 to PD14. On PD1, during maternal separation, pups were weighed and culled to litters of eight with approximately four male and four female pups per litter. Maternal separation was conducted between 17:00 and 20:30 daily. During each session, dams were removed from the home cage and placed in a separate cage in the same room with only water available. Pups were then removed and placed together as an entire litter in a separate cage in the same room with a heating pad underneath, maintaining the temperature within the litter at approximately 33–35°C. After 3 h, the pups were returned to the home cage, followed by the dams. Control dams and litters were left undisturbed during this period. Pups were weighed again on PD14. Bedding in all cages was changed 1 or 2 days before PD0, then again during the separation period on PD7 and after maternal separation on PD14. Bedding was then changed twice during the following week, after which pups were weaned on PD21 and housed in a separate cage with their littermates. On PD28, two of the four male pups from each litter were paired in one cage and transferred to a separate colony room. The remaining pairs of male pups were used for different experiments not reported here.
Intracranial Self-Stimulation Apparatus and Procedures
Electrode Implantation Surgery
Electrode implantation surgery was conducted at 8 weeks of age. Rats were anesthetized with a 2% isoflurane/oxygen vapor mixture and secured on a stereotaxic frame (Kopf Instruments, Tujunga, CA). Stainless steel bipolar stimulating electrodes (11 mm length, model MS303/2, Plastics One, Roanoke, VA) were implanted in the posterior lateral hypothalamus (anterior/posterior, −0.5 mm from bregma; medial/lateral, ±1.7 mm; dorsal/ventral, −8.3 mm from dura; incisor bar set +5.0 mm above the interaural line) according to the atlas of Paxinos and Watson (1998). Electrodes were secured to the skull with four stainless steel jeweler’s screws and dental acrylic. Rats were administered antibiotics and analgesics and allowed 1 week to recover before testing.
ICSS Apparatus
ICSS training and testing occurred in Plexiglas operant chambers (25 × 31 × 24 cm3; Med Associates, St. Albans, VT). Each testing chamber was enclosed within a light- and sound-attenuated chamber (62 × 63 × 43 cm3; San Diego Instruments, San Diego, CA). One wall of the operant chamber contained a metal wheel manipulandum (5 cm width) that extended 3 cm from the wall and required approximately 0.2 N of force to rotate one-quarter turn. Intracranial stimulation was delivered by constant current stimulators (Stimtek model 1200, San Diego Instruments, San Diego, CA). Animals were connected to the stimulation circuit through flexible bipolar leads (Plastics One, Roanoke, VA) attached to gold-contact swivel commutators (model SL2C, Plastics One, Roanoke, VA) mounted above the chamber. The stimulation parameters, data collection, and all test session functions were controlled by a computer.
ICSS Procedure
Reward function was assessed using a discrete-trial current threshold procedure based on the procedure originally designed by Kornetsky et al. (Kornetsky et al., 1979) and described in detail by Markou and Koob (1992, 1993). Rats were placed in the operant testing chambers, and electrodes were connected via a flexible lead (Plastics One, Roanoke, VA) to the constant current stimulators. Subjects were initially trained to turn the wheel manipulandum on a fixed-ratio 1 schedule of reinforcement, during which each quarter turn of the wheel resulted in the delivery of a contingent electrical pulse delivered to the posterior lateral hypothalamus, an area of the brain’s reward circuitry. The electrical reinforcer had a train duration of 500 ms and consisted of 0.1 ms rectangular cathodal pulses that were delivered with 100 Hz frequency. The current delivered was adjusted for each animal and typically ranged from 100 to 250 μA. After successful acclimatization to this procedure (i.e., two sessions of 100 reinforcers in less than 20 min), the rats were gradually trained on the following discrete-trial, current threshold procedure.
At the beginning of each trial, rats received a noncontingent stimulus (current varied; see below). Rats were then given the opportunity during a 7.5 s limited hold period to respond on the wheel manipulandum. A quarter turn of the wheel manipulandum was considered a response and resulted in the delivery of a contingent stimulus that was identical in all parameters to the previous noncontingent stimulus. An intertrial interval (ITI) averaging 10 s (7.5–12.5 s) followed each response or response-free limited hold period. After completion of this ITI period, a new trial began with the delivery of a new noncontingent stimulus. To discourage responding on the manipulandum that was not time-linked to the presentation of the noncontingent stimulus, responding during the ITI resulted in the resetting of the ITI period to 12.5 s, which exceeded or was equal to the original random duration of the ITI.
By systematically varying the current of the contingent and noncontingent electrical stimuli, the ICSS threshold was determined for each individual subject. The current of the stimuli was varied in 5 μA steps in a pattern of alternating descending and ascending series of currents with each current presented in three consecutive trials (within each trial, all parameters of the stimulus were the same). Each set of three trials was considered a “positive” response if the rat responded in at least two of the three trials in the set. Conversely, if the rat responded to less than two of the three trials in the set, this was considered a “negative” response, suggesting that this particular current was not reinforcing. Two consecutive sets of “negative” trials resulted in termination of the descending series and initiation of the next ascending series. Similarly, two consecutive sets of “positive” trials resulted in the termination of the ascending series and the initiation of a descending series. During each test session, rats were exposed to two descending and two ascending trials, starting with the descending series and alternating between ascending and descending series. The first descending series started with the current set 30–40 μA above the rat’s estimated average reward threshold; all subsequent series began with the current set 5 μA below (ascending series) or above (descending series) the last stimulus delivered in the previous series. The rat’s reward threshold for a given series was defined as the average current between the last two “positive” sets of trials in the series and the two “negative” sets of trials at the end of the series. The overall reward threshold for a test session was defined as the average of the reward threshold values for the two descending and two ascending series completed in that session. Elevations in a rat’s reward threshold indicated that stimulus intensities previously perceived as reinforcing were no longer rewarding, reflecting a decrease in reward function and suggesting an anhedonic or depression-like state. Conversely, lowering of reward thresholds reflected increased reward function. Average response latencies were also recorded during each session.
After training in this procedure, rats were tested daily until they achieved stable baseline thresholds, defined as <10% variation in reward thresholds over 5 consecutive baseline days. The duration of each session was 30–40 min. Rats with stable ICSS current thresholds were exposed to each of the manipulations below.
First Amphetamine Challenge
Rats were administered either 0.5 mg/kg D-amphetamine sulfate (Sigma, St. Louis, MO) or vehicle (sterile 0.9% saline) intraperitoneally (i.p.) in a counterbalanced design 15 min before initiating ICSS testing. ICSS testing was conducted again 24 h later without any injections. Forty-eight hours after the initial injection, rats that previously received amphetamine were administered saline and vice versa, and ICSS thresholds were assessed 15 min later. Thus, all maternally separated and control rats received one amphetamine and one saline administration. ICSS testing continued daily with no further manipulations for 1 week. In the first cohort only, half of the maternally separated and control rats were administered amphetamine (5 mg/kg/day for 7 days via surgically implanted subcutaneous osmotic minipumps; 10.3 ± 0.3 μl/hr pumping rate; 2063 ± 51 μl fill volume; Alzet Osmotic Pump Model 2ML1, Cupertino, CA), whereas the other half received saline-filled minipumps. ICSS current thresholds were assessed daily during amphetamine exposure and for 1 week after minipumps were removed. The subsequent social defeat procedure was conducted in rats that were treated with saline minipumps only, whereas rats treated with chronic amphetamine were exposed to the non-stress condition to prevent the possible confounding effects of chronic amphetamine treatment on the subsequent stress response. Data were analyzed as described below and indicated no difference in ICSS thresholds during chronic amphetamine exposure between maternally separated and control rats (data not shown). We have previously shown that repeated daily injections of 4 mg/kg amphetamine to result in a similar lowering of thresholds in naïve rats compared to the continuous infusion method used here without the development of sensitization or tolerance (Lin et al., 2000). Thus, it is unlikely that a different administration procedure would have yielded different ICSS thresholds between maternally separated and non-handled rats. Therefore, chronic amphetamine treatment was not repeated in the second cohort of maternally separated rats.
Repeated Social Defeat Exposure
Rats were then exposed to either social defeat or no stress in a counterbalanced design 5–10 min before the initiation of ICSS testing. Social defeat was conducted in a separate colony room than where the experimental rats were housed. During social defeat, each female resident was removed from the cage, a wire mesh partition was placed in the resident’s cage, and the experimental rat (either maternally separated or non-handled) was placed behind the partition to physically separate it from the male resident. After 10 min, the partition was removed, allowing for physical defeat to occur, characterized by a supine posture by the experimental rat during an attack by the resident. As soon as a single attack occurred, the two rats were separated, and the experimental rat was returned behind the wire partition for an additional 45 min. ICSS testing was conducted immediately afterward. Control rats were briefly handled prior to ICSS testing. After ICSS testing, rats were returned to their home cages, and the social defeat procedure was repeated the following day with a different resident/intruder pairing for a total of 7 consecutive days. ICSS testing continued daily for 1 additional week under no stress conditions for all rats.
Second Amphetamine Challenge
All rats were administered amphetamine (0.5 mg/kg, i.p.) then vehicle 48 h later or vice versa, and ICSS thresholds were assessed exactly as described above.
Statistical Analyses
Postnatal body weights and daily baseline ICSS current thresholds were analyzed using mixed three-way analysis of variance (ANOVA), with Early Life Stress and Cohort as the between-subjects factors and Time as the within-subjects factor. Adult body weights and average baseline ICSS thresholds were analyzed using a two-way ANOVA with Early Life Stress and Cohort as the between-subjects factors. Daily maternal behavior counts were calculated across the first and second postnatal weeks and analyzed using a four-way ANOVA with Early Life Stress, Behavior, and Cohort as the between-subjects factors and Time as the within-subjects factor. For ICSS experiments, absolute current values at baseline varied from 55 μA to 250 μA as a result of a number of factors, including individual electrode placements and other poorly understood individual subject characteristics. Thus, changes in ICSS thresholds in response to the drug and stress manipulations were expressed as a percentage of the 3 day average baseline threshold preceding any manipulation (e.g., first amphetamine challenge, social defeat, second amphetamine challenge). Analyzing the data in this manner yields similar results compared to analyzing absolute values, but greatly decreases the variability associated with individual differences in absolute current thresholds. Percent changes were analyzed using either: 1) a two-way ANOVA with Early Life Stress and Cohort as the between-subjects factors (first amphetamine challenge experiment); 2) a four-way ANOVA with Early Life Stress, Social Defeat, and Cohort as the between-subjects factors and Time as the within-subjects factor (repeated social defeat experiment); or 3) a three-way ANOVA with Early Life Stress, Social Defeat, and Cohort as the between-subjects factors (second amphetamine challenge experiment). Response latencies were analyzed exactly as ICSS thresholds for each experiment, other than they were expressed as absolute values. It should be noted that none of these analyses indicated a significant main effect of Cohort or a significant interaction between Cohort and any other independent variables, reflecting the statistical similarity in behavioral responses between the two cohorts tested (detailed statistics not shown). Thus, data from both cohorts of rats are reported here as combined, unless otherwise stated below, to increase statistical power. Significant main and interaction effects were further analyzed using the Newman-Keuls post hoc test to assess differences between treatment groups. Statistical outliers, indicated by data two standard deviations beyond the group mean, were excluded from analyses. Where statistical outliers were excluded for primary analyses, a Tukey’s bisquare estimator (TBE) was also calculated with no data excluded for comparison with the unweighted sample means. The level of significance was set at 0.05.
RESULTS
From the first cohort, 16 maternally separated and 11 control rats were successfully trained in the ICSS procedure and achieved baseline thresholds that were considered stable to proceed with subsequent experimentation. From the second cohort, 11 maternally separated and 9 control rats were successfully trained and stabilized in the ICSS procedure. Thus, a total of 27 maternally separated rats from 18 litters and 20 control rats from 14 litters were included in the analyses below. No more than two male rats were used from each litter and each experimental group contained only one male rat from a particular litter.
Body Weights
In the first cohort of rats, all male pups were weighed on PD1 and PD14. The average male pup weight per litter was calculated and analyzed. A mixed two-way ANOVA revealed a significant main effect of Time (F1,28 = 2317, p < 0.0001) but no effect of Early Life Stress and no interaction between the two variables. No significant difference in body weights was observed between rats before maternal separation treatment (7.09 g ± 0.20 [SEM], n = 15) and rats in the non-handled condition (7.51 g ± 0.17, n = 15; i.e., PD1). Similarly, no significant difference in body weights was observed on PD14 between maternally separated rats (32.66 g ± 0.92, n = 15) and non-handled rats (33.97 g ± 0.83, n = 15). In both cohorts, male rats used for ICSS testing were weighed on PD70. An unpaired t-test revealed no significant difference in body weights between maternally separated rats (410.6 g ± 9.28, n = 27) and non-handled rats (407.3 g ± 11.25, n = 20).
Maternal Behaviors
Maternal behaviors were scored for all dams in both cohorts of rats, but only data from rats with successful ICSS surgeries and training were analyzed to directly compare neonatal experience with behavioral responses in adulthood. Maternal behaviors were recorded daily before the separation procedure, and average daily counts for each behavior were analyzed separately across the first (i.e., PD1-7) and second (i.e., PD8-14) weeks of postnatal life. A mixed three-way ANOVA revealed a strong trend for a significant interaction between Early Life Stress, Behavior, and Time (F4,139 = 2.123, p = 0.0811). A total of 12 of the 320 data points were removed as statistical outliers. Since measures of active maternal care (i.e., licking, grooming, and arched-back nursing) and not passive maternal care are often found to be altered after maternal separation or brief handling (Liu et al., 1997; Macri et al., 2004), post hoc analyses were conducted at both time points (i.e., weeks 1 and 2) for licking, grooming and arched-back nursing behaviors. During the first postnatal week, post hoc analyses revealed significantly less licking and grooming of pups in the maternally separated litters (unweighted mean = 1.317; TBE = 1.125; n = 18 litters) compared with non-handled control litters (unweighted mean = 1.808; TBE = 1.832; n = 14 litters; Fig. 2A). During the second postnatal week, post hoc analyses revealed significantly less licking and grooming (unweighted mean = 1.437; TBE = 1.012; n = 18 litters) and significantly less arched-back nursing (unweighted mean = 3.333; TBE = 2.631; n = 18 litters) of pups in the maternally separated group compared with licking and grooming (unweighted mean = 1.612; TBE = 1.496; n = 18 litters) and arched-back nursing (unweighted mean = 4.125; TBE = 4.438; n = 18 litters) in non-handled controls, respectively (Fig. 2B).
Figure 2.
Mean (± SEM) frequency of maternal behaviors scored daily prior to maternal separation during the (A) first and (B) second postnatal weeks. NC, no contact; LG, licking and grooming; ABN, arched-back nursing; BN, blanket nursing; PN, passive nursing. *p < 0.05, significantly different from non-handled controls.
ICSS Current Thresholds
Baseline Values
Baseline ICSS current thresholds were recorded for at least 14 days prior to the initiation of drug and stress exposure in maternally separated rats (n = 27) and non-handled rats (n = 20; Fig. 3A). A mixed two-way ANOVA revealed no main effects of Early Life Stress or Time and no Early Life Stress × Time interaction, although a very small trend was found toward maternally separated rats having higher thresholds than control rats. An unpaired t-test comparing the 14-day mean ICSS thresholds between both groups also revealed no significant difference in baseline thresholds (Fig. 3B).
Figure 3.

(A) Daily ICSS current threshold values (mean ± SEM) during 14 days of baseline assessment in maternally separated and non-handled rats. (B) Average ICSS current threshold values (mean ± SEM) under baseline conditions in maternally separated and non-handled rats.
First Acute Amphetamine Injection
An unpaired t-test indicated no difference in baseline thresholds for the 3 days prior to amphetamine or saline administration between maternally separated rats (130.4 μA ± 9.89) and control rats (125.3 μA ± 11.84). An unpaired t-test revealed that acute amphetamine administration lowered ICSS thresholds in maternally separated rats (unweighted mean = 75.846; TBE = 75.112; n = 26; one statistical outlier was removed) to a greater extent than in non-handled controls (unweighted mean = 81.664; TBE = 82.081; n = 18; two statistical outliers were removed; t42 = 2.737, p < 0.01; Fig. 4). No effect of saline administration was found in either group (data not shown), and no effect of maternal separation was observed on response latencies (data not shown).
Figure 4.
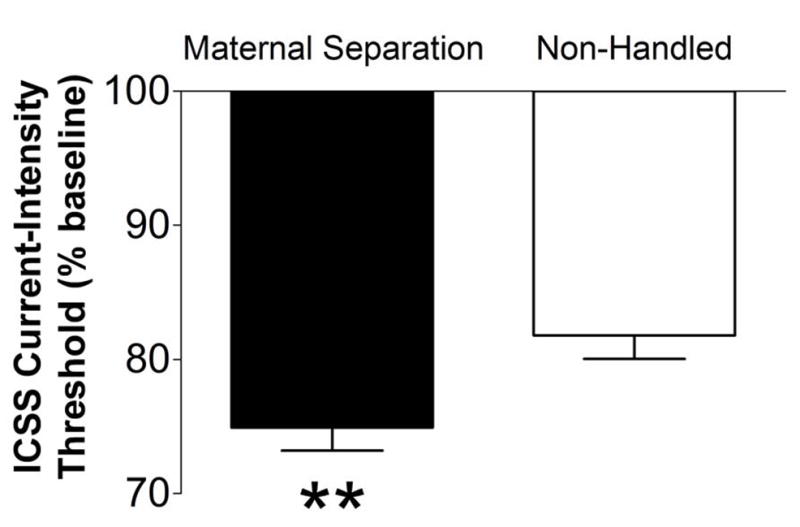
Mean (± SEM) change in ICSS current thresholds, expressed as a percentage of baseline threshold values, in response to amphetamine (0.5 mg/kg, i.p.) in maternally separated and non-handled rats. **p < 0.01, significantly different from non-handled controls.
Repeated Social Defeat Exposure
A two-way ANOVA indicated no difference in baseline thresholds for the 3 days prior to social defeat between groups. Maternally separated rats were exposed to social defeat (n = 14; baseline threshold = 133.03 μA ± 14.48) or no stress (n = 13; 136.07 μA ± 13.82), and non-handled controls were also exposed to social defeat (n = 10; 133.06 μA ± 21.84) or no stress (n = 10; 134.67 μA ± 14.45). A mixed three-way ANOVA revealed a significant Early Life Stress × Social Defeat × Time interaction (F1,38 = 8.850, p < 0.01; one statistical outlier was removed in the maternal separation/social defeat group from week 1; one statistical outlier was removed from each group from week 2). Post hoc analyses indicated that in non-handled rats, acute social defeat significantly elevated thresholds (unweighted mean = 107.676; TBE = 106.869) compared with non-stressed controls (unweighted mean = 99.158; TBE = 103.055). However, in maternally separated rats, social defeat did not significantly elevate thresholds (unweighted mean = 105.262; TBE = 98.734) compared with non-stressed controls (unweighted mean = 100.729; TBE = 97.930; Fig. 5A). After the seventh consecutive day of social defeat, post hoc analyses revealed a significant stress-induced threshold elevation in maternally separated rats after repeated social defeat (unweighted mean = 114.642; TBE = 109.261) compared to no social defeat (unweighted mean = 100.942; TBE = 97.970), but only a nonsignificant stress-induced elevation in thresholds in non-handled rats (unweighted mean = 108.758; TBE = 106.640) compared to no social defeat (unweighted mean = 97.876; TBE = 99.366; Fig. 5B). No effect of Early Life Stress or Social Defeat was found on response latencies at either time point (data not shown).
Figure 5.
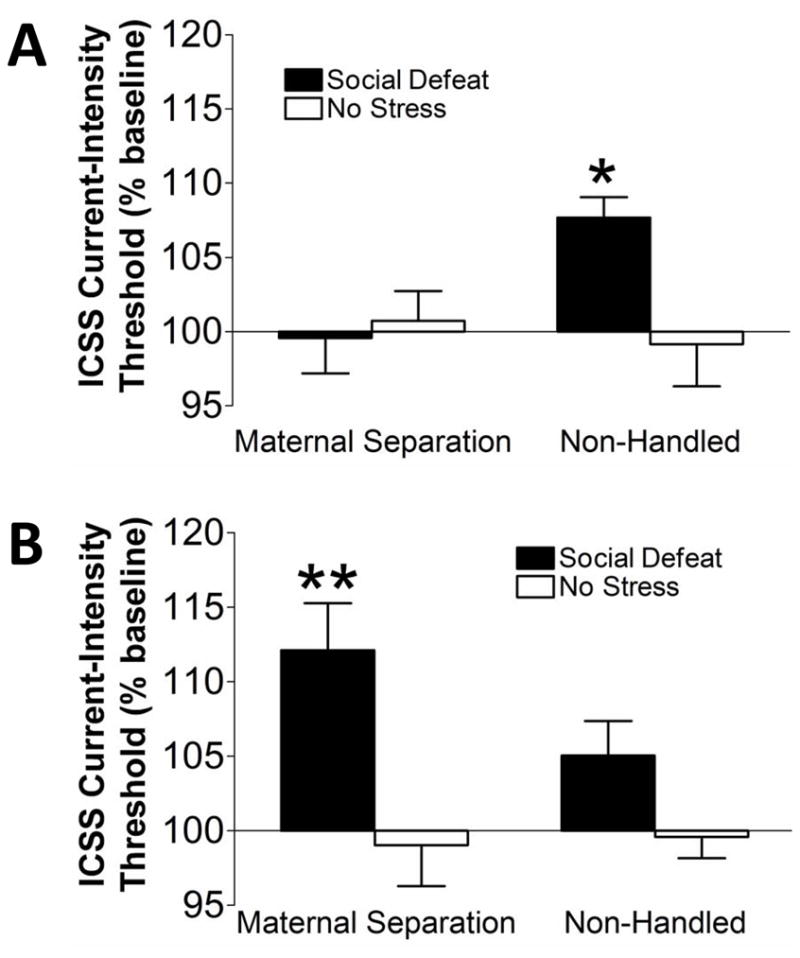
Mean (± SEM) change in ICSS current thresholds, expressed as a percentage of baseline threshold values, in response to the (A) first and (B) seventh repeated social defeat sessions. *p < 0.05, significantly different from non-handled/no stress rats and from maternal separation/social defeat rats. **p < 0.01, significantly different from maternal separation/no stress rats.
Second Acute Amphetamine Injection
A two-way ANOVA indicated no difference in baseline thresholds between groups (maternal separation/social defeat: 130.67 μA ± 13.62; maternal separation/no stress: 135.98 μA ± 14.98; non-handled/social defeat: 133.94 μA ± 22.85; non-handled/no stress: 135.24 μA ± 16.43). One week after repeated social defeat exposure, the second amphetamine challenge again lowered ICSS thresholds in maternally separated rats, but prior social defeat did not affect amphetamine-induced threshold changes (Fig. 6). These effects were revealed by a two-way ANOVA, which indicated a main effect of Early Life Stress (F1,42 = 5.375, p < 0.05), such that amphetamine administration significantly lowered thresholds in maternally separated rats (unweighted mean = 70.239; TBE = 70.813) compared with non-handled controls (unweighted mean = 74.733; TBE = 76.109; one statistical outlier was removed). However, no main effect of prior Social Defeat exposure and no Early Life Stress × Social Defeat interaction were found. No effect of saline administration was observed in any group (data not shown), and no effect of Early Life Stress or Social Defeat was found on response latencies (data not shown).
Figure 6.

Mean (± SEM) change in ICSS current thresholds, expressed as a percentage of baseline values, in response to amphetamine (0.5 mg/kg, i.p.) in maternally separated and non-handled control rats 1 week after repeated social defeat. *p < 0.05, significantly different from non-handled controls.
DISCUSSION
The results of the present studies indicate that maternal separation produced decreased levels of maternal care toward the offspring in the form of reduced licking, grooming, and arched-back nursing by the dams. Offspring that experienced maternal separation later showed no alterations in brain reward function during adulthood under baseline conditions, reflected in ICSS current thresholds, but did exhibit a greater reward-enhancing effect after acute amphetamine administration, a blunted anhedonic response to acute social defeat, and an exacerbated anhedonic response to repeated social defeat. Repeated social defeat did not alter the subsequent response to a second acute amphetamine challenge.
Maternal separation did not affect the growth of offspring, reflected by identical body weights at birth, at the end of the separation procedure, and at adulthood compared with non-handled control rats. Body weight is a gross measure of physical development and indicates that the separation procedure did not result in malnutrition of the offspring or altered physical development that would otherwise affect the ability of rats to perform the subsequent behavioral tasks. Longer separation procedures than those used here are often termed “maternal deprivation,” in which the lack of physical maternal care and nutrition might affect physical and psychosocial development. When left completely undisturbed, the amount of maternal care naturally varies between individual litters, and licking and grooming of the pups are maternal behaviors that are critical for the normal development of the pups (Champagne et al., 2003). Dams that lick and groom their pups less tend to have offspring with an altered HPA response to stress (Champagne and Meaney, 2001), increased anxiety-like behavior and “fearfulness” (Caldji et al., 1998), and deficits in spatial learning and memory (Liu et al., 2000). The separation procedure used here produced significant changes in critical maternal behaviors during the first two weeks of postnatal life. Maternally separated pups received significantly less licking and grooming from their dams throughout the two week period compared with non-handled controls. Arched-back nursing of maternally separated pups also decreased, although this effect was significant only during the second week of observation. These results are in contrast to those reported by Macri et al. (2004), where neonatal maternal separation increased licking, grooming, and arched-back nursing behaviors toward the pups compared to non-handled control treatment. In either case, it is impossible to determine whether changes in maternal care are directly responsible for the changes in behavior observed during adulthood, as a number of factors may have contributed to the differences in maternal care observed during the maternal separation procedure. The key differences between the procedure used here and that used by Macri and colleagues (2004) are slightly different separation lengths (3 hr here vs. 4 hr), time of day during initiation of maternal separation (end of dark period here vs. beginning of dark period), and placement of the pups during separation (in a separate cage here vs. remaining in the home cage). Thus, it is possible that any of these differences contributed to the differences in maternal care. In addition, we recorded maternal behaviors immediately prior to separation and 20 hr after any disruption, toward the end of the dark period when these behaviors are reported to peak during the diurnal cycle in undisturbed rats (Champagne et al., 2003). This time point approximately corresponds to the period after the dams are reunited with their pups in the study by Macri and coworkers (2004). It is possible that had we observed maternal behaviors after reuniting the dams and pups, we might have also observed an increase in maternal care from the dams that had been separated. Indeed, diurnal patterns of maternal care might also contribute to differences in neonatal development. Nonetheless, we did not measure maternal behaviors at any other time point nor at any time point did Macri and colleagues (2004) observe decreased maternal care from separated dams relative to non-handled controls. Thus, any differences observed in adulthood of maternally separated offspring in the present study can be attributed to the maternal separation procedure, which in our design produced decreased maternal care at a time of day when quantitative levels of care are naturally elevated. Whether or not decreased maternal care contributed to the behavioral changes in adulthood cannot be determined.
During adulthood, anhedonia is often assessed in maternally separated rodents by measuring the intake of or motivation for palatable food rewards, such as sucrose. However, quantification of anhedonia involving a sucrose reinforcer under baseline conditions has yielded inconsistent results. For example, most published reports indicate that maternal separation in rodents does not affect sucrose consumption or preference (Matthews et al., 1996; Shalev and Kafkafi, 2002; Matthews and Robbins, 2003; Ruedi-Bettschen et al., 2005), though few reports have indicated an increase in sucrose consumption and preference in maternally separated rats (Vazquez et al., 2005; Michaels and Holtzman, 2007). Regarding motivated behavior, maternal separation has been shown to either reduce the motivation for a sucrose reward (Ruedi-Bettschen et al., 2005; Ruedi-Bettschen et al., 2006; Leventopoulos et al., 2009) or have no effect on motivated responding (Shalev and Kafkafi, 2002), assessed using a progressive-ratio schedule of reinforcement. In the present study, ICSS current thresholds were slightly, but nonsignificantly, higher in maternally separated rats during the baseline assessment compared with controls, suggesting a mild anhedonic effect. This slight difference is unlikely to indicate an anhedonic profile in maternally separated rats under baseline conditions because a number of extraneous factors may account for differences in absolute ICSS current threshold values between individual animals and groups of animals, such as slight variations in exact electrode placement that are difficult to quantify, affecting the minimal stimulation required for a response. Thus, such small differences between groups cannot be considered significant unless replicated consistently. Indeed, neither cohort of maternally separated rats assessed here displayed significantly elevated thresholds at baseline compared with non-handled controls. Furthermore, data from Matthews and colleagues (2003) also indicated no baseline differences in ICSS thresholds between maternally separated and control rats using an ICSS testing procedure identical to the one used here.
In response to a single amphetamine challenge that typically induces a lowering of ICSS thresholds that reflects reward enhancement (Lin et al., 1999; Lin et al., 2002; Matthews and Robbins, 2003; Todtenkopf et al., 2009), maternally separated rats exhibited a greater lowering of thresholds compared with controls. Increased sensitivity to the reward-enhancing properties of drugs of abuse may indicate a heightened vulnerability to repeated use and dependence (Wise, 1998; Gardner, 2005). These results contrast with those by Matthews and colleagues (2003), who found no effect of maternal separation with any of three amphetamine doses tested, including the dose used in our studies (0.5 mg/kg), on ICSS thresholds. The discrepancy in these findings may be attributable to the very different maternal separation procedures used in these studies. The maternal separation procedure used by Matthews and coworkers (2003) involved 6 h of separation per day over 10 days spaced randomly between PD5 and PD20. Because it has been shown that natural variations in maternal care are most pronounced during the first week postpartum (Champagne et al., 2003), it is possible that disruption of the dams and pups during this initial postnatal period is necessary to alter development.
Other studies investigating the effects of early life stress on other behaviors induced by administration of psychostimulants, such as amphetamine, also indicate an enhancing effect of the drug in maternally separated rats. Specifically, rats that are maternally separated (Brake et al., 2004) or artificially reared with minimal maternal-like stimulation (Lovic et al., 2006) exhibit a potentiated locomotor response to an amphetamine challenge in adulthood. Furthermore, maternal separation increases oral amphetamine consumption (Vazquez et al., 2006) and leads to the acquisition of cocaine self-administration at lower doses compared with non-handled controls (Moffett et al., 2006). These enhancing effects of maternal separation on the stimulating properties of amphetamine are hypothesized to be mediated by changes in striatal dopamine transmission. Dopamine transporter levels in the nucleus accumbens are greatly reduced in maternally separated rats (Meaney et al., 2002), which may confer greater sensitivity to drugs that block dopamine transport, such as amphetamine. Accordingly, amphetamine induces a greater dopamine response in the nucleus accumbens of maternally separated rats compared to control rats (Hall et al., 1999), which may account for the increased reward-enhancing effect of amphetamine in maternally separated rats observed in the present study.
Exposure to social defeat using the standard resident/intruder procedure is known to produce anhedonic signs in various behavioral assessments (Von Frijtag et al., 2000; Rygula et al., 2005; Miczek et al., 2008). In the present study, a single exposure to social defeat also produced anhedonia, reflected in elevated ICSS current thresholds in non-handled control rats. This effect slightly dissipated over time. Repeated daily exposure to social defeat continued to elevate ICSS current thresholds in non-handled control rats, but this threshold elevation was no longer significant after the seventh consecutive day of exposure, reflecting habituation to the stressor. Interestingly, a single exposure to social defeat had no effect on ICSS thresholds in maternally separated rats, indicating that these rats did not become anhedonic in response to an acute stressor challenge. In contrast, after repeated daily exposure to the stressor, ICSS thresholds were significantly elevated in maternally separated rats, indicating an exacerbated response to the stressor. Little is known regarding the neurophysiological response to chronic psychosocial stress in animals exposed to early life stress. One recent report indicated that neonatal maternal separation altered the hypothalamic-pituitary-adrenal axis response to a chronic psychosocial stressor (i.e., housing with a larger, dominant mouse for 19 days) in adulthood (Veenema et al., 2008). Specifically, maternally separated mice exposed to the chronic stressor had greater corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus compared with maternally separated/non-stressed mice and non-maternally separated/stressed mice. Thus, while neonatal maternal separation may appear to initially protect against the anhedonic effect of acute exposure to a social stressor, early life stress is shown to sensitize both the neurophysiological response (Veenema et al., 2008) and the anhedonic response to repeated social stress in adulthood.
Exposure to stress, such as social defeat in rodents, can also enhance the behavioral and neurochemical responses to drugs of abuse (Antelman et al., 1980; Robinson, 1988; Kalivas and Stewart, 1991; Sorg and Kalivas, 1991). Moreover, high comorbidity exists between stress- and drug-related disorders, such as MDD and substance use disorder (Kosten et al., 1998; Leventhal et al., 2008). To assess the effect of stress exposure on the reward-enhancing effect of abused drugs, rats were again administered amphetamine (0.5 mg/kg, i.p.) 1 week after exposure to social defeat or no stress treatment. As before, amphetamine lowered ICSS thresholds to a greater extent in maternally separated rats compared with non-handled controls, but prior exposure to repeated social defeat did not alter this response in either maternally separated or non-handled rats. Stress-induced sensitization to the rewarding properties of drugs is highly context- and time-sensitive (Shaham et al., 1995). Drug administration or seeking often occurs either in the same environment as the stressor exposure or immediately after stressor exposure. In the present study, stress and drug administrations occurred in separate contexts and were separated by 1 week, which may have accounted for the lack of a stress-induced effect on amphetamine-induced changes in ICSS thresholds.
In conclusion, the present data suggest that exposure to early life stress may not necessarily alter mood or affect under non-stressful baseline conditions in adulthood. Early life stress may instead confer vulnerability to challenges in adulthood, such as exposure to repeated psychosocial stressors or drugs of abuse. Our data indicate that exposure to early life stress in the form of neonatal maternal separation can differentially alter reward sensitivity depending on the challenges encountered in adulthood. Stress-induced anhedonia gradually dissipated over time in non-handled controls. In contrast, maternally separated rats that were seemingly protected from the anhedonic effect of a single social defeat exposure subsequently developed greater anhedonia after repeated stress exposure, and this exaggerated response to repeated stress may confer vulnerability to depressive disorders. Concurrently, the reward-enhancing properties of amphetamine also increased in maternally separated rats, and this response may confer vulnerability to substance abuse. Thus, neonatal maternal separation and neglect may represent a rodent procedure with which to investigate the neurobiological mechanisms that underlie comorbid depressive and substance use disorders precipitated by early life stress.
Acknowledgments
This work was supported by National Institutes of Health grants U01 MH69062 and 2R01 MH62527 to AM and National Research Service Award Individual Postdoctoral fellowship F32MH080585 to AD from the National Institute of Mental Health. The authors wish to thank Mr. Michael Arends for editorial assistance.
ABREVIATIONS
- ANOVA
analysis of variance
- ICSS
intracranial self-stimulation
- ITI
intertrial interval
- MDD
major depressive disorder
- PD
postnatal day
- TBE
Tukey’s bisquare estimator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207 (4428):329–31. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Arellano CM. Child maltreatment and substance use: a review of the literature. Subst Use Misuse. 1996;31 (7):927–35. doi: 10.3109/10826089609063963. [DOI] [PubMed] [Google Scholar]
- Association AP. Diagnostic and Statistical Manual of Mental Disorders IV. Washington D.C.: American Psychiatric Press; 1994. [Google Scholar]
- Bifulco A, Brown GW, Adler Z. Early sexual abuse and clinical depression in adult life. Br J Psychiatry. 1991;159:115–22. doi: 10.1192/bjp.159.1.115. [DOI] [PubMed] [Google Scholar]
- Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A. Influence of early postnatal rearing conditions on mesocorticolimbic dopamine and behavioural responses to psychostimulants and stressors in adult rats. Eur J Neurosci. 2004;19 (7):1863–74. doi: 10.1111/j.1460-9568.2004.03286.x. [DOI] [PubMed] [Google Scholar]
- Brown GR, Anderson B. Psychiatric morbidity in adult inpatients with childhood histories of sexual and physical abuse. Am J Psychiatry. 1991;148 (1):55–61. doi: 10.1176/ajp.148.1.55. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95 (9):5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetti L, Bachar E, Galili-Weisstub E, De-Nour AK, Shalev AY. Parental bonding and mental health in adolescence. Adolescence. 1997;32 (126):381–94. [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2 (11):2987–95. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79 (3):359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004(225):re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Dobkin PL, Tremblay RE, Sacchitelle C. Predicting boys’ early-onset substance abuse from father’s alcoholism, son’s disruptiveness, and mother’s parenting behavior. J Consult Clin Psychol. 1997;65 (1):86–92. doi: 10.1037//0022-006x.65.1.86. [DOI] [PubMed] [Google Scholar]
- Feehan M, McGee R, Stanton WR, Silva PA. Strict and inconsistent discipline in childhood: consequences for adolescent mental health. Br J Clin Psychol. 1991;30(Pt 4):325–31. doi: 10.1111/j.2044-8260.1991.tb00953.x. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81 (2):263–84. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Hall FS, Wilkinson LS, Humby T, Robbins TW. Maternal deprivation of neonatal rats produces enduring changes in dopamine function. Synapse. 1999;32 (1):37–43. doi: 10.1002/(SICI)1098-2396(199904)32:1<37::AID-SYN5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Holmes SJ, Robins LN. The influence of childhood disciplinary experience on the development of alcoholism and depression. J Child Psychol Psychiatry. 1987;28 (3):399–415. doi: 10.1111/j.1469-7610.1987.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Holmes SJ, Robins LN. The role of parental disciplinary practices in the development of depression and alcoholism. Psychiatry. 1988;51 (1):24–36. doi: 10.1080/00332747.1988.11024377. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16 (3):223–44. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62 (6):617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU, McLean S, Jacobson JO. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch Gen Psychiatry. 1979;36 (3):289–92. doi: 10.1001/archpsyc.1979.01780030055004. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Markou A, Koob GF. Depression and stimulant dependence: neurobiology and pharmacotherapy. J Nerv Ment Dis. 1998;186 (12):737–45. doi: 10.1097/00005053-199812000-00001. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Stone K, Young D, Chelminski I, et al. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. Am J Addict. 2008;17 (3):218–23. doi: 10.1080/10550490802019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventopoulos M, Russig H, Feldon J, Pryce CR, Opacka-Juffry J. Early deprivation leads to long-term reductions in motivation for reward and 5-HT1A binding and both effects are reversed by fluoxetine. Neuropharmacology. 2009;56 (3):692–701. doi: 10.1016/j.neuropharm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Lin D, Bruijnzeel AW, Schmidt P, Markou A. Exposure to chronic mild stress alters thresholds for lateral hypothalamic stimulation reward and subsequent responsiveness to amphetamine. Neuroscience. 2002;114 (4):925–33. doi: 10.1016/s0306-4522(02)00366-4. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat--interactions between the two drugs. Psychopharmacology (Berl) 1999;145 (3):283–94. doi: 10.1007/s002130051060. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Time-dependent alterations in ICSS thresholds associated with repeated amphetamine administrations. Pharmacol Biochem Behav. 2000;65 (3):407–17. doi: 10.1016/s0091-3057(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3 (8):799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277 (5332):1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lovic V, Fleming AS, Fletcher PJ. Early life tactile stimulation changes adult rat responsiveness to amphetamine. Pharmacol Biochem Behav. 2006;84 (3):497–503. doi: 10.1016/j.pbb.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Macri S, Mason GJ, Wurbel H. Dissociation in the effects of neonatal maternal separations on maternal care and the offspring’s HPA and fear responses in rats. Eur J Neurosci. 2004;20(4):1017–24. doi: 10.1111/j.1460-9568.2004.03541.x. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav. 1992;51 (1):111–9. doi: 10.1016/0031-9384(92)90211-j. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. Behavioural Neuroscience: A Practical Approach. Sahgal A. Oxford: IRL Press; (Intracranial self-stimulation thresholds as a measure of reward.). Intracranial self-stimulation thresholds as a measure of reward; pp. 93–115. [Google Scholar]
- Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27 (1–2):45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- Matthews K, Wilkinson LS, Robbins TW. Repeated maternal separation of preweanling rats attenuates behavioral responses to primary and conditioned incentives in adulthood. Physiol Behav. 1996;59 (1):99–107. doi: 10.1016/0031-9384(95)02069-1. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27 (1–2):127–38. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- Michaels CC, Holtzman SG. Enhanced sensitivity to naltrexone-induced drinking suppression of fluid intake and sucrose consumption in maternally separated rats. Pharmacol Biochem Behav. 2007;86 (4):784–96. doi: 10.1016/j.pbb.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120 (2):102–28. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett MC, Harley J, Francis D, Sanghani SP, Davis WI, Kuhar MJ. Maternal separation and handling affects cocaine self-administration in both the treated pups as adults and the dams. J Pharmacol Exp Ther. 2006;317 (3):1210–8. doi: 10.1124/jpet.106.101139. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Robinson TE. (Stimulant drugs and stress: factors influencing individual differences in the susceptibility to sensitization.). Stimulant drugs and stress: factors influencing individual differences in the susceptibility to sensitization. In: Kalivas PW, Barnes CD, editors. Sensitization in the Nervous System. Caldwell, NJ: Telford Press; pp. 145–73. [Google Scholar]
- Ruedi-Bettschen D, Pedersen EM, Feldon J, Pryce CR. Early deprivation under specific conditions leads to reduced interest in reward in adulthood in Wistar rats. Behav Brain Res. 2005;156 (2):297–310. doi: 10.1016/j.bbr.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Ruedi-Bettschen D, Zhang W, Russig H, Ferger B, Weston A, Pedersen EM, et al. Early deprivation leads to altered behavioural, autonomic and endocrine responses to environmental challenge in adult Fischer rats. Eur J Neurosci. 2006;24 (10):2879–93. doi: 10.1111/j.1460-9568.2006.05158.x. [DOI] [PubMed] [Google Scholar]
- Russek LG, Schwartz GE. Narrative descriptions of parental love and caring predict health status in midlife: a 35-year follow-up of the Harvard Mastery of Stress Study. Altern Ther Health Med. 1996;2 (6):55–62. [PubMed] [Google Scholar]
- Russek LG, Schwartz GE. Feelings of parental caring predict health status in midlife: a 35-year follow-up of the Harvard Mastery of Stress Study. J Behav Med. 1997;20 (1):1–13. doi: 10.1023/a:1025525428213. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162 (1):127–34. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Kelsey JE, Stewart J. Temporal factors in the effect of restraint stress on morphine-induced behavioral sensitization in the rat. Psychopharmacology (Berl) 1995;117 (1):102–9. doi: 10.1007/BF02245104. [DOI] [PubMed] [Google Scholar]
- Shalev U, Kafkafi N. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharmacol Biochem Behav. 2002;73 (1):115–22. doi: 10.1016/s0091-3057(02)00756-6. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Kalivas PW. Effects of cocaine and footshock stress on extracellular dopamine levels in the ventral striatum. Brain Res. 1991;559 (1):29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, O’Neill KS, Kriksciukaite K, Turncliff RZ, Dean RL, Ostrovsky-Day I, et al. Route of administration affects the ability of naltrexone to reduce amphetamine-potentiated brain stimulation reward in rats. Addict Biol. 2009;14 (4):408–18. doi: 10.1111/j.1369-1600.2009.00161.x. [DOI] [PubMed] [Google Scholar]
- Vazquez V, Farley S, Giros B, Dauge V. Maternal deprivation increases behavioural reactivity to stressful situations in adulthood: suppression by the CCK2 antagonist L365,260. Psychopharmacology (Berl) 2005;181 (4):706–13. doi: 10.1007/s00213-005-0029-0. [DOI] [PubMed] [Google Scholar]
- Vazquez V, Giros B, Dauge V. Maternal deprivation specifically enhances vulnerability to opiate dependence. Behav Pharmacol. 2006;17 (8):715–24. doi: 10.1097/FBP.0b013e3280116e6f. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Reber SO, Selch S, Obermeier F, Neumann ID. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149 (6):2727–36. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Reijmers LG, Van der Harst JE, Leus IE, Van den Bos R, Spruijt BM. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res. 2000;117 (1–2):137–46. doi: 10.1016/s0166-4328(00)00300-4. [DOI] [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51 (1–2):13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]